Abstract
Endophytic bacteria reside within plant hosts without causing disease symptoms. In this study, 853 endophytic strains were isolated from aerial tissues of four agronomic crop species and 27 prairie plant species. We determined several phenotypic properties and found approximately equal numbers of gram-negative and gram-positive isolates. In a greenhouse study, 28 of 86 prairie plant endophytes were found to colonize their original hosts at 42 days postinoculation at levels of 3.5 to 7.7 log10 CFU/g (fresh weight). More comprehensive colonization studies were conducted with 373 corn and sorghum endophytes. In growth room studies, none of the isolates displayed pathogenicity, and 69 of the strains were recovered from corn or sorghum seedlings at levels of 8.3 log10 CFU/plant or higher. Host range greenhouse studies demonstrated that 26 of 29 endophytes were recoverable from at least one host other than corn and sorghum at levels of up to 5.8 log10 CFU/g (fresh weight). Long-range dent corn greenhouse studies and field trials with 17 wild-type strains and 14 antibiotic-resistant mutants demonstrated bacterial persistence at significant average colonization levels ranging between 3.4 and 6.1 log10 CFU/g (fresh weight) up to 78 days postinoculation. Three prairie and three agronomic endophytes exhibiting the most promising levels of colonization and an ability to persist were identified as Cellulomonas, Clavibacter, Curtobacterium, and Microbacterium isolates by 16S rRNA gene sequence, fatty acid, and carbon source utilization analyses. This study defines for the first time the endophytic nature of Microbacterium testaceum. These microorganisms may be useful for biocontrol and other applications.
Endophytic bacteria are bacteria that live in plant tissues without doing substantive harm or gaining benefit other than residency (20, 21). Bacterial endophytes can be isolated from surface-disinfected plant tissue or extracted from internal plant tissue (17). As cited in the extensive review of Kobayashi and Palumbo (21), both gram-positive and gram-negative bacterial endophytes have been isolated from several tissue types in numerous plant species. Furthermore, several different bacterial species have been isolated from a single plant (21). Endophytes enter plant tissue primarily through the root zone; however, aerial portions of plants, such as flowers, stems, and cotyledons, may also be used for entry (21). Specifically, the bacteria enter tissues via germinating radicles (14), secondary roots (1), stomates (36), or as a result of foliar damage (25). Endophytes inside a plant may either become localized at the point of entry or spread throughout the plant (17). These microorganisms can reside within cells (19), in the intercellular spaces, (31) or in the vascular system (3).
Significant variations in the populations of both indigenous and introduced endophytes have been reported. These variations are attributed to plant source, plant age, tissue type, time of sampling, and environment. Generally, bacterial populations are larger in roots and decrease in the stems and leaves (24). Natural endophyte concentrations can vary between 2.0 and 6.0 log10 CFU per g for alfalfa, sweet corn, sugar beet, squash, cotton, and potato, as described by Kobayashi and Palumbo (21). Similar results were obtained for endophytic bacteria inoculated by root or seed drenching, with the population levels reaching between 3.0 and 5.0 log10 CFU/g of plant tissue for tomato and potato (21). The levels of colonization by nonpathogenic endophytes tend to be far less than the levels of colonization by pathogenic bacteria; the concentrations of the latter organisms range from 7.0 to 10.0 log10 CFU/g (fresh weight) of tissue in susceptible infected plants (15, 45).
Our research goal was to determine the prevalence, properties, persistence, and types of endophytic bacteria in agronomic and native plants. In this study, 853 different endophytic colonizing bacterial strains were isolated from four agronomic crop species and 27 prairie plant species. Six of the most promising colonizing strains were identified taxonomically as species of Cellulomonas, Clavibacter, Curtobacterium, and Microbacterium by fatty acid, carbon source utilization, and 16S rRNA gene sequence analyses.
MATERIALS AND METHODS
Bacterial strains.
The type strain of Microbacterium testaceum, IFO 12675, was received from Mariko Takeuchi, Institute for Fermentation, Osaka, Japan. A total of 853 endophytic strains were isolated from diverse hosts. The following strains were given special designations: CE648, isolated from dent corn; LB030, isolated from little bluestem; PD039, isolated from prairie dropseed; SE017 and SE034, isolated from sorghum; and SG041, isolated from sideoats grama.
Plant sources.
A broad range of agronomic and prairie plants common to the midwestern United States were surveyed for the presence of potential endophytic bacteria. Plants were selected based either on their economic importance to agriculture or on their perennial nature and thus their potential ability to support stable bacterial ecosystems (Table 1). The agronomic plants screened were maize (corn), sorghum, soybeans, and wheat. The prairie plants tested included various native species of grasses, forbs, legumes, and prairie wildflowers. The agronomic plants were harvested from field plots located 300 miles apart in Nebraska, and the prairie plants were collected from three virgin prairies and an established prairie grass plot within 25 miles of Lincoln, Nebr.
TABLE 1.
Phenotypic characterization of endophytic bacteria from agronomic crops and prairie plants
| Plant species (common name) | No. of plantsa | No. of isolatesb,c | Gram reactionc
|
||
|---|---|---|---|---|---|
| Positive | Negative | NDd | |||
| Agronomic crops | |||||
| Glycine max (soybean) | 60 | 17 | 17e | ||
| Sorghum bicolor (sorghum) | 120 | 353 (151) | 137 (75) | 173 (76) | 43 (0)f |
| Triticum aestivum (wheat) | 48 | 28 | 28 (0)e | ||
| Zea mays (corn) | 90 | 336 (222) | 164 (134) | 136 (88) | 36 (0)f |
| Total | 318 | 734 (373) | 301 (209) | 309 (164) | 124 (0) |
| Prairie plants | |||||
| Agropyron elongatum (tall wheatgrass) | 1 | 1 | 1 | ||
| Agropyron intermedium (intermediate wheatgrass) | 1 | 1 | 1 | ||
| Amorpha canescens (leadplant) | 5 | 4 | 1 | 3e | |
| Andropogon gerardi (big bluestem) | 6 | 7 | 3 | 4 | |
| Andropogon scoparius (little bluestem) | 6 | 12 | 2 | 8 | 2e |
| Artemisia ludoviciana (cudweed sagewort) | 3 | 2 | 1 | 1 | |
| Asclepias syriaca (milkweed) | 28 | 20 | 20e | ||
| Asclepias verticillata (whorled milkweed) | 4 | 2 | 1 | 1 | |
| Baptisia leucantha (white false indigo) | 3 | 3 | 1 | 2 | |
| Bouteloua curtipendula (sideoats grama) | 4 | 12 | 5 | 6 | 1e |
| Bouteloua gracilis (blue grama) | 2 | 8 | 2 | 6 | |
| Bromus biebersteinii (meadow brome) | 1 | 1 | 1 | ||
| Bromus inermis (smooth brome) | 1 | 1 | 1 | ||
| Buchloe dactyloides (buffalograss) | 2 | 6 | 1 | 4 | 1e |
| Callirhoe involucrata (purple poppy mallow) | 2 | 3 | 2 | 1e | |
| Dicanthelium oligosanthes (panicgrass) | 6 | 3 | 3 | ||
| Euphorbia podperae (leafy spurge) | 3 | 2 | 1 | 1 | |
| Koeleria pyramidata (Junegrass) | 4 | 3 | 2 | 1e | |
| Lathyrus latifolius (perennial pea) | 1 | 1 | 1 | ||
| Lespedeza capitata (roundhead lespedeza) | 2 | 2 | 2 | ||
| Panicum virgatum (switchgrass) | 7 | 7 | 2 | 4 | 1e |
| Petalostemon purpureus (purple prairieclover) | 4 | 3 | 1 | 2e | |
| Phalaris arundinacea (reed canarygrass) | 1 | 1 | 1 | ||
| Psoralea tenuiflora (wild alfalfa) | 4 | 3 | 1 | 2e | |
| Sorghastrum nutans (indiangrass) | 7 | 7 | 2 | 4 | 1e |
| Sporobolus asper (prairie dropseed) | 6 | 3 | 1 | 2 | |
| Vicia villosa (hairy vetch) | 2 | 1 | 1 | ||
| Total | 116 | 119 | 25 | 59 | 35 |
Total number of individual plants surveyed.
