Abstract
A previously undescribed plant-microbe interaction between a root-colonizing Streptomyces species, S. lydicus WYEC108, and the legume Pisum sativum is described. The interaction is potentially of great importance to the health and growth in nature of this nodulating legume. The root-colonizing soil actinomycete S. lydicus WYEC108 influences pea root nodulation by increasing root nodulation frequency, possibly at the level of infection by Rhizobium spp. S. lydicus also colonizes and then sporulates within the surface cell layers of the nodules. Colonization leads to an increase in the average size of the nodules that form and improves the vigor of bacteroids within the nodules by enhancing nodular assimilation of iron and possibly other soil nutrients. Bacteroid accumulation of the carbon storage polymer, poly-β-hydroxybutyrate, is reduced in colonized nodules. Root nodules of peas taken from agricultural fields in the Palouse hills of northern Idaho were also found to be colonized by actinomycete hyphae. We hypothesize that root and nodule colonization is one of several mechanisms by which Streptomyces acts as a naturally occurring plant growth-promoting bacterium in pea and possibly other leguminous plants.
Streptomyces lydicus WYEC108 is a root-colonizing actinomycete originally isolated and studied for its properties as an antifungal biocontrol agent. This strain is capable of mycoparasitic colonization of fungal root pathogens and excretion of antifungal metabolites within plant rhizospheres (16, 62). Recently, we demonstrated that strain WYEC108 is also a plant growth-promoting bacterium in the absence of fungal pathogen challenge. This may be due to the ability of strain WYEC108 to produce hydroxamate-type siderophores and/or other plant growth-promoting metabolites in the rhizosphere (25). Streptomyces spp. have been previously described as rhizosphere-colonizing bacteria (37, 38), antifungal biocontrol agents useful in controlling fungal root diseases (51), in vitro siderophore producers, and in vitro producers of plant growth-promoting hormones (25). Plant root exudates stimulate rhizosphere growth of actinomycetes that are strongly antagonistic to fungal pathogens, while the actinomycetes utilize root exudates for growth and synthesis of antimicrobial substances (16, 62). In addition, actinomycetes synthesize an array of biodegradative enzymes, including chitinases (9, 23, 35), glucanases (18, 26, 29, 32, 59, 60), peroxidases (48), and other enzymes possibly involved in mycoparasitic activity. Yet, the overall importance, physiological activities, and symbiotic roles of actinomycetes in situ within plant rhizospheres remain little studied at the biochemical or mechanistic levels. We believe that Streptomyces are far more important rhizosphere bacteria than has been generally recognized.
Important to the symbiotic relationship between plants and microbes is the acquisition of iron. Although abundant in nature, under aerobic conditions at a neutral or alkaline pH, iron is found in highly insoluble forms not readily available for cellular uptake (42, 43). Plants and bacteria have evolved various scavenging systems to acquire iron from the environment (22, 40). These systems often utilize low-molecular-weight iron-scavenging molecules, siderophores, which have high affinities for iron (12). Iron is found in proteins such as nitrogenase, ferredoxins, cytochromes, and leghemoglobin, all of which are important in the symbiotic relationship between legumes and Rhizobium spp. (44, 49, 50, 56). Soil Streptomyces has been reported to produce hydroxamate-type siderophores (25, 40, 41).
S. lydicus WYEC108 was originally isolated from a rhizosphere soil of linseed (16). It was found to produce a number of extracellular chitinases (35); a low-molecular-weight, hydrophilic antifungal compound particularly active against oomycete fungi (46); and hydroxamate-type siderophores (25). This strain is also a plant growth-promoting bacterium in the absence of fungal pathogen stress, in plants such as carrots and beets (25).
Recently, we observed serendipitously that when S. lydicus WYEC108 colonizes the roots of young pea seedlings, it specifically influences root nodulation by Rhizobium. Vegetative hyphae of S. lydicus WYEC108 colonize the surface of emerging nodules and then sporulate within root surface cell layers. S. lydicus WYEC108 appears to develop a beneficial relationship with nodule bacteroids. Experiments were undertaken to investigate the significance of these observations, which turn out to be a potentially important plant-microbe interaction not previously known. In this previously undescribed apparent symbiosis, the streptomycete receives nutrients from the plant in the form of exudates and/or plant cell biomass. The streptomycete promotes nodulation, enhances nodule growth and bacteroid differentiation, and aids the bacteroids in assimilating iron and possibly other inorganic nutrients from the soil. The result is enhanced overall growth of the plant.
MATERIALS AND METHODS
Soil composition, plant, and bacterial strains.
Experiments were conducted in nonsterile Palouse silt loam (PSL) in a growth chamber. The soil had the following characteristics: pH 5.7; total carbon, 19.5 g/kg; organic carbon, 19.2 g/kg; total nitrogen, 1.6 g/kg; clay, 211 g/kg; sand, 88 g/kg (39). Dry peas and lentils are major crops in this region of northern Idaho, and the soils are naturally abundant in Rhizobium. The legume used in these studies was the pea plant (Pisum sativum); S. lydicus WYEC108 was used as the root-colonizing actinomycete (16). Inoculum was prepared as a spore suspension in sterile talc (108 CFU/g). Spores were scraped from the surface of sporulation agar plates (62) and aseptically transferred and mixed into presterilized talc.
Seed inoculation, plant growth, and harvest.
Individual pea seeds were inoculated with 1 g of spore suspension. Seeds were sowed by the removal of soil to create a cavity 2.0 to 2.5 cm deep. The spore formulation was placed into the cavity, the seed was then placed on top of the formulation, and soil was replaced to cover the seed. Control seeds were treated with 1 g of sterile talc. All seeds were planted in polyvinyl chloride containers (50 cm long with an inside diameter of 3.75 cm; schedule 40 polyvinyl chloride pipe). Seeds were germinated, and plants were grown in a growth chamber (Conviron) at a relative humidity of 96% with 16 h of daylight. Chamber temperature was maintained at 28°C during the lighted hours and 20°C during the dark period. Plants were harvested after intervals of up to 30 days. Various characterizations of the harvested plants were performed, including determinations of plant wet weights, root wet weights, average shoot lengths, average root lengths, average number of nodules per root, and average nodule weight.
Light microscopy, transmission electron microscopy (TEM), and scanning electron microscopy (SEM).
Nodules from inoculated and control plants were washed several times in distilled water and then surface sterilized with 70% ethanol. The nodules were then individually crushed in 1.0-ml aliquots of sterile distilled water, and smears from the resulting suspensions were prepared on microscope slides and stained as previously described (20) and viewed by light microscopy at a magnification of ×1,000.
Roots and nodules from inoculated and control plants were also examined by electron microscopy. For SEM, washed nodules were fixed in 2 to 3 ml of 1.5% glutaraldehyde in 0.2 M cacodylate buffer (10) and then rinsed with the same buffer for three 10-min intervals. Osmium tetroxide (1 to 2%) was added to the samples. They were left in the solution for 12 to 16 h at 4°C. Next, the samples were rinsed with 0.2 M cacodylate buffer, three times for 10 min each, and then dehydrated in a graded series of ethanol. These samples were then critical point dried, coated with gold, and observed under a scanning electron microscope (model 1830; Amray Inc., Bedford, Mass.). For TEM, the samples were processed as above, until the dehydration stage (10). Samples were dehydrated in a graded series of acetone and infiltrated in Spurrs (resin polymer) overnight (for 12 h at 60°C) with five changes before the samples were embedded in flat molds (Ted Pella, Redding, Calif.). Thin sections were made with a Bromma 8800 Ultratome III (LKB Co., Bromma, Sweden) by using a Diatome diamond knife (Diatome Ltd., Biel, Switzerland). The resulting sections were viewed under a JEOL 1200 EX II transmission electron microscope (Japan Electronic Optics Laboratory).