Total number of bacterial isolates recovered from each plant species surveyed.
Number of isolates (number of corn and sorghum endophyte isolates utilized in more extensive phenotypic characterizations).
ND, not determined.
Gram reaction was not performed on the isolates due to culture loss in storage.
The Gram reaction test could not definitively determine the classification for the isolates.
Isolation of endophytic bacteria.
Putative endophytic bacterial strains were defined as isolates that were obtained from surface-sterilized plants, displayed differentiable colony morphologies, and were recovered from the initial survey of agronomic crops and prairie grasses. For corn and sorghum, endophytic populations were collected from the pith tissue of stalks. Samples of dent corn (cultivars Mo17 × B73 and RN11) and sorghum (cultivars RS626 and Dekalb 61) were randomly collected during the growing season (June to September) from 10 healthy mature plants per site at four different geographical locations. Individual plants were severed aseptically 3 cm above the soil level, and the stalks were stripped of leaves, put into plastic bags, and kept on ice until further processing. In the laboratory, the stalks were wiped with 70% ethanol and flame sterilized, and each stalk was dissected into a segment containing the third, fourth, and fifth nodes. A crosscut through the stalk 2 cm above the third node was made, and a sterile no. 8 cork borer was inserted to a depth of at least 2 cm. The outer stalk was removed, exposing a cylinder of tissue inside the cork borer.
Soybean, wheat, and prairie plants were collected in the field during early summer (May and June) as described above. Plant leaves and stems were surface sterilized for 10 s with 2% sodium hypochlorite containing 0.1% Tween 20 (Sigma-Aldrich Co., St. Louis, Mo.). To remove the disinfectant, sections were rinsed five times each in two washes of nonsterile deionized distilled water and a wash of sterile water; the sections were dried with sterile paper towels. All agronomic crop and prairie plant samples were placed into polyethylene bags (Associated Bag Co., Milwaukee, Wis.) and either comminuted using a rolling press machine (Precision Machine Co., Lincoln, Nebr.) or dissected into ca. 1-cm pieces and macerated with either a sterile mortar and pestle or a sterile Polytron homogenizer (Brinkman Instruments, Westbury, N.Y.). Tissue extracts were then serially diluted in 12.5 mM potassium phosphate buffer (pH 7.1) (phosphate buffer) and plated in triplicate to recover any bacterial endophytes present in the plant tissue.
Preliminary studies were conducted to evaluate the efficacy of the decontamination procedures. Stalks of corn and sorghum plants were sprayed with a suspension containing 7.0 log10 CFU of the orange-pigmented organism Clavibacter michiganensis subsp. nebraskensis per ml before the sterilization procedure described above was performed. Subsequent colony counting demonstrated that the external bacterial recovery levels were 2.5 log10 CFU/g (fresh weight) or less. Without this procedure, virtually all the colonies recovered (6.0 to 7.0 log10 CFU/g [fresh weight]) were colonies of the sprayed bacteria. Thus, our decontamination procedure effectively reduced potential external contamination by more than 10,000-fold.
Bacterial growth conditions.
All bacteria were grown on plates at 27°C for 48 to 72 h. Liquid cultures were grown for 16 h in a PsycroTherm shaking incubator (New Brunswick Scientific, New Brunswick, N.J.) at 250 rpm. Bacto Agar (Difco Laboratories, Detroit, Mich.) was added as a solidifying agent at a concentration of 15 g/liter when required. For initial isolation and phenotypic characterization, the bacteria were grown on nutrient broth-yeast extract medium (NBY medium), Clavibacter michiganensis subsp. nebraskensis selective medium (CNS), or a modified CNS medium without lithium chloride (16, 48). NBY broth and agar were used to grow bacterial cultures for plant inoculation. Bacterial isolation in greenhouse studies and field trials was performed by using NBY agar containing 40 μg of cycloheximide (Sigma-Aldrich Co.) per ml, modified CNS agar, and/or antibiotic-supplemented NBY agar. For genotypic characterization, bacteria were grown in tryptic soy broth (Difco Laboratories) or on tryptic soy broth agar.
Phenotypic characterization of bacterial isolates.
Colonies of bacterial isolates were characterized 48 and 96 h postinoculation for the following traits: color, form, elevation, margin, diameter, surface, opacity, and texture. Motility, morphology, size, and division mode were also evaluated by performing phase-contrast microscopy with a Zeiss universal microscope (Carl Zeiss, Inc., Thornwood, N.Y.) at a magnification of ×1,000 as described previously (8). The Gram reaction was performed as described previously (41) by using a 3% KOH test. Chitinase activity was assessed with shrimp chitin (Sigma-Aldrich Co.) by the method of Kole and Altosaar (22). Fatty acid analysis was performed by MIDI Laboratories using the Sherlock Microbial Identification System TSBA 3.0, TSBA 4.1, and CORYNE 1.2 software (MIDI, Newark, Del.), and carbon source utilization was determined by using Biolog GP2 microplates comparing outputs to the MicroLog System 2 database, release 4.01B (Biolog, Inc., Hayward, Calif.). Prototype strains used in taxonomic comparisons were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSM), the Institute for Fermentation at Osaka (IFO), and the American Type Culture Collection (ATCC).
Antimicrobial agent resistance was tested individually on agar plates containing antibiotics at the following concentrations: kanamycin, rifampin, streptomycin, and tetracycline, 10 μg/ml; and ampicillin, chloramphenicol, gentamicin, and hygromycin, 50 μg/ml. Bacterial isolates were plated onto NBY agar with or without antibiotic supplementation. Most antibiotics were purchased from Sigma-Aldrich; the only exception was hygromycin, which was obtained from Roche Molecular Biochemicals, Indianapolis, Ind. Bacteria were considered sensitive to an antibiotic at the concentration tested if no visible growth was observed on plates containing the antibiotic when there was visible growth on control plates after 48 h of incubation at 27°C.
Inoculation of plants with bacterial endophytes.
Bacteria were grown to the mid-log phase, pelleted by centrifugation (3,440 × g, 10 min, 4°C), washed twice, and suspended in phosphate buffer. To confirm inoculation density and purity, an aliquot of each culture was serially diluted in phosphate buffer and plated on NBY medium. Corn and sorghum plants were inoculated in triplicate when a minimum height of 7.6 cm was reached and the stalks were at least 0.5 cm in diameter, which corresponded to 7 to 14 days after seed germination. A 26-gauge needle attached to a tuberculin syringe containing a bacterial suspension was passed horizontally through the seedling stem approximately 1 cm above the crown of the plant. A 5-μl droplet of suspension was formed at the tip of the needle, which was withdrawn through the plant stem. The plant was rotated 90°, the needle was reintroduced perpendicular to the first wound channel, and the inoculation procedure was repeated. Thus, 10 μl corresponded to an inoculum of either ca. 6.0 log10 CFU/plant for growth room studies or 7.0 log10 CFU/plant for greenhouse studies and field trials.
For soybean, wheat, host range, and prairie plant inoculations, bacterial suspensions were prepared and quantified as described above. Plants were grown for 3 to 4 weeks until they reached the size described above. A 26-gauge needle was utilized for most inoculations; the only exception was the geranium inoculations, for which the calibrated eye of a no. 20 tapestry needle was used. Inoculation of nine replicates with ca. 6.0 log10 CFU of bacteria/plant (host range studies) and inoculation of seven replicates with 8.0 log10 CFU of bacteria/plant (homologous studies) were conducted in the same manner, with minor modifications. Monocots lacking a dominant stem were inoculated in one to three stems per plant, and wheat crowns were injected with 100 μl of inoculum.
Isolation of endophytic bacteria from experimentally inoculated plants.
For the initial time point (1 day postinoculation), whole-plant samples of corn and sorghum were obtained as described above. For subsequent samples, partial plant sections were collected from the lower main stem, the lowest nonsenescent leaf, a midstem leaf, and a newly expanded leaf. The only exception was for whole-plant samples of seedlings in the growth room studies. Plant sections were surface sterilized as described above for soybean, wheat, and prairie plants. Before samples were pooled in polyethylene bags, a section that was 1 by 4 cm was cut aseptically from the interior stem tissue and a 5-cm-long sample was dissected aseptically from the middle portion of the longitudinal axis of each leaf. Plant tissues were weighed, processed by the rolling press method, diluted, and plated as described above.
As a control for all inoculation studies to test for the presence of indigenous endophytic bacteria, all agronomic crops and prairie plants were sham inoculated with phosphate buffer by using the methods used for the experimental plants. All bacterial endophytes recovered were compared qualitatively, which included isolation on selective media and use of antibiotic resistance markers, and quantitative recovery data were compared with control data to distinguish growth of introduced bacteria from the presence of indigenous microorganisms.
Growth room studies.
Corn and sorghum endophytic bacterial strains were tested in both aseptically grown cultivar Mo17 × B73 dent corn and cultivar RS626 sorghum seedlings to screen for potential pathogenicity to host plants and to assess the ability of these bacteria to colonize agronomically significant crops. In conjunction with these assays, pathogenicity assays were also conducted in a greenhouse study. To prepare plants for in vitro inoculation, seeds were surface sterilized by immersion for 20 min in a solution containing 0.80% sodium hypochlorite and 0.05% Tween 20 with shaking at 250 rpm, followed by a 30-s dip in 70% ethanol and two rinses in sterile distilled water. The seeds were then placed embryo side up on NBY agar at 25°C until germination was observed in 2 to 4 days. Seedlings were exposed to a photoperiod consisting of 16 h of light and 8 h of darkness. Seedlings that did not exhibit fungal or bacterial contamination and had a coleoptile which was approximately 1 cm long were aseptically transferred to test tubes; each test tube contained a seed support chromatography paper wick (Whatman Inc., Clifton, N.J.) resting in Murashige and Skoog's nutrient medium at pH 5.8 (Sigma-Aldrich Co.). Plants were harvested immediately after inoculation and at 1 and 8 days postinoculation.
Homologous and host range greenhouse studies.
All prairie plant species and wheat (cultivar Centurk) were evaluated for the ability to support colonization in the greenhouse of bacterial endophytes previously isolated from the same host. In addition, a host range study was conducted to determine the ability of corn and sorghum endophytes to colonize cultivars of cotton (Coker 404), cucumber (SMR-18), geranium (Aurora), milkweed (Natural Fiber Co.), onion (Spanish Yellow), potato (Red Kennebec), tobacco (Xanthi), tomato (Super Sioux), soybean (Amsoy 71), and wheat (Centurk). In both studies we utilized a randomized complete block design. To ensure that only replicating populations were recovered in both studies, the distal 15 cm of the main axis without the seed head was harvested from each plant 42 days postinoculation. Plants that were flowering or showing vegetative growth were preferentially selected.
Dent corn greenhouse studies and field trials.
Wild-type bacterial strains from corn and sorghum that colonized plant tissue at moderate to high concentrations without any apparent deleterious effects on seedlings grown in vitro were evaluated by using a randomized complete block experimental design for long-range colonization (>50 days postinoculation). Cultivar Mo17 × B73 dent corn was used in greenhouse studies (University of Nebraska-Lincoln) and in two separate row-irrigated field trials located 32 miles apart at the University of Nebraska Agricultural Research and Development Center (Mead, Nebr.; field trial I) and the University of Nebraska Agronomy Farm (Lincoln, Nebr.; field trial II). In addition, antibiotic-resistant mutants were included in these studies. Spontaneous antibiotic-resistant mutants were isolated from 14 bacterial endophytes by the gradient plate method (29). Putative antibiotic-resistant mutants were confirmed by the same method and replicated onto NBY media containing 50 μg of rifampin per ml, 25 μg of kanamycin per ml, or 12.5 μg of tetracycline per ml to confirm resistance. Marker stability and growth rate (efficiency of plating) were determined in vitro and in planta by isolation on NBY media and by three consecutive transfers to NBY media without antibiotics and subsequent transfer to NBY media supplemented with the appropriate antibiotics. After inoculation, plants were harvested in the field after 24 h and at approximately 2-week intervals (five sampling periods) and in the greenhouse on day 1 and after about 2 and 7 weeks (three collection times).
Statistical analysis.
The data were analyzed by using SAS, version 8 (SAS Institute Inc., Cary, N.C.), analysis of variance, as well as by using least-square means to test for pairwise differences when overall effects were present.
16S rRNA gene amplification and sequencing.
Genomic DNA was isolated by using standard bacterial procedures (38). The following primers were used for PCR amplification of the 16S ribosomal DNA: p515FPL (5′-GTGCCAGCAGCCGCGGTAA-3′) (35), p13B (5′-AGGCCCGGGAACGTATTCAC-3′) (34), and PCR-1 (5′-AGTTTGATCCTGGCTCAGGA-3′). Each reaction mixture contained Taq DNA polymerase (Promega, Madison, Wis.), 1.5 mM magnesium chloride, each deoxynucleoside triphosphate at a concentration of 0.1 mM, 10% (vol/vol) dimethyl sulfoxide (Fisher Scientific, Pittsburgh, Pa.), 0.4 mM spermidine (Sigma-Aldrich Co.), each primer (Integrated DNA Technologies, Coralville, Iowa) at a concentration of 10 pM, and 10 ng of DNA per μl. The thermocycling conditions consisted of a denaturation step at 94°C for 3 min, 30 amplification cycles of 94°C for 1 min, 57°C for 1 min, and 72°C for 2 min, and a final polymerization step of 72°C for 4 min with a GeneAmp PCR System 9600 (Perkin-Elmer, Norwalk, Conn.). PCR products were visualized on 0.8% agarose gels, and the products were excised and purified either with glassmilk (GENECLEAN; Bio 101, Vista, Calif.) or with the Promega Wizard PCR Preps system (Madison, Wis.) by following the manufacturers' instructions prior to sequencing.
DNA sequencing was performed with an ABI 377 Prism DNA sequencer (PE Applied Biosystems Inc., Foster City, Calif.). The following primers were used for sequencing: p91E (5′-TCAAA[G/T]GAATTGACGGGGGC-3′) (35), SEQ-1 (5′-ACGTATTCACCGCAGCGTTG-3′), SEQ-2 (5′-GGCCTTCGGGTTGTAAACCT-3′), and SEQ-3 (5′-CCAACATCTCACGACACGAG-3′). Also, MIDI Laboratories sequenced 500 bp of the 5′ end using proprietary primers and procedures. Sequences were aligned, and a consensus sequence was computed with fragment assembly tools in the Genetics Computer Group software package (Madison, Wis.). Nucleotide sequence similarities were determined by using BLAST, version 2.0 (National Center for Biotechnology Information databases). A phenogram was created by using PHYLIP software, version 3.5.