Negative staining was performed by resuspending cell pellets in 100 μl of cacodylate buffer. A 200-mesh copper grid (Ted Pella) was dipped into 5 μl of sample suspension, allowed to dry for 5 s, and then introduced into 0.5% (wt/vol) phosphotungstic acid, dried for 5 s, and then rinsed in distilled water for 30 s (10). The sample was air dried and viewed using TEM.
Enumeration of bacteroids.
To enumerate bacteroids, measurement bars in the micrographs were used as guides. A square with an area of 4 cm2 was cut, and four transects were conducted on each of the micrographs at random locations (see Fig. 3). The bacteroids present within each square were counted, and the average number per square micrometer was calculated. The differences between nodules from control plants and those from inoculated plants were statistically analyzed to determine their level of significance.
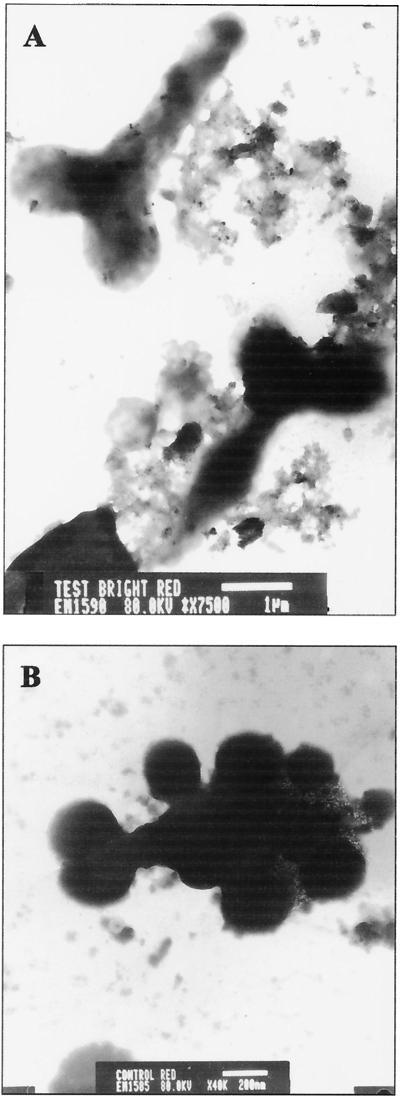
FIG. 3.
TEM micrographs of root nodule contents. (A) Thirty-day-old colonized plant nodule with well-differentiated bacteroids (bar = 1 μm). (B) Thirty-day-old control plant nodule with a less-differentiated bacteroid (bar = 200 nm).
EDXS.
In conjunction with the electron microscopy studies, the distribution of iron (Fe) and molybdenum (Mo) within the roots, nodules, and bacteroids was determined by energy-dispersive X-ray spectroscopy (EDXS). Sample preparation was the same as that described for SEM. The samples were analyzed using an X-ray analyzer (Norann Inc., Madison, Wis.).
Sample preparation for EDXS of clay particles.
Ten grams of the rhizosphere soil (soil physically removed from the roots) was added to 100 ml of sterile distilled water, and the suspension was allowed to settle for 10 min. A loopful of the supernatant was placed on a microscope slide and air dried. The rest of the processing was the same as above, except that the sample was not fixed.
In vitro production of siderophores by S. lydicus WYEC108.
For detection of siderophores, the universal chrome azurol sulfate (CAS) indicator solution for siderophore detection was prepared as previously described by Schwyn and Neilands (54), with modifications. Modified solutions used included 10 mM hexadecyltrimethylammonium bromide (HDTMA) in double-distilled water (ddH2O) (solution I); 1 mM FeCl3 in 10 mM HCl (solution II); 2 mM CAS in ddH2O (solution III); and 4.3 g of piperazine dissolved in a small amount of H2O (∼20 ml) to which 12 M HCl (∼6.7 ml) was added to help dissolve the piperazine (solution IV). Six milliliters of solution I and 1.5 ml of solution II were added to 20 ml of H2O and gently mixed. Next, 7.5 ml of solution III was slowly added with gentle mixing. Finally, solution IV was added and the volume was increased to 100 ml with ddH2O. The pH of the CAS indicator solution was then adjusted to 5.6. To 0.5 ml of culture supernatant, 0.5 ml of CAS indicator solution was added, and the percent transmittance at 630 nm was recorded.
Siderophore excretion by Streptomyces was detected by growth on freshly prepared CAS agar plates (1). The growth medium used was the rhizosphere siderophore medium (RSM) (11). One hundred milliliters of the following CAS solution was added to 900 ml of sterile, molten RSM agar (1.5% wt/vol): 10 ml of 1 mM FeCl3 · 6H2O (in 10 mM HCl) mixed with 50 ml of aqueous solution of CAS (1.21 g/liter). The mixture was then autoclaved, cooled to 50°C, and added to the molten RSM agar. Agar plates were immediately poured. Cultures positive for siderophore production produced a halo of orange around the colony where siderophores had chelated iron that had been bound to the dye. S. lydicus WYEC108, a strain known to excrete hydroxamate-type siderophores (11, 25), was used as a positive control.
Hydroxamate siderophores were assayed by the method of Csaky (17). To 0.5 ml of culture supernatant, 0.5 ml of 6 M H2SO4 was added; the mixture was autoclaved in a glass-stoppered tube at 121°C for 30 min and allowed to cool, and 1.0 ml of 1% (wt/vol) sulfanilic acid in 30% (vol/vol) acetic acid and 0.5 ml of 1.3% (wt/vol) iodine in 30% (vol/vol) acetic acid were added. After 5 min at room temperature, excess I2 was eliminated by the addition of 1.0 ml of 2% (wt/vol) Na3AsO2 solution. A solution of α-naphthylamine (0.3% [wt/vol] in 30% acetic acid [vol/vol]; 1 ml) was added, and the total volume was increased to 10 ml with distilled water. After 30 min at room temperature, absorbance at 526 nm was recorded. The assay is quantitative for hydroxamic acids. Hydroxylamine hydrochloride was used as a standard.
Catechol siderophores were assayed using the method of Arnow (4). One milliliter of the culture supernatant was decanted to a test tube and reagents were added in the following order, with mixing after each addition: 1 ml of 0.5 M HCl, 1 ml of nitrite-molybdate reagent, 1 ml of 1 M sodium hydroxide, and ddH2O to increase the volume to 5 ml. Absorbance at 510 nm was recorded.
Using a Biochemicals Kit (catalog no. 139 076; Boehringer Mannheim, Indianapolis, Ind.), the presence of another possible iron chelator, citric acid, was assayed.
Characterization of 16S rRNA genes from microbial DNA present within nodules.