Nucleotide sequence accession numbers.
Partial sequence data for the 16S rRNA genes have been deposited in the EMBL/GenBank/DDBJ nucleotide sequence data libraries. Data for endophtyic strains have been deposited under the following accession numbers: CE648, AF474330; LB030, AF474326; PD039, AF474328; SE017, AF474325; SE034, AF474327; and SG041, AF474329.
RESULTS
Isolation and phenotypic characterization of bacterial endophytes.
To evaluate populations of potentially endophytic bacteria in agronomic crops and prairie plants, a total of 853 isolates were collected over a 6-year period from all of the healthy plants surveyed. For isolation of bacterial strains, plants were harvested and processed as described in Materials and Methods. Following surface sterilization, wheat, soybean, and prairie plant samples were collected from the entire aerial portions of plants. The pith tissues of corn and sorghum stalks were chosen for isolation in order to assess potential relationships to the development of stalk rot disease, a common late season disease attributed to multiple microorganisms (50). The population sizes depended on the date of sampling and varied from 0 to 6.0 log10 CFU/tissue sample (mean, 3.0 log10 CFU/tissue sample). A majority of the microorganisms isolated (689 strains) were from corn and sorghum; 45 strains were recovered from soybean and wheat, and 119 strains were obtained from 27 different host species of grasses, forbs, legumes, and wildflowers (Table 1). As a whole, fewer isolates were recovered from perennial plants than from the agronomic crops. Preliminary characterization of these bacteria showed that approximately equal percentages of gram-positive (41%) and gram-negative (42%) bacteria were recovered from the agronomic crop plants, whereas gram-negative bacteria (50%) were isolated more frequently than gram-positive bacteria (21%) from prairie plants. Gram reactions were not determined for the remaining isolates due to culture loss in storage or the variability of Gram reaction results.
A more extensive phenotypic characterization was carried out with 373 culturable microorganisms recovered from corn (222 isolates) and sorghum (151 isolates). A total of 209 of these endophytes were confirmed to be gram-positive organisms, and 164 were gram-negative organisms (Table 1). As expected, more gram-negative bacteria than gram-positive bacteria were motile. Chitin utilization was positive for 157 gram-positive and 117 gram-negative isolates (data not shown). Analysis of resistance to various antibiotics showed that 20% of the corn and sorghum isolates were sensitive to all of the antibiotics at the concentrations tested (data not shown). For gram-negative bacteria, resistance to ampicillin was most common (75%), followed by resistance to kanamycin (70%), resistance to streptomycin (36%), and resistance to tetracycline (27%). Gram-positive strains demonstrated a similar pattern of antibiotic resistance, although they were more likely to be resistant to kanamycin (51%) than to ampicillin (39%). Ninety percent of both gram-positive and gram-negative strains were sensitive to rifampin.
Colonization studies with endophytic bacteria.
To determine which of the endophytic bacteria had the ability to colonize and persist at high levels in plant hosts, we carried out studies with experimentally inoculated plants. In all colonization studies, controls were included to verify that the inoculated bacteria were recovered. In general, the representative control plants inoculated with phosphate buffer yielded no indigenous bacteria. In addition, the colony morphologies of the endophytes recovered after experimental inoculation were indistinguishable from the colony morphologies of the inoculated organisms.
For the prairie endophytes, 86 isolates were inoculated into the homologous plant hosts and grown in the greenhouse. Of these, 28 strains had colonized the plants at levels ranging from 3.5 to 7.7 log10 CFU/g (fresh weight) at 42 days postinoculation (Table 2). The highest proportions of endophytes that were able to colonize the original hosts in greenhouse studies were obtained from little bluestem, switchgrass, and prairie dropseed. Prairie endophytes LB030, PD039, and SG041 (see Materials and Methods) were able to colonize the original hosts in a consistent manner at titers between 3.8 and 5.7 log10 CFU/g (fresh weight) (data not shown). In the same homologous greenhouse study, none of the 14 wheat endophytes tested was able to colonize wheat to any significant extent.
TABLE 2.
Bacterial endophyte recovery from agronomic crops and prairie plants grown in either the greenhouse or the growth room
| Plant host species | No. of isolates tested | No. of isolates recovereda | Colonization levelb (avg ± SD) |
|---|---|---|---|
| Greenhouse studiesc | |||
| Agronomic crop | |||
| Wheat | 14 | 0 | |
| Prairie plants | |||
| Intermediate wheatgrass | 1 | 0 | |
| Big bluestem | 7 | 3 | 3.6 ± 0.2 (3.5-3.9) |
| Little bluestem | 10 | 6 | 4.7 ± 1.3 (4.7 ± 1.3 (3.6-7.0) |
| Cudweed sagewort | 2 | 1 | 4.5 |
| Milkweed | 15 | 0 | |
| Whorled milkweed | 2 | 0 | |
| Sideoats grama | 11 | 3 | 5.0 ± 0.9 (4.0-5.7) |
| Blue grama | 8 | 3 | 5.3 ± 1.5 (3.6-6.4) |
| Meadow brome | 1 | 0 | |
| Smooth brome | 1 | 0 | |
| Buffalograss | 5 | 2 | 5.2 ± 0.0 (5.2-5.2) |
| Leafy spurge | 2 | 0 | |
| Perennial pea | 1 | 0 | |
| Roundhead lespedeza | 2 | 0 | |
| Switchgrass | 6 | 5 | 4.6 ± 0.9 (4.0-5.5) |
| Purple prairieclover | 1 | 0 | |
| Reed canarygrass | 1 | 1 | 3.9 |
| Indiangrass | 6 | 2 | 6.2 ± 2.1 (4.8-7.7) |
| Prairie dropseed | 3 | 2 | 5.5 ± 0.1 (5.5-5.6) |
| Hairy vetch | 1 | 0 | |
| Total | 100 | 28 | |
| Growth room studiesd | |||
| Agronomic crops | |||
| Corn | 217e | 207 (95) | 7.1 ± 1.4 (3.2-9.4) |
| 145f | 134 (92) | 6.8 ± 1.5 (3.0-9.6) | |
| Sorghum | 69g | 67 (97) | 6.5 ± 1.3 (3.1-8.7) |
| 39h | 37 (95) | 6.7 ± 1.6 (3.1-8.7) | |
| Total | 470 | 445 (95) |
Number of endophytic bacterial strains that were confirmed to colonize plant tissue. The numbers in parentheses are percentages.
Titer for growth chamber studies at 8 days postinoculation expressed as log10 CFU/plant and titer for greenhouse studies at 42 days postinoculation expressed as log10 CFU/g (fresh weight). The values in parentheses are the lowest and highest average titers.
All bacterial isolates were reintroduced into the same plant species at a level of 8.0 log10 CFU/plant.
All bacterial isolates were reintroduced at a level of 6.0 log10 CFU/plant.
Number of corn endophytes that were reinoculated into corn seedlings.
Number of sorghum endophytes that were reinoculated into corn seedlings.
Number of corn endophytes that were reinoculated into sorghum seedlings.
Number of sorghum endophytes that were reinoculated into sorghum seedlings.
A large number of endophytes were isolated from corn and sorghum, and we first performed preliminary growth room and greenhouse assays to determine whether any of the isolates were pathogenic in dent corn and sorghum. The results indicated that none of the 373 corn and sorghum isolates produced any disease symptoms or abnormalities in corn and sorghum. Subsequently, to evaluate the colonization ability of corn and sorghum endophytes, all 373 isolates were tested with dent corn and sorghum seedlings which belong to the Andropogoneae tribe. Of the 222 corn endophytes tested in corn seedlings, 46 colonized corn seedlings at 8 days postinoculation at levels of 8.3 log10 CFU/plant or higher (Table 2). Similarly, only 15 of the 151 sorghum endophytes tested in corn seedlings colonized at these levels. In contrast, only 5 of 69 corn endophytes and 3 of 39 sorghum endophytes reached these colonization levels in sorghum seedlings; five of these isolates colonized both corn and sorghum seedlings. Of the endophytes inoculated into corn and sorghum seedlings, six strains appeared to be specific for sorghum and one strain appeared to be specific for corn. In summary, 69 strains were recovered from either corn or sorghum seedlings at desirable concentrations (8.3 log10 CFU/plant or higher).
Based on the growth room assays described above, we selected 19 corn endophytes and 10 sorghum endophytes to perform host range studies in the greenhouse in order to test the ability of these microorganisms to colonize diverse plant species, including those listed in Table 3. Cucumber and tomato plants supported growth of about 60% of the corn and sorghum endophytes, respectively. Most endophytes (26 of 29 strains) were able to colonize at least one species different from the original host at levels ranging from 0.1 to 5.8 log10 CFU/g (fresh weight), and two endophytes were able to colonize five or six plant species.
TABLE 3.
Corn and sorghum endophyte populations in a range of hosts determined in greenhouse studies
| Plant host species (common name) | Corn endophytes
|
Sorghum endophytes
|
||
|---|---|---|---|---|
| No. of isolatesa | Colonization levelb (avg ± SD) | No. of isolatesa | Colonization levelb (avg ± SD) | |
| Allium cepa (onion) | 17 (8) | 3.9 ± 1.2 (2.4-5.8) | 7 (2) | 3.5 ± 0.8 (2.9-4.1) |
| Asclepias syriaca (milkweed) | 19 (0) | 0 | 10 (2) | 1.3 ± 0.4 (1.0-1.5) |
| Cucumis sativus (cucumber) | 19 (11) | 1.5 ± 1.2 (0.1-3.8) | 10 (5) | 1.3 ± 1.3 (0.4-3.5) |
| Pelargonium × hortorum (geranium) | 19 (2) | 1.9 ± 0.1 (1.8-1.9) | 10 (4) | 3.2 ± 1.3 (1.4-4.3) |
| Glycine max (soybean) | NDc | ND | 7 (0) | 0 |
| Lycopersicon esculentum (tomato) | 19 (4) | 1.7 ± 1.0 (1.0-3.1) | 10 (6) | 2.0 ± 1.6 (0.8-4.6) |
| Nicotiana tabacum (tobacco) | 16 (4) | 3.0 ± 1.0 (2.3-4.5) | 10 (3) | 2.7 ± 0.2 (2.5-2.8) |
| Solanum tuberosum (potato) | 19 (6) | 2.8 ± 0.3 (2.5-3.2) | 10 (5) | 3.0 ± 0.4 (2.7-3.6) |
| Triticum aestivum (wheat) | 15 (2) | 3.1 ± 1.5 (2.0-4.1) | 5 (2) | 4.6 ± 0.1 (4.5-4.7) |
Number of isolates tested (inoculated at a level of 6.0 log10 CFU/plant). The numbers in parentheses are the numbers of isolates recovered.
Titer at 42 days postinoculation expressed as log10 CFU/g (fresh weight). The values in parentheses are the lowest and highest average titers.
ND, not determined.
The corn and sorghum endophytes CE648, SE017, and SE034 (see Materials and Methods) were able to consistently colonize corn seedlings at titers ranging from 9.0 to 9.6 log10 CFU/plant (data not shown). Likewise, all of these isolates colonized sorghum seedlings at levels of 6.6 to 8.6 log10 CFU/plant. These isolates also exhibited colonization levels of 0.8 to 4.7 log10 CFU/g (fresh weight) over an extended host range. All three colonized geranium and cucumber; in addition, SE017 colonized wheat, potato, and tomato, while SE034 grew in tomato and milkweed (data not shown).
Extensive long-range greenhouse studies and field trials were performed with nine bacterial endophytes from corn and eight bacterial endophytes from sorghum showing significant colonization levels in at least one of the types of seedlings. These strains were introduced into greenhouse-grown dent corn in order to determine the titers achievable throughout most of the corn plant life cycle, through day 67 postinoculation (Table 4). All strains were recovered at the highest levels early in the study (days 1 and 11), and the average colonization levels were 6.1 ± 0.1 log10 CFU/g (fresh weight). By day 67, the colonization levels had decreased by about 10-fold (Table 4). The colonization levels for corn and sorghum endophytes were not significantly different over the course of the study.
TABLE 4.
Recovery of wild-type agronomic crop endophytic bacteria from dent corn
| Location | Daya | Corn endophytes
|
Sorghum endophytes
|
||
|---|---|---|---|---|---|
| No. of isolatesb | Colonization levelc (avg ± SD) | No. of isolatesb | Colonization levelc (avg ± SD) | ||
| Greenhouse | |||||
| 1 | 9 (9) | 6.1 ± 0.1 (4.7-6.8)d,e | 8 (8) | 5.7 ± 0.1 (4.9-6.7)e | |
| 11 | 9 (9) | 5.1 ± 0.2 (4.5-6.3)f | 8 (7) | 5.7 ± 0.2 (4.9-6.7)f | |
| 67 | 9 (3) | 4.4 ± 0.3 (2.6-5.9) | 8 (3) | 4.3 ± 0.3 (3.5-5.2) | |
| Field trial I | |||||
| 1 | 5 (5) | 5.4 ± 0.2 (4.2-6.6)g | 8 (7) | 5.0 ± 0.2 (3.8-6.0)g | |
| 9 | 5 (5) | 4.1 ± 0.2 (3.4-5.1) | 8 (8) | 4.2 ± 0.2 (3.4-5.4)h | |
| 21 | 5 (2) | 4.0 ± 0.3 (3.5-4.4) | 8 (6) | 3.8 ± 0.2 (3.0-4.4) | |
| 46 | 5 (4) | 3.8 ± 0.2 (2.9-4.7) | 8 (8) | 3.8 ± 0.2 (2.7-4.7) | |
| 71 | 5 (3) | 3.8 ± 0.2 (3.1-4.9) | 8 (6) | 3.7 ± 0.2 (2.3-4.3) | |
| Field trial II | |||||
| 1 | 5 (5) | 4.5 ± 0.2 (3.4-5.7)i | 8 (7) | 4.4 ± 0.2 (2.7-5.8)i | |
| 14 | 5 (2) | 4.2 ± 0.3 (2.9-5.1) | 8 (7) | 4.1 ± 0.2 (3.2-4.9)j | |
| 26 | 5 (2) | 3.4 ± 0.3 (3.0-3.8) | 8 (5) | 3.6 ± 0.2 (2.4-4.2) | |
| 52 | 5 (3) | 3.8 ± 0.3 (3.0-5.4) | 8 (5) | 3.8 ± 0.2 (3.4-4.6) | |
| 78 | 5 (2) | 3.5 ± 0.3 (2.5-4.2) | 8 (5) | 3.4 ± 0.2 (2.6-4.3) | |
Number of days between inoculation and plant harvest.
Number of isolates tested (inoculated at a level of 7.0 log10 CFU/plant). The numbers in parentheses are the numbers of isolates recovered.
Titer for each day expressed as log10 CFU/g (fresh weight). The values in parentheses are the lowest and highest average titers.
Significant difference (P < 0.05) between days 1 and 11.
Significant difference (P < 0.05) between days 1 and 67.