These analyses were carried out using PCR amplification and denaturing gradient gel electrophoresis (DGGE) analysis to isolate and characterize the bacterial 16S ribosomal DNA (rDNA) (2, 33). Washed and surface-sterilized nodules were individually crushed in 1.0-ml aliquots of sterile distilled water and centrifuged at 16,000 × g (Eppendorf centrifuge model 5415C) for 3 min, and the supernatant was removed. The cell pellet was resuspended in 50 μl of sterile distilled water. Next, 1 μl of this suspension was added to the PCR mixture. The forward primer was 338F (5′-ACT CCT ACG GGA GGC AGC-3′) (2), which corresponds to a conserved region of the 5′ end of bacterial 16S rDNA, and the reverse primer was 907R (5′-CCG TCA ATT CMT TTR AGT TT-3′) (33), which corresponds to a conserved region of the 3′ end of bacterial 16S rDNA. A GC clamp was added to the forward primer. PCR was performed in a PTC 100 thermocycler (MJ Research, Inc.), under the following conditions: initial denaturation at 99°C for 15 min and an intermediate hold stage at 80°C for 10 min. Thirty cycles of denaturation (30 s at 94°C), annealing (30 s at 55°C), and extension (45 s at 72°C) were employed, with a final extension for 45 s at 72°C. The 50-μl reaction volume contained 1 μl of a 1× PCR buffer solution (Gibco-BRL, Life Technologies), 1 mM MgCl2 (Gibco-BRL), 0.5× bovine serum albumin (New England Biolabs), a 2 μM concentration of each primer, a 0.05 mM concentration of each deoxynucleoside triphosphate (Gibco-BRL), and 0.25 μl of Taq polymerase (Gibco-BRL). The PCR-amplified products were further separated by DGGE (Bio-Rad Labs, Richmond, Calif.) on a 60-to-80% denaturant gradient (5.6 M urea and 4.2 M formamide) for 21 h at 60 V and at a temperature of 60°C. Bands obtained by DGGE were excised with a sterile scalpel blade and processed by the crush-and-soak method (52). One microliter of the purified band was amplified by PCR, and the resultant product was sequenced by the Laboratory for Biotechnology and Bioanalysis (School of Molecular Biosciences, Washington State University, Pullman).
Plant growth trials.
Trials were conducted in a growth chamber as well as in a greenhouse. In the chamber, each run consisted of five plants, of which three were inoculated and two were noninoculated. The data presented are pooled from three experiments since there was homogeneity of variances between trials and since the environmental conditions did not vary between trials. The greenhouse trials involved 30 test and 30 control plants for every experiment; the conditions in the greenhouse were not as stringent as those in the laboratory. The relative humidity varied from 50 to 60%. Pooled data are presented since there was homogeneity of variances and since the environmental conditions did not vary between trials.
Nitrogenase assay.
Laboratory-grown plants were assayed for their nitrogenase activity. For greenhouse-grown plants, five control and five test plants were selected at random and assayed for nitrogenase activity. Preparation of the plants and generation of acetylene for the assay were done as described previously (57). The roots were assayed in 30-ml Balch tubes. Three milliliters of acetylene in an air mixture was injected into the tubes. Incubation was for 1 h at room temperature. Three hundred microliters of the headspace gas was withdrawn from the tubes and assayed for nitrogenase activity. Calibration of the gas chromatograph (HP 5890 series II) and integration of the ethylene peaks were done as previously described (57). A Porapak N column (1.9 m by 0.3125 cm; Alltech part no. 2716C) was used at a temperature of 100°C; the injector temperature was 140°C. The carrier gas (helium), air, and hydrogen gas flow rates were maintained as previously described (57)
Statistical analysis.
All results were subjected to the univariate procedure and the Proc T test of the SAS (SAS Institute, Cary, N.C.) analysis program.
RESULTS
After 30 days of growth, both control and S. lydicus WYEC108-inoculated pea plants were healthy (Fig. 1), but the inoculated plants had longer shoots, higher average plant and root wet weights, higher average nodule weight, and significantly higher average nitrogenase activities compared to the uninoculated control plants (Table 1 and Table 2). All of the differences were statistically significant. Visual observations showed that the numbers of lateral roots and root hairs were also higher in the inoculated plants than in the control plants (data not presented). Roots from inoculated plants also contained a greater number of root nodules, and the average nodule weight was significantly greater for inoculated plants than for controls (Tables 1 and 2). In inoculated plants, root nodules were present as clusters of two to four arising from the same node on the root hair, while in controls, generally single nodules emerged from a node. Nodules from inoculated plants were pinker than control plant nodules, which were white or greenish white (data not shown).
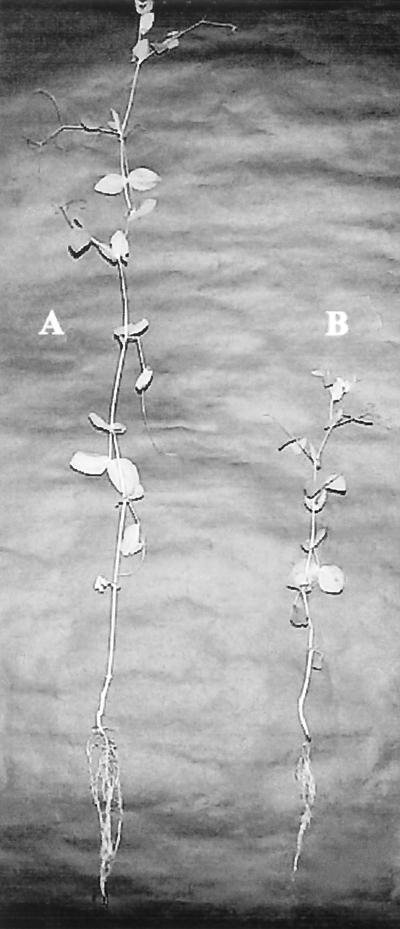
FIG. 1.
Photograph of 30-day-old inoculated and uninoculated pea plants. (A) Thirty-day-old inoculated pea plant, showing the enhanced shoot and root length of the plant. (B) Healthy 30-day-old control plant.
TABLE 1.
Results of growth chamber trials for S. lydicus WYEC108-colonized and noncolonized pea plants after 30 days (n = 15)
| Response variable | Value for variable (mean ± SD)
|
t ratio | P | |
|---|---|---|---|---|
| Control | Test | |||
| Avg shoot length (cm) | 32.37 ± 8.31 | 59.5 ± 15.91 | −3.74 | 0.0032 |
| Avg root length (cm) | 9.08 ± 5.97 | 16.20 ± 6.43 | −2.07 | 0.0631 |
| Avg plant wet wt (g) | 1.59 ± 0.37 | 2.56 ± 0.56 | −3.69 | 0.0039 |
| Avg root wet wt (g) | 0.44 ± 0.27 | 0.84 ± 0.41 | −2.05 | 0.067 |
| Avg no. of nodules | 7.33 ± 4.18 | 29.14 ± 16.77 | −3.09 | 0.0103 |
| Avg nodule wet wt (g) | 0.004 ± 0.003 | 0.037 ± 0.023 | −3.45 | 0.0055 |
| Avg nitrogenase activity (μmol of ethylene produced/h/plant) | 0.47 ± 0.43 | 52.08 ± 3.39 | −36.98 | 0.0001 |
TABLE 2.
Results of greenhouse trials (n = 134) and nitrogenase assays (n =30) for S. lydicus WYEC108-colonized and noncolonized pea plants after 30 days of growth
| Response variable | Value for variable (mean ± SD)
|
t ratio | P | |
|---|---|---|---|---|
| Control | Test | |||
| Avg shoot length (cm) | 26.15 ± 7.25 | 35.08 ± 8.42 | −6.23 | 0.0001 |
| Avg root length (cm) | 13.66 ± 4.84 | 17.29 ± 5.79 | −3.54 | 0.0001 |
| Avg plant wet wt (g) | 1.90 ± 0.74 | 2.96 ± 1.09 | −6.15 | 0.0001 |
| Avg root wet wt (g) | 0.98 ± 0.46 | 1.49 ± 0.78 | −4.35 | 0.0001 |
| Avg no. of nodules | 23.32 ± 20.55 | 37.82 ± 26.53 | −3.36 | 0.0011 |
| Avg nodule wet wt (g) | 0.01 ± 0.0105 | 0.036 ± 0.0359 | −5.48 | 0.0001 |
| Avg nitrogenase activity (μmol of ethylene produced/h/plant) | 0.91 ± 1.64 | 8.07 ± 7.85 | −3.46 | 0.0018 |
Light microscopy showed that bacteroids within the Sudan black B-stained preparations of crushed nodules from S. lydicus WYEC108-colonized nodules were markedly different from those within preparations of control nodules (Fig. 2).