Significant difference (P < 0.05) between days 11 and 67.
Significant difference (P < 0.05) when day 1 was compared to days 9, 21, 46, and 71.
Significant difference (P < 0.05) when day 9 was compared to days 21 and 71.
Significant difference (P < 0.05) when day 1 was compared to days 26, 52, and 78.
Significant difference (P < 0.05) between days 14 and 78.
Since bacterial endophytes could be recovered from artificially inoculated corn plants in the greenhouse over an extended period of time, another study was conducted in field trials, which provided a fluctuating environment. In two separate field trials, five corn endophytes and eight sorghum isolates were tested. All of these strains were recovered from growing dent corn plants through day 71 postinoculation (field trial I) or day 78 postinoculation (field trial II) (Table 4). Similar to the greenhouse experiments, the highest colonization levels at both sites were observed on day 1, and at the remaining times moderate levels of colonization were consistently observed. The colonization levels were maintained throughout all major growth stages of the plants, and the levels of recovery were similar or only slightly reduced over the sampling period for 9 of the 13 bacterial strains in each field trial. The average population levels for the remaining four isolates were less than 2.0 log10 CFU/g (fresh weight). There was no significant difference between corn and sorghum endophytes in both field trials.
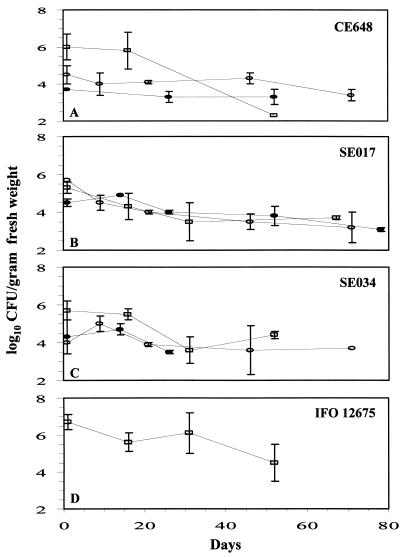
Long-range quantitative population data for selected corn (strain CE648) and sorghum (strains SE017 and SE034) endophytes in greenhouse- and field-grown corn are shown in Fig. 1A to C. Isolates CE648 and SE034 displayed the greatest colonization titers for early samples in the greenhouse studies (Fig. 1A and C). The levels for all of the isolates in one or both field trials were 3.1 log10 CFU/g (fresh weight) or greater after at least 50 days (Fig. 1A to C).
FIG. 1.
Persistence of selected endophytic isolates in dent corn. Strains CE648 (A), SE017 (B), and SE034 (C) were tested in long-range greenhouse studies and two separate field trials using a randomized complete block design. Strain IFO 12675 (D) is the M. testaceum type strain, and it was tested only in the greenhouse. Symbols: □, greenhouse; ○, field trial I; •, field trial II. The error bars indicate standard deviations (n ≥ 3). The minimum detection limit was 102 CFU/g (fresh weight).
Considering the potential use of endophytes exhibiting selectable phenotypes in other applications (6, 37), we isolated spontaneous kanamycin-, rifampin-, and tetracycline-resistant mutants from the corn and sorghum endophytes that exhibited the highest colonization levels. These mutants were tested in long-range colonization studies performed with dent corn in both greenhouse studies and field trials. The results, including the levels of colonization, were similar to those obtained with the corresponding wild-type strains, but there were some differences (data not shown). Levels for antibiotic-resistant isolates were significantly lower than those for wild-type isolates during field trial I (days 9, 21, and 46) and during field trial II (day 14).
Taxonomic identification of selected bacterial endophytes.
A number of agronomic crop and prairie plant endophytes were selected for taxonomic studies based on the colonization studies described above. Isolates LB030, PD039, and SG041 were gram positive, resistant to 50 μg of hygromycin per ml, and susceptible to 10 μg of chloramphenicol per ml. Isolate SG041 was positive for motility and chitin utilization. In addition, the fatty acid analysis identified the prairie plant endophyte LB030 as Microbacterium esteraromaticum (equivalent to DSM 8609, IFO 3751, and ATCC 8091), PD039 as Clavibacter michiganensis subsp. nebraskensis (equivalent to DSM 7483 and ATCC 27794), and SG041 as Curtobacterium flaccumfaciens subsp. flaccumfaciens (equivalent to DSM 20129, IFO 12156, and ATCC 6887). Carbon source utilization tests identified LB030 as M. testaceum, PD039 as Clavibacter michiganensis subsp. insidiosus (equivalent to DSM 20157 and ATCC 10253), and SG041 as Curtobacterium citreum (equivalent to DSM 20528, IFO 12677, and ATCC 15828).
To confirm the results obtained by these procedures, we analyzed the entire 16S rRNA gene sequence by PCR amplification using the primer sets described in Materials and Methods. As determined by this method, the LB030 16S rRNA gene was 96% homologous to the Microbacterium arabinogalactanolyticum type strain DSM 8611 (= IFO 14344 = ATCC 51926) 16S rRNA gene (39), and the strain LB030 16S rRNA gene was 96% homologous to the partial 16S rRNA genes of Microbacterium sp. strain VKM Ac-1807 and Microbacterium phyllosphaerae. The PD039 16S rRNA gene exhibited 99% homology to the 16S rRNA gene of Clavibacter michiganensis type strain DSM 46364 (= IFO 12471) (32), whereas the SG041 16S rRNA gene exhibited 99% homology to the 16S rRNA gene of Curtobacterium luteum type strain DSM 20542 (=IFO 12676 = ATCC 15830) (32).
Preliminary taxonomic identification of an additional 52 corn and sorghum endophytes was carried out by performing a fatty acid analysis. Only 36 of these strains could be classified in a genus yielding a similarity index greater than 0.3 with the Sherlock Microbial Identification System. The 15 genera identified were Agrobacterium, Bacillus, Bradyrhizobium, Cellulomonas, Clavibacter, Corynebacterium, Enterobacter, Erwinia, Escherichia, Klebsiella, Microbacterium, Micrococcus, Pseudomonas, Rothia, and Xanthomonas. Of these, Bacillus, Corynebacterium, and Microbacterium were the most prevalent genera, with eight, five, and nine assigned isolates, respectively.
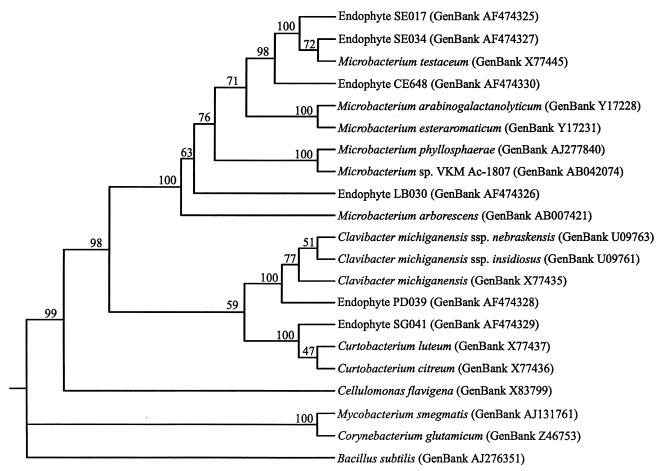
The three corn and sorghum endophytes (CE648, SE017, and SE034) were gram positive, resistant to 50 μg of hygromycin per ml and 50 μg of gentamicin per ml, and susceptible to 10 μg of chloramphenicol per ml. Isolate SE034 was positive for motility and chitin utilization, while CE648 was positive only for chitin utilization. Fatty acid analysis identified the agronomic crop endophyte CE648 as Microbacterium arborescens (equivalent to DSM 20754, IFO 3750, and ATCC 4358), SE017 as Cellulomonas flavigena (equivalent to DSM 20109T, IFO 3754, and ATCC 482), and SE034 as M. testaceum (equivalent to DSM 20166, IFO 12675, and ATCC 15829). The carbon source utilization analysis identified all three corn and sorghum endophytes as M. testaceum. Similarly, the CE648, SE017, and SE034 16S rRNA genes exhibited 99% homology to the 16S rRNA gene of the M. testaceum type strain (32). A phenogram reflecting the relationships among the endophytic and candidate strains used in all of the analyses described above was constructed based on the 16S rRNA gene sequences as described in Materials and Methods (Fig. 2). Because the corn and sorghum endophytes were all identified as M. testaceum isolates by the 16S rRNA gene sequence analysis, we tested the type strain of M. testaceum in long-range greenhouse colonization studies. The results demonstrated that this organism was able to colonize dent corn at levels similar to the levels of colonization by the agronomic crop endophyte strains isolated in our study (Fig. 1D).