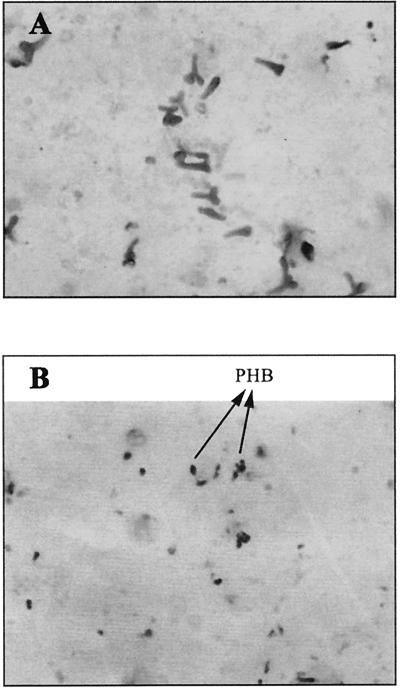
FIG. 2.
Sudan black B-stained preparation of root nodule contents (magnification, ×1,000). (A) Thirty-day-old colonized plant nodule bacteroids, with negligible amounts of intracellular PHB (black) accumulation noted, indicating vigorous bacteroids. (B) Thirty-day-old control plant nodule bacteroids, with greater amounts of intracellular PHB (black) accumulation noted, indicating bacteroids responding to a high C/N ratio.
There were greater numbers of bacteroids in the nodules from inoculated plants; they were more differentiated, and they contained less poly-β-hydroxybutyrate (PHB) than did those from controls. Control bacteroids contained considerable amounts of PHB (an indicator of high C/N stress), and cellular morphology was less defined.
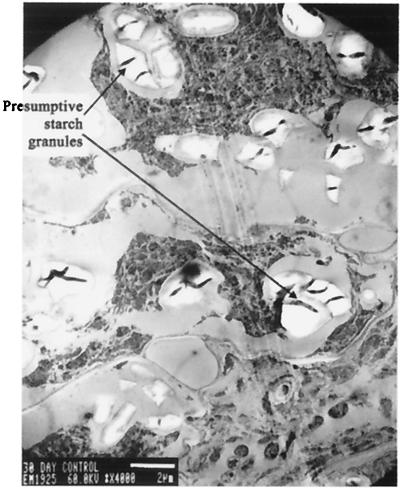
TEM analyses of nodule cross-sections confirmed and extended the observations made by light microscopy. Bacteroids from control nodules were fewer, contained more PHB, and were more senescent than the more numerous and vigorous bacteroids that contained less PHB in nodules from S. lydicus WYEC108-inoculated plants (Fig. 3 and 4). In typical TEM pictures, the number of bacteroids (mean ± standard deviation) was 0.94 ± 0.75 and 2.1 ± 0.25 per μm2 (t ratio = −3.02; P = 0.0235), respectively, for nodules from control and inoculated plants. Bacteroids in the nodules from 30-day control plants were already senescent, while those from inoculated plants were vigorous and well differentiated (Fig. 4). In addition, control nodules contained presumptive starch deposits, which were absent in the colonized nodules (Fig. 5).
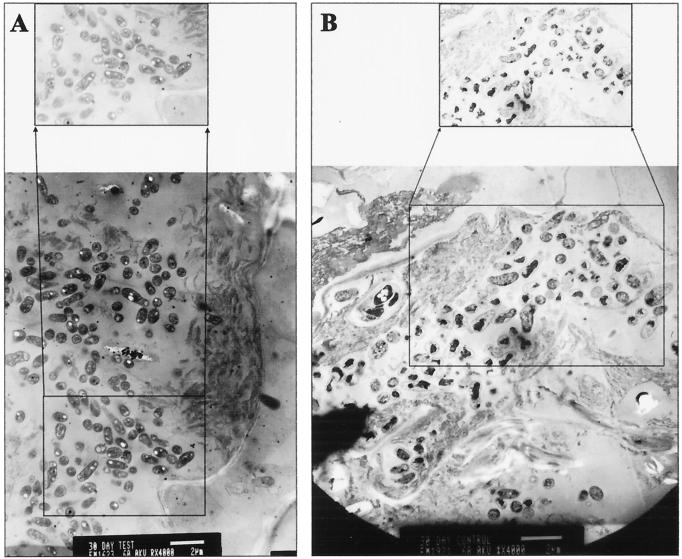
FIG. 4.
TEM micrographs of root nodule contents (bar = 2 μm). The expanded area depicts in greater detail the morphology and number of bacteroids. (A) Bacteroids from a colonized plant nodule appear vigorous and more numerous. (B) Bacteroids from a control plant nodule appear senescent, and vacuole-laden tissue is present.
FIG. 5.
TEM micrograph of the cellular components of a control nodule with starch deposits (bar = 2 μm).
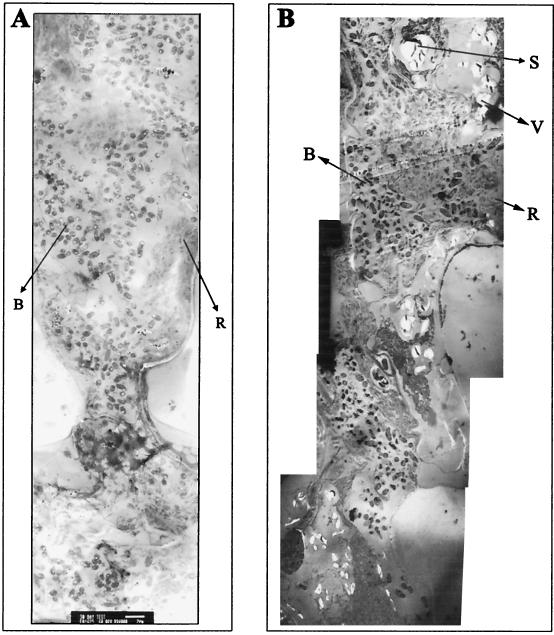
TEM micrographs (Fig. 6) of both the Streptomyces-inoculated and control nodules revealed remarkable differences between the colonized and control nodules, with respect to bacteroid morphology as well as the cytology of the nodules. The Streptomyces-colonized nodules were larger; contained more bacteroids; and had less accumulation of rough endoplasmic reticulum (RER), fewer polysomes (determined visually, but not quantified), and virtually no starch granules or vacuoles. In contrast, control nodules were smaller than colonized nodules, had fewer bacteroids, were generally senescent, had greater accumulation of RER, had more polysomes, and had numerous vacuoles as well as starch granules.
FIG. 6.
TEM micrographs of root nodule contents (bar = 2 μm). (A) Thirty-day-old colonized nodule, with vigorous bacteroids, a lack of vacuoles, and less accumulation of RER and polysomes. (B) Thirty-day-old control nodule with senescent bacteroids, more vacuoles, and greater RER and polysome accumulation. Abbreviations: B, bacteroids; R, RER and polysomes; S, starch deposits; V, vacuoles.
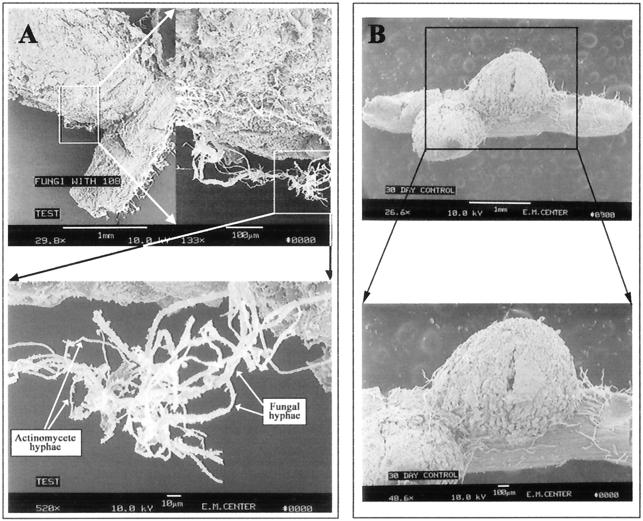
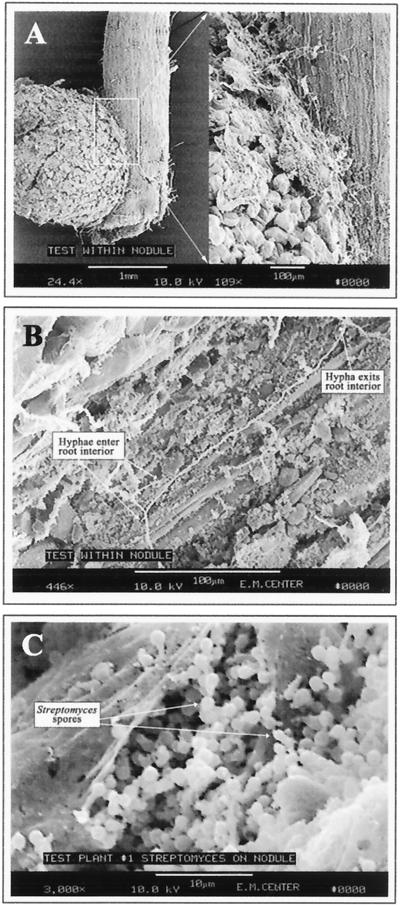
SEM revealed a remarkable degree of preferential colonization of nodules relative to roots by S. lydicus WYEC108 (Fig. 7 to 9). Nodulated roots from inoculated plants exhibited significant surface colonization by Streptomyces (Fig. 7A), while those from uninoculated control plants did not (Fig. 7B). Observations based on numerous SEM preparations indicated that colonization was initially nodule specific. The Streptomyces hyphae moved from the nodule onto the root at the base of the nodule. Once on the nodule and root surface, the hyphae spread and actually penetrated the surface cell layer (Fig. 8). Figure 8B shows hyphae entering and then exiting the surface cell layer of a nodule. An additional observation from the SEM analyses was that Streptomyces hyphae were occasionally observed in close association with the few fungal hyphae present (Fig. 7A). This observation did not provide information about the nature of the fungi or its relationship with the Streptomyces but warrants further study.
FIG. 7.
SEM micrographs of the root nodule surface. (A) Thirty-day-old colonized nodule, with intense surface colonization by actinomycete hyphae (top panel; bar = 1 mm) and fungal hyphae interlocked with actinomycete hyphae on the colonized root surface (bottom panel; bar = 10 μm). (B) Thirty-day-old control nodule, with absence of colonization by any actinomycete; the filaments are root hairs (bar = 1 mm [top panel] and 100 μm [bottom panel]).
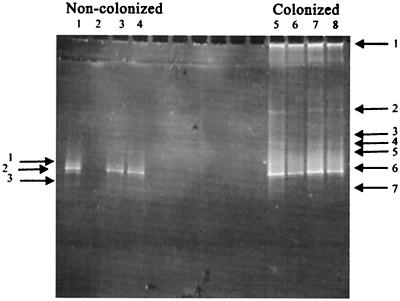
FIG. 9.
DGGE analysis of 16S rDNA bands obtained from noncolonized and colonized plant nodules. Different lanes are replicate samples; lane 2 is a blank lane.
FIG. 8.
SEM micrographs of the root nodule surface and its interior. (A) Actinomycete hypha moving from nodule to root surface and penetrating the surface cell layers of the root (bar = 100 μm). (B) Actinomycete colonizing the interior of the nodule; the bacterium appears to be moving from the external surface of the root cells into the interior of the root cells, intermittently (bar = 100 μm). (C) Thirty-day-old colonized nodule with subsurface sporulation of the Streptomyces on colonized nodule (bar = 10 μm).
EDXS analyses of whole-nodule preparations showed that nodules from 30-day-old inoculated plants contained more Fe and Mo by both atom percent and weight percent than did bacteroids from control plants (Table 3). The differences were statistically significant. These differences between colonized and control nodules were not observed in nodule preparations from 10- and 20-day-old plants (data not shown). EDXS analysis, conducted on both groups in conjunction with TEM analysis, showed that more than 90% of the Fe and Mo present in the nodule accumulated in the zone of bacteroid growth. This indicates the significance of Fe and Mo in the growth and function of bacteroids and the striking effect of Streptomyces nodule colonization on the accumulation of these metals.
TABLE 3.
Typical values from EDXS analysis of nodules, bulk, and rhizoplane PSL soil
| Response variable | Value for variable (mean ± SD) in:
|
t ratio | P | ||
|---|---|---|---|---|---|
| 30-Day nodule
|
Clay particles | ||||
| Control | Colonized | ||||
| Avg Fe (atom%) | 1.23 ± 0.35 | 5.04 ± 1.62 | 0.73 | −3.94 | 0.0479 |
| Avg Fe wt (%) | 1.98 ± 0.56 | 8.39 ± 2.69 | 0.42 | −3.99 | 0.0472 |
| Avg Mo (atom%) | 0.55 ± 0.16 | 3.06 ± 0.95 | 0.20 | −3.51 | 0.0391 |
| Avg Mo wt (%) | 1.54 ± 0.43 | 8.7 ± 2.42 | 0.69 | −3.93 | 0.0294 |
Elemental analysis of the bulk soil by the University of Idaho Analytical Laboratory showed an Fe content of 24,000 μg/g and an Mo content of <3.8 μg/g. The PSL was also analyzed by EDXS for its content of Fe and Mo (Table 3). It is possible that any adhering soil (clay) particles might influence the results of the Fe and Mo determinations, even though the nodules were surface washed prior to EDXS analysis. EDXS of the clay particles from PSL that might adhere to the nodules in spite of vigorous washings showed that the Fe content was 0.73 on an atom percent basis and 1.42 on a weight percent basis. Since elemental analysis of the bulk soil showed Fe to be present at a concentration of 24,000 μg/g and Mo to be present at <3.8 μg/g, there was little Fe or Mo present in clay particles that might adhere to roots.
The EDXS data indicated that S. lydicus WYEC108 may be involved in supplying Fe and/or Mo to the bacteroids within the nodules. Therefore, the ability of this strain to produce siderophores was evaluated in vitro. The various siderophore characterization assays showed that S. lydicus WYEC108 produces one or more hydroxamate siderophores but not catechol siderophores or citrate (data not shown).
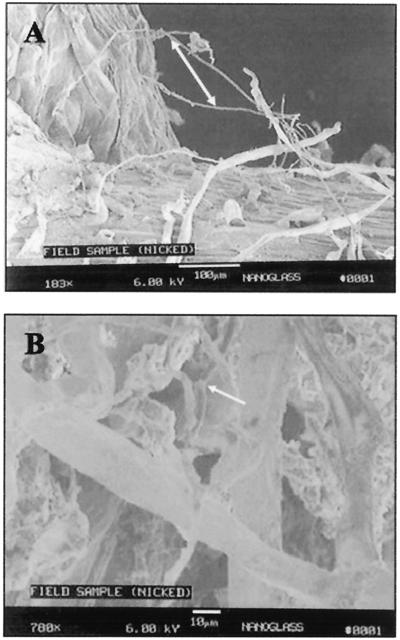
The bacterial DNA present in the nodules from inoculated and control plants was analyzed for diversity of 16S rDNA using PCR amplification, DGGE analysis, and sequencing to isolate and identify the bacterial 16S rDNA (2, 33). PCR-DGGE analysis of DNA from colonized nodules revealed seven amplified DNA bands, while analysis of noncolonized nodules revealed three bands (Fig. 9). Not surprisingly, a BLAST search of the sequences of bands 1 and 2 of the noncolonized nodules and bands 1, 3, 4, 5, and 6 of the colonized nodules obtained from these PCR products indicated the presence of Rhizobium species in nodules from both the noncolonized controls and colonized plants. In the colonized nodules, band 2 displayed 92% sequence homology with Streptomyces isolated from a rapeseed rhizosphere (accession no. AJ295528). Band 3 of the noncolonized nodules and band 7 of the colonized nodules showed 97% homology to the soybean 18S ribosomal gene. Finally, in order to determine if pea root and nodule colonization by Streptomyces is a natural event, young pea plants that were growing rapidly, but which had not yet flowered, were collected from a local field near Moscow, Idaho. Root nodules were examined by SEM, and, as shown in Fig. 10, the nodules from these plants were indeed colonized by actinomycete hyphae, confirming that the colonization reported here is not a laboratory artifact.