FIG. 2.
Phenogram expressing the relationships of identified bacterial endophytes to taxonomically similar microorganisms based on the 16S rRNA gene sequences. The unrooted phenogram was constructed as described in Materials and Methods. The GenBank accession number is given in parentheses for each organism. The positions of the endophyte strains are based on the best match for genus and species. The numbers at the nodes are bootstrap values based on 100 replications. The 16S rRNA gene sequence of Bacillus subtilis was utilized as an outgroup.
DISCUSSION
Our research goals were to survey agronomic crops and prairie plants for the presence of endophytic bacteria and to determine their phenotypic properties, their taxonomic positions, and their colonization levels in experimentally inoculated plants. In this study, we isolated several hundred bacterial strains from dent corn, sorghum, soybean, wheat, perennial grasses, forbs, legumes, and prairie wildflowers (Table 1). Similarly, other workers have reported isolation of indigenous endophytic bacteria from yellow dent type corn (7), sweet corn (12, 28), and alfalfa (14). To our knowledge, our study is the first to describe indigenous bacterial endophytes isolated from sorghum, soybean, wheat, and native perennial plants. There appears to be significant variation in the types of indigenous bacteria isolated from diverse host plant species. Several factors may explain these differences, including host specificity, geographical distribution, plant age, and tissue type (21).
In our colonization studies of prairie plant endophytes in the greenhouse, we demonstrated that most of the introduced endophytic strains could recolonize the original plant host at moderate to high levels through 42 days postinoculation (Table 2). To our knowledge, this is the first experimental greenhouse study of colonization of prairie plants by bacterial endophytes. However, other workers have conducted similar studies in growth chambers. For example, the rhizobacterium Pseudomonas aureofaciens was inoculated and was recovered after 29 days from tall fescue leaves and pea and bean stems at a level of 2.3 log10 CFU/g (fresh weight) (24).
The ability of corn and sorghum endophytes to colonize and persist in dent corn and sorghum was initially shown by using seedlings (Table 2). Based on an inoculum level of 6.0 log10 CFU/plant, some multiplication of the endophytes occurred in many plants. This is the first demonstration of endophytic colonization of experimentally inoculated sorghum seedlings. A subset of endophytes identified in the growth room studies that yielded high levels of colonization were analyzed in more comprehensive long-range greenhouse studies and field trials in which dent corn was used as the host. As expected, the colonization rates observed in the greenhouse were higher than those achieved in the field. In the field, there is increased microbial competition, the cultural practices, such as irrigation, are less consistent, and there may be unfavorable environmental conditions, such as high temperatures. In contrast, the sterile seedling inoculation experiments resulted in population levels that were usually 10-fold higher than the levels in the greenhouse. Most of the corn and sorghum endophytes persisted at significant average colonization levels ranging between 3.4 and 6.1 log10 CFU/g (fresh weight) in the greenhouse and fields for up to 67 and 78 days, respectively (Table 4 and Fig. 1). Antibiotic-resistant mutants obtained from selected strains of these endophytes could also colonize plants at levels similar to the levels of wild-type strains in these greenhouse and field experiments. In this context, similar results were obtained in other plant species for antibiotic-resistant mutants, as reviewed by Kobayashi and Palumbo (21), for Bacillus subtilis, Erwinia sp., and Pseudomonas. In one of these studies, Frommel et al. (13) obtained higher average population levels of rifampin- and nalidixic acid-resistant mutants of Pseudomonas sp. in potato roots in both greenhouse studies and field trials. This result may be a reflection of the ability of Pseudomonas sp. to establish high population densities in the rhizosphere.
Other investigators have performed bacterial endophyte colonization studies with corn or other agronomic crops, obtaining levels comparable to those assessed here. Lamb et al. (24) tested the ability of the rhizobacterium P. aureofaciens to colonize corn, wheat, oats, and cotton. Microorganisms were recovered at levels of 2.5 to 4.7 log10 CFU/g (fresh weight) from the aerial portions of these plants. Similarly, inoculation of Clavibacter xyli subsp. cynodontis onto corn seeds by using a pressure bomb resulted in recovery levels as high as 9.0 log10 CFU/g (fresh weight) of tissue (11). In contrast, there have been few population studies of the levels of recovery and colonization of indigenous endophytes in the aerial portions of corn. McInroy and Kloepper (27) isolated naturally occurring endophytic bacteria from sweet corn grown in the greenhouse and field and reported population levels ranging from 3.0 to 7.0 log10 CFU/g (fresh weight). Fisher et al. (12) recovered bacteria from field-grown sweet corn and reported colonization levels of 2.3 to 6.5 log10 CFU/g (wet weight) of tissue. From the latter reports and our studies, it is clear that indigenous bacterial endophytes can be isolated from corn plants throughout the entire growing season in many different environments with different corn varieties. In addition, the population levels for endophytes (single or mixed) are fairly consistent and are between 2.0 and 7.0 log10 CFU/g of tissue.
In our host range study, endophytes obtained from the monocots corn and sorghum were shown to colonize and persist in both monocot and dicot plant species (Table 3). The highest endophyte population levels were obtained for the monocot hosts (onion and wheat). Other workers have also looked at the recovery of endophytes from monocot plants (corn and banana) after the organisms were originally isolated from monocot species (21). The previous studies include Enterobacter cloacae, Burkholderia cepacia, and Clavibacter xyli subsp. cynodontis isolated from many different plant species, suggesting that these bacteria have developed an evolutionary niche within plants.
Six endophytes with the most promising levels of colonization and the ability to persist in a range of host plants were chosen for taxonomic identification based on 16S rRNA gene sequence, commercial fatty acid, and carbon utilization analyses. Tang et al. (44) compared these methods for identification of gram-negative bacilli to the genus and species levels and reported that 16S rRNA gene sequence analysis was 10 and 20% more accurate than the commercial fatty acid and carbon utilization analysis systems, respectively. In our study, all analyses resulted in the same genus level identity for all endophytes except SE017, which was identified as a Cellulomonas strain by fatty acid analysis and as a Microbacterium strain by the other two methods. The endophytes PD039 (Clavibacter michiganensis) and SE034 (M. testaceum) were each identified as the same organism at the genus and species levels by all tests.