FIG. 10.
SEM micrographs of the root nodule surface. (A) Native actinomycete colonizing the surface of a root nodule of a pea plant taken from an agricultural field in north Idaho; arrow indicates the actinomycete hyphae (bar = 100 μm). (B) Close-up view of surface colonization of a nodule from a field plant colonized by native actinomycete hyphae; arrow indicates the hyphae (bar = 10 μm).
DISCUSSION
We are confident that we have discovered a novel plant-microbe interaction, which is at least specific to S. lydicus and P. sativum growing in PSL soil. Numerous observations were made that show the importance of this interaction to the health of this legume when it is growing in this soil.
PHB is produced by microbial cells in response to nitrogen limitation relative to carbon (47). The importance of tricarboxylic acid cycle regulatory events like PHB synthesis and alanine synthesis also depends on the overall need to balance the oxidation-reduction state of pyridine nucleotides with the total carbon and nitrogen pools in the bacteroids (47). Either factor might affect PHB accumulation in the bacteroids of the control and inoculated plants. Regardless, nodule colonization by S. lydicus clearly changes the metabolite pool within the nodules in a way that represses PHB synthesis. Our hypothesis is that the effect is related to more-vigorous and long-lived bacteroids and higher levels of nitrogen fixation by bacteroids within colonized nodules. This changes their soluble C/N ratio in a manner that signals the regulatory system to repress PHB synthesis.
Senescent nodules showed accumulation of starch grains (amyloplasts), a situation considered to be diagnostic of an ineffective nodule (45). The numerous senescent nodules within the 30-day-old control plants indicate that they were ineffective and nitrogen starved. The senescent bacteroids would most likely be ineffective at fixing nitrogen compared to the vigorous bacteroids from the Streptomyces-colonized nodules. The nodules from the inoculated plants were also pinker, indicating the presence of more leghemoglobin, giving the bacteroids within them the ability to fix greater amounts of nitrogen than the bacteroids within the nodules from the control plants (8). This observation is clearly reflected in the significantly higher nitrogenase activity for the test plants than for the control plants.
Visual estimates showed that bacteroids from the 30-day-old inoculated plant nodules contained a three to five times greater volume of living tissue within them than those from control plants, in which bacteroids were often mostly depleted of cellular material. This observation is similar to what Chen and Thornton (15) reported for effective versus ineffective nodules relative to nitrogen fixation abilities. The increased accumulation of RER in the control nodules reflects increased metabolic activity in response to nitrogen starvation (27, 34).
The pattern of colonization by S. lydicus appears to involve nodulation sites first. The vegetative hyphae on the nodule then move from the enlarging nodules onto the root hairs. This is accompanied by hyphal penetration of cell surface layers and by sporulation of the streptomycete. This pattern, along with the more numerous nodules in inoculated roots, leads us to hypothesize that the initial colonization of root hairs by S. lydicus plays some role in promoting a site-specific Rhizobium infection that helps initiate nodule formation. Various factors are thought to be involved in the complex process of nodulation. It is known that lipo-chitin signals induce nodule formation and that they mimic endogenous plant growth regulators (19, 53, 58, 61). Soil microorganisms such as Agrobacterium tumefaciens (13) or Bacillus subtilis (3) can also stimulate nodulation, through the production of bioactive molecules. This is supported by the observations that plant growth regulators like gibberellin and other plant hormones are produced by bacteria and actinomycetes (5-7, 28, 31). Roots also excrete phenolic compounds (55). Soil microorganisms, including rhizobia and actinomycetes, have the ability to degrade phenolic compounds (21, 55), indicating that phenolic compounds may be attractants for both. We hypothesize that S. lydicus colonizes young root hairs of germinating pea plants and that rhizobia may use these colonization sites as preferential or opportunistic infection sites. Though S. lydicus WYEC108 excretes antimicrobial compounds, antagonism tests indicate that strain WYEC108 does not inhibit the growth of Rhizobium leguminosarum on solid media (data not shown). Thus, S. lydicus appears to be compatible with Rhizobium.
The electron micrographs show that Streptomyces sporulates within the surface cell layer of the nodules and that vegetative hyphae penetrate surface root cell layers. Additional TEM studies are needed to determine if S. lydicus hyphae are present deeper within the nodules. Chaintreuil et al. (14) have reported intense bacterial colonization of the root surface and even bacterial invasion into deeper intercellular spaces. They also noted a few intracellular bacterial infections in epidermal cells filled with bradyrhizobia. Also, actinomycetes like Frankia spp. form nodules with leguminous plants like Myrica spp., Alnus spp., and Shepherdia sp. (30). Gurney and Mantle (24) isolated an antibiotic-producing endophytic Streptomyces sp. from the perennial ryegrass. Considering these reports, the colonization of legume nodules by Streptomyces, though not reported previously, is not surprising.
The intimate association of S. lydicus WYEC108 hyphae with a root-colonizing fungus is also an interesting observation of as-yet-undetermined significance. S. lydicus is a producer of chitinases and other extracellular hydrolytic enzymes, and it has mycoparasitic properties (35, 62). The micrograph depicting the fungus-streptomycete association, however, may be indicative of the streptomycete parasitizing the fungus or existing in a mutualistic Streptomyces-fungus association.
The elevated Fe and Mo levels in the bacteroids of the colonized nodules are reflective of their vigor. Clearly, the bacteroids benefit from the presence of S. lydicus. One of the probable mechanisms by which S. lydicus enhances and lengthens bacteroid vigor is through the elaboration of Fe and/or Mo chelators that assimilate and then transfer the metals to the bacteroids. A number of other root-colonizing Streptomyces spp. produce hydroxamate siderophores (25). The EDXS data and the known ability of S. lydicus to produce hydroxamate-type siderophores support this conclusion. The PSL soil used in the present study contained limited amounts of Mo. Also, the amount of bioavailable iron may be low, since nodules in the control plants showed signs of Fe and/or Mo stress. This was not the case for the vigorous and well-differentiated S. lydicus-colonized nodules. This, too, supports the involvement of the streptomycete in sequestering metals for the bacteroids.
Further research is also needed to examine the possibility that other Streptomyces strains might be equally good colonizers of legume roots, especially in light of the fact that strain WYEC108 was not originally isolated from a pea rhizosphere (16) and that it is a plant growth-enhancing bacterium in plants such as carrot and beet (25). It is also important to determine if this S. lydicus symbiosis is highly specific for pea, or if it will extend to other leguminous plants.
The plant-microbe interaction described here has not been reported previously. The implications of the discovery are significant, particularly if further research confirms that Streptomyces-legume root and nodule associations are common in nature, especially under conditions of low Fe and/or Mo availability. This appears to be the case for peas growing in agricultural fields within the Palouse area of northern Idaho, since we have found actinomycete-colonized nodules in field plants.
Overall, these results lead us to the conclusion that the nitrogen-fixing function of bacteroids within legume nodules may be dependent on or aided by external colonization by metal-chelating symbionts such as Streptomyces. The long-term significance of this discovery in terms of agricultural application might be in delaying senescence of bacteroids to improve N2 fixation during seed formation when plants require nitrogen the most (36).
Acknowledgments
We thank Dena Yoder of California State University, Chico, for providing the nodule crush procedure; Dave Newcombe and Evan Griffiths for their guidance in conducting DGGE analysis; Andrzej Paszczynski for his help in conducting the nitrogenase assay; Bahman Shafii and William Price for help in conducting the statistical analysis; Melinda Crawford for help in preparing the manuscript; and Mike Mortimer and Adriana Parra at the School of Biological Chemistry, Washington State University, for advice regarding the nitrogenase assay. We acknowledge the assistance of Chris M. Davitt, Valerie Lynch Holmes, and Vince Franceschi at the Electron Microscopy Center, Washington State University, Pullman, and of Sheri Wardwell and Josh Smith at the University of Idaho.
This research was supported in part by grant DAAD190110428 from the U.S. Department of Defense, Army Research Office; by seed grant KDY012 from the University of Idaho Research Council; and by the Idaho Agricultural Experiment Station.
REFERENCES
- 1.Abd-Alla, M. H. 1999. Growth and siderophore production in Bradyrhizobium (Lupin) strains under iron limitation. Folia Microbiol. 44:196-200. [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereaux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo, F. F., and M. Hungria. 1995. Comportamento a campo e casa de vegetacao de soja inoculada com Bacillus e Bradyrhizobium, p. 456-461. In M. Hungria, E. L. Balota, A. Colozzi-Filho, and D. S. Andrade (ed.), Microbilogia do solo: desafios para o seculo XXI. IAPAR/EMBRAPA-CNPSo, Londrina, PR, Brazil.
- 4.Arnow, L. E. 1937. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine-tyrosine mixtures. J. Biol. Chem. 118:531-537. [Google Scholar]
- 5.Atzorn, R. A., C. T. Crozier, C. T. Weeler, and G. Sanberg. 1988. Production of gibberellins and indole-3-acetic acid by Rhizobium phaseoli in relation to nodulation of Phaseolus vulgaris roots. Plant 175:532-536. [DOI] [PubMed] [Google Scholar]
- 6.Bakker, P. A. H. M., J. G. Lamers, A. W. Bakker, J. D. Marugg, P. J. Weisbeek, and B. Schippers. 1986. The role of siderophores in potato tuber yield increase by Pseudomonas putida in a short rotation of potato. Neth. J. Plant Pathol. 92:249-256. [Google Scholar]
- 7.Becker, J. O., and R. J. Cook. 1988. Role of siderophores in suppression of Pythium species and production of increased-growth response of wheat by fluorescent pseudomonads. Phytopathology 78:778-782. [Google Scholar]
- 8.Beringer, J. E., N. Brewin, A. W. Johnston, H. M. Schulman, and D. A. Hopwood. 1979. The rhizobium-legume symbiosis. Proc. R. Soc. Lond. Ser. B 2043:219-233. [DOI] [PubMed] [Google Scholar]
- 9.Blaak, H., J. Schnellmann, S. Walter, B. Henrissat, and H. Schrempf. 1993. Characteristics of an exochitinase from Streptomyces olivaceoviridis, its corresponding gene, putative protein domains and relationship to other chitinases. Eur. J. Biochem. 214:659-669. [DOI] [PubMed] [Google Scholar]
- 10.Bozzola, J. J., and L. D. Russell. 1992. Electron microscopy: principles and techniques for biologists, 2nd ed. Jones and Bartlett Publishers, Inc., Sudbury, Mass.
- 11.Buyer, J. S., L. J. Sikora, and R. L. Chaney. 1989. A new growth medium for the study of siderophore-mediated interactions. Biol. Fertil. Soils 8:97-101. [Google Scholar]
- 12.Cabrera, G., A. Xiong, M. Uebel, V. K. Singh, and R. K. Jayaswal. 2001. Molecular characterization of the iron-hydroxamate uptake system in Staphylococcus aureus. Appl. Environ. Microbiol. 67:1001-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caetano-Anolles, G., and W. D. Bauer. 1988. Enhanced nodule initiation on alfalfa by wild type Rhizobium meliloti co-inoculated with nod gene mutants and other bacteria. Planta 174:385-395. [DOI] [PubMed] [Google Scholar]
- 14.Chaintreuil, C., E. Giraud, Y. Prin, J. Lorquin, A. Bâ, M. Gillis, P. de Lajudie, and B. Dreyfus. 2000. Photosynthetic bradyrhizobia are natural endophytes of the African wild rice Oryza breviligulata. Appl. Environ. Microbiol. 66:5437-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, H. K., and H. G. Thornton. 1940. The structure of “ineffective” nodules and its influence on nitrogen fixation. Proc. R. Soc. Lond. Ser. B 129:208-229. [Google Scholar]
- 16.Crawford, D. L., J. M. Lynch, J. M. Whipps, and M. A. Ousley. 1993. Isolation and characterization of actinomycete antagonists of a fungal root pathogen. Appl. Environ. Microbiol. 59:3899-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Csaky, T. Z. 1948. On the estimation of bound hydroxylamine in biological materials. Acta Chem. Scand. 2:450-454. [Google Scholar]
- 18.Damude, H. G., N. R. Gilkes, D. G. Kilburn, R. C. Miller, Jr., and R. A. Warren. 1993. Endoglucanase CasA from alkalophilic Streptomyces strain KSM-9 is a typical member of family B of beta-1,4-glucanases. Gene 123:105-107. [DOI] [PubMed] [Google Scholar]
- 19.De Jong, A. J., R. Heidstra, H. P. Spaink, M. V. Hartog, E. A. Meijer, T. Hendriks, F. L. Schiavo, M. Terzi, T. Bisseling, A. V. Kammen, and S. C. deVries. 1993. Rhizobium lipooligosaccharides rescue a carrot somatic embryo mutant. Plant Cell 5:615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doetsch, R. N. 1981. Determinative methods of light microscopy, p. 21-33. In P. Gerhardt, R. G. E. Murray, R. N. Costilow, E. W. Nester, W. A. Wood, N. R. Krieg, and G. B. Phillips (ed.), Manual of methods for general bacteriology. American Society for Microbiology, Washington, D.C.
- 21.Golovleva, L. A., O. Zaborina, R. Pertsova, B. Baskunov, Y. Schurukhin, and S. Kuzmin. 1991. Degradation of polychlorinated phenols by Streptomyces rochei 303. Biodegradation 2:201-208. [DOI] [PubMed] [Google Scholar]
- 22.Guerinot, M. L. 1994. Microbial ion transport. Annu. Rev. Microbiol. 48:743-772. [DOI] [PubMed] [Google Scholar]
- 23.Gupta, R., R. K. Saxena, P. Chaturvedi, and J. S. Virdi. 1995. Chitinase production by Streptomyces viridificans: its potential in fungal cell wall lysis. J. Appl. Bacteriol. 78:378-383. [DOI] [PubMed] [Google Scholar]
- 24.Gurney, K. A., and P. G. Mantle. 1993. Biosynthesis of 1-N-methylalbonourosin by an endophytic Streptomyces sp. isolated from perennial ryegrass. J. Nat. Products 56:1194-1198. [Google Scholar]
- 25.Hamby, M. K. 2001. M.S. thesis. University of Idaho, Moscow.
- 26.Harchand, R. K., and S. Singh. 1997. Characterization of cellulase complex of Streptomyces albaduncus. J. Basic Microbiol. 37:93-103. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch, A. M., M. Bang, and F. M. Ausubel. 1983. Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of R. meliloti. J. Bacteriol. 155:367-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofte, M., K. Y. Seong, E. Jurkevitch, and W. Verstraete. 1991. Pyoverdin production by the plant growth beneficial Pseudomonas strain 7NSK2. Plant Soil 130:249-257. [Google Scholar]
- 29.Hopwood, D. 1990. Antibiotic biosyntheisis in Streptomyces, p. 129-148. In D. A. Hopwood and K. Chater (ed.), Genetics of bacterial diversity. Academic Press, London, United Kingdom.
- 30.Huguet, V., J. M. Batzli, J. F. Zimpfer, P. Normand, J. O. Dawson, and M. P. Fernandez. 2001. Diversity and specificity of Frankia strains in nodules of sympatric Myrica gale, Alnus incana, and Shepherdia canadensis determined by rrs gene polymorphism. Appl. Environ. Microbiol. 67:2116-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katznelson, H., and S. G. Cole. 1965. Production of gibberellin like substances by bacteria and actinomycetes. Can. J. Microbiol. 11:733-741. [DOI] [PubMed] [Google Scholar]
- 32.Konovalov, S. A., S. P. Vorotilo, and L. A. Ziukova. 1974. The biosynthesis of glucanases and mannanases, going into a lytic complex, by a heat tolerant strain of Actinomyces griseinus. Mikrobiologiia 43:261-266. [PubMed] [Google Scholar]
- 33.Lane, D. J., B. Pace, G. J. Olson, D. A. Stahl, M. L. Sogin, and N. R. Pace. 1985. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analysis. Proc. Natl. Acad. Sci. USA 82:6955-6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackenzie, C. R., and D. C. Jordan. 1974. Ultrastructure of root nodules formed by ineffective strains of Rhizobium meliloti. Can. J. Microbiol. 20:755-758. [DOI] [PubMed] [Google Scholar]
- 35.Mahadevan, B., and D. L. Crawford. 1996. Purification of chitinase from the biocontrol agent Streptomyces lydicus WYEC108. Enzyme Microb. Technol. 20:489-493. [Google Scholar]
- 36.Manen, J. F., P. Simon, J. C. V. Slooten, M. Osteras, S. Frutiger, and G. J. Hughes. 1991. A nodulin specifically expressed in senescent nodules of winged bean is a protease inhibitor. Plant Cell 3:259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, J. J., E. Liljeroth, G. Heinken, and J. A. V. Veen. 1990. Fluctuations in the fluorescent pseudomonad and actinomycete populations of rhizosphere and rhizoplane during the growth of spring wheat. Can. J. Microbiol. 36:254-258. [Google Scholar]
- 38.Miller, J. J., E. Liljeroth, M. J. E. I. M. Williamsen-De Klein, and J. A. V. Veen. 1990. The dynamics of actinomycetes and fluorescent pseudomonads in wheat rhizoplane and rhizosphere. Symbiosis 9:389-391. [Google Scholar]
- 39.Morra, M. J., R. R. Blank, L. L. Freeborn, and B. Shafii. 1991. Size fractionation of soil organo-mineral complexes using ultrasonic dispersion. Soil Sci. 152:294-303. [Google Scholar]
- 40.Muller, G., B. F. Matzanke, and K. N. Raymond. 1984. Iron transport in Streptomyces pilosus mediated by ferrichrome siderophores, rhodotorulic acid, and enantio-rhodotorulic acid. J. Bacteriol. 160:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller, G., and K. N. Raymond. 1984. Specificity and mechanism of ferrioxamine-mediated iron transport in Streptomyces pilosus. J. Bacteriol. 160:304-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neilands, J. B. 1981. Microbial iron compounds. Annu. Rev. Biochem. 50:715-731. [DOI] [PubMed] [Google Scholar]
- 43.Neilands, J. B. 1995. Siderophores: structure and function of microbial ion transport compound. J. Biol. Chem. 270:26723-26726. [DOI] [PubMed] [Google Scholar]
- 44.Noya, F., A. Arias, and E. Fabiano. 1997. Heme compounds as iron sources for nonpathogenic Rhizobium bacteria. J. Bacteriol. 179:3076-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nutman, P. S. 1959. Some observations on root hair infection by nodule bacteria. J. Exp. Bot. 10:250-263. [Google Scholar]
- 46.Ploeger, K. L. 1998. Ph.D. thesis. University of Idaho, Moscow.
- 47.Poole, P., and D. Allaway. 2000. Carbon and nitrogen metabolism in Rhizobium. Adv. Microb. Physiol. 43:119-150. [DOI] [PubMed] [Google Scholar]
- 48.Ramachandra, M., D. L. Crawford, and G. Hertel. 1988. Characterization of an extracellular lignin peroxidase of the lignocellulolytic actinomycete Streptomyces viridosporus. Appl. Environ. Microbiol. 54:3057-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reigh, G., and M. O'Connell. 1993. Siderophore-mediated iron transport correlates with the presence of specific iron-regulated proteins in the outer membrane of Rhizobium meliloti. J. Bacteriol. 175:94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rioux, C. R., D. C. Jordan, and J. B. Rattray. 1986. Iron requirement of Rhizobium leguminosarum and secretion of anthranilic acid during growth on an iron-deficient medium. Arch. Biochem. Biophys. 248:175-182. [DOI] [PubMed] [Google Scholar]
- 51.Rothrock, C. S., and D. Gottlieb. 1984. Role of antibiosis in antagonism of Streptomyces hygroscopicus var. geldanus to Rhizoctonia solanii in soil. Can. J. Microbiol. 30:1440-1447. [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 53.Schmidt, J., H. Rohrig, M. John, U. Weineke, G. Stacey, C. Koncz, and J. Schell. 1993. Alternation of plant growth and development by Rhizobium nodA and nodB genes involved in the synthesis of oligosaccharide signal molecules. Plant J. 4:651-658. [Google Scholar]
- 54.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 55.Siqueira, J. O., M. G. Nair, R. Hammerschmidt, and G. R. Safir. 1991. Significance of phenolic compounds in plant-soil-microbial systems. Crit. Rev. Plant Sci. 10:63-121. [Google Scholar]
- 56.Smith, M. J., J. N. Schoolery, B. Schwyn, I. Holden, and J. B. Neilands. 1985. Rhizobactin, a structurally novel siderophore from R. meliloti. J. Am. Chem. Soc. 107:1739-1743. [Google Scholar]
- 57.Somasegaran, P., and H. J. Hoben. 1994. Handbook for rhizobia: methods in legume-rhizobium technology. Springer-Verlag, New York, N.Y.
- 58.Spaink, H. P., A. H. M. Wijfjes, T. B. V. Vliet, J. W. Kijne, and B. J. J. Lugtenberg. 1993. Rhizobial lipo-oligosaccharide signals and their role in plant morphogenesis: are analogous lipophilic chitin derivatives produced by the plant? Aust. J. Plant Physiol. 20:381-392. [Google Scholar]
- 59.Thomas, L., and D. L. Crawford. 1998. Cloning of clustered S. viridosporus T7A lignocellulose catabolism genes encoding peroxidase and endoglucanase and their extracellular expression in Pichia pastoris. Can. J. Microbiol. 44:364-372. [DOI] [PubMed] [Google Scholar]
- 60.Trejo-Estrada, S. R., A. Paszczynski, and D. L. Crawford. 1998. Antibiotics and enzymes produced by the biological control agent Streptomyces violaceusniger YCED-9. J. Ind. Microbiol. Technol. 21:81-90. [Google Scholar]
- 61.Truchet, G., D. G. Barker, S. Camut, F. de Billy, J. Vasse, and T. Huguet. 1989. Alfalfa nodulation in the absence of Rhizobium. Mol. Gen. Genet. 219:65-68. [Google Scholar]
- 62.Yuan, W. M., and D. L. Crawford. 1995. Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Appl. Environ. Microbiol. 61:3119-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]