The close association observed between soil and plant environments suggests a potential endophytic role for Cellulomonas and Microbacterium strains. Our studies identified for the first time the endophytic nature of M. testaceum for various plant hosts. Cellulomonas flavigena has been isolated as an epiphytic bacterium from the surfaces of olive leaves (10), while M. testaceum was isolated from Chinese rice paddies (23). Researchers have identified microbial antagonists belonging to the genus Microbacterium that decrease nematode populations for up to 96 days in the greenhouse and throughout the following growing season in field trials (18, 46). The chitinolytic microorganism M. testaceum has been isolated from cotton rhizospheres only when chitin has been added to the soil (18). A second coryneform bacterium, M. esteraromaticum, has been isolated from soybean rhizospheres after use of a nematicide-producing velvetbean cropping system (46). Curtobacterium citreum and Curtobacterium luteum were originally isolated from Chinese rice paddies (23). Strains of Curtobacterium flaccumfaciens cause bacterial diseases (47) but also have been recovered from the phyllosphere of wheat (26) and have been shown to be a biocontrol agent for cucumber (33). These three bacterial species have been isolated as endophytes from red clover nodules, and Curtobacterium luteum consistently promotes plant growth alone or in combination with Rhizobium spp. (40). The Clavibacter michiganensis group contains subspecies that can cause diseases in their natural hosts, such as bacterial wilt of alfalfa, bacterial canker of tomato and pepper, and Goss's wilt of corn (47). Naturally occurring nonpathogenic strains, such as our strain PD039, have not been reported previously.
Based on the 16S rRNA gene sequence analysis results, we constructed a phenogram for the six endophytes mentioned above and related microorganisms (Fig. 2). Endophytes SE017, SE034, CE648, and LB030 fall into the Microbacterium group containing M. testaceum, M. arabinogalactanolyticum, M. arborescens, and M. esteraromaticum (39, 42, 43, 52). The first three strains were definitively identified as M. testaceum, whereas the fourth isolate could not be clearly identified to the species level. Endophyte PD039 belongs to the genus Clavibacter and is most closely related to the species Clavibacter michiganensis, including Clavibacter michiganensis subsp. nebraskensis and Clavibacter michiganensis subsp. insidiosus (32). Endophyte SG041 fits well into the Curtobacterium group consisting of Curtobacterium citreum, Curtobacterium flaccumfaciens, and Curtobacterium luteum (9, 32).
Modification of plants to obtain organisms with improved genetic capabilities and tolerance to different environmental conditions is generally carried out by plant breeding and by integrating foreign DNA into plant genomes to produce transgenic plants (53). Although successful for certain plants, these methods are costly, are dependent on the plant variety being studied, and take several years to reach market. As an alternative approach, beneficial endophytes have been used to express and secrete useful products without requiring integration of foreign DNA into the plant genome. Endophytic bacteria have a multitude of applications that enhance agricultural production; they enhance wheat growth through production of phytohormones (2), increase rice production by increasing mineral availability (30), increase cotton disease resistance (5), contribute to corn pest management (11), fix nitrogen in rice and wheat (49), decrease susceptibility to frost damage (51), and increase potato tuber formation under heat stress conditions (4). Environmentally, the use of specific endophytes may be preferable to the use of nonspecific chemical fertilizers and pesticides because of cost, time effectiveness, and contributions to sustainable agricultural systems.
This study demonstrated the occurrence and diversity of culturable endophytes in a large number of plant species. The successful colonization of several crops with such microbes suggests that they can be utilized in future applications, such as delivery of degradative enzymes for controlling certain plant diseases or other useful products.
Acknowledgments
We thank Pam Gering for technical assistance. We thank M. Takeuchi for providing the type strain of M. testaceum. We thank Julie Berg for assistance with statistical analysis of data concerning the isolation of inoculated bacterial endophytes from greenhouse- and field-grown corn.
Crop Genetics International (Columbia, Md.), Kamterter II, L.L.C. (Lincoln, Nebr.), and the University of Nebraska Center for Biotechnology (Lincoln, Nebr.) contributed funds for this research. Z.F. is a recipient of a Maude Hammond Fling-Bukey Memorial Fund Fellowship from Graduate Studies, University of Nebraska-Lincoln.
Footnotes
This is a contribution of the University of Nebraska Agricultural Research Division, Lincoln, Journal Series no. 13445.
REFERENCES
- 1.Agarwal, S., and S. T. Shende. 1987. Tetrazolium reducing microorganisms inside the root of Brassica species. Curr. Sci. 56:187-188. [Google Scholar]
- 2.Barbieri, P., T. Zanelli, E. Galli, and G. Zanetti. 1986. Wheat inoculation with Azospirillum brasilense Sp6 and some mutants altered in nitrogen fixation and indole-3-acetic acid production. FEMS Microbiol. Lett. 36:87-90. [Google Scholar]
- 3.Bell, C. R., G. A. Dickie, W. L. G. Harvey, and J. W. Y. F. Chan. 1995. Endophytic bacteria in grapevine. Can. J. Microbiol. 41:46-53. [Google Scholar]
- 4.Bensalim, S., J. Nowak, and S. K. Asiedu. 1998. A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. Am. J. Potato Res. 75:145-152. [Google Scholar]
- 5.Chen, C., E. M. Bauske, G. Musson, R. Rodríguez Kábana, and J. W. Kloepper. 1995. Biological control of fusarium wilt on cotton by use of endophytic bacteria. Biol. Control 5:83-91. [Google Scholar]
- 6.Compeau, G., B. J. Al-Achi, E. Platsouka, and S. B. Levy. 1988. Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl. Environ. Microbiol. 54:2432-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Araujo, J. M., A. C. da Silva, and J. L. Azevedo. 2000. Isolation of endophytic actinomycetes from roots and leaves of maize (Zea mays L.). Braz. Arch. Biol. Technol. 43:447-451. [Google Scholar]
- 8.Doetsch, R. N. 1981. Determinative methods of light microscopy, p. 21-33. In P. Gerhardt, R. G. E. Murray, R. N. Costilow, E. W. Nester, W. A. Wood, N. R. Krieg, and G. B. Phillips (ed.), Manual of methods for general bacteriology. American Society for Microbiology, Washington, D.C.
- 9.Dopfer, H., E. Stackebrandt, and F. Fiedler. 1982. Nucleic acid hybridization studies on Microbacterium, Curtobacterium, Agromyces and related taxa. J. Gen. Microbiol. 128:1697-1708. [DOI] [PubMed] [Google Scholar]
- 10.Ercolani, G. L. 1991. Distribution of epiphytic bacteria on olive leaves and the influence of leaf age and sampling time. Microb. Ecol. 21:35-48. [DOI] [PubMed] [Google Scholar]
- 11.Fahey, J. W., M. B. Dimock, S. F. Tomasino, J. M. Taylor, and P. S. Carlson. 1991. Genetically engineered endophytes as biocontrol agents: a case study from industry, p. 401-411. In J. H. Andrews and S. S. Hirano (ed.), Microbial ecology of leaves. Springer-Verlag, London, United Kingdom.
- 12.Fisher, P. J., O. Petrini, and H. M. Lappin Scott. 1992. The distribution of some fungal and bacterial endophytes in maize (Zea mays L.). New Phytol. 122:299-305. [DOI] [PubMed] [Google Scholar]
- 13.Frommel, M. I., J. Nowak, and G. Lazarovits. 1993. Treatment of potato tubers with a growth promoting Pseudomonas sp.: plant growth responses and bacterium distribution in the rhizosphere. Plant Soil 150:51-60. [Google Scholar]
- 14.Gagne, S., C. Richard, H. Rousseau, and H. Antoun. 1987. Xylem-residing bacteria in alfalfa roots. Can. J. Microbiol. 33:996-1000. [Google Scholar]
- 15.Grimault, V., and P. Prior. 1994. Invasiveness of Pseudomonas solanacearum in tomato, eggplant and pepper: a comparative study. Eur. J. Plant Pathol. 100:259-267. [Google Scholar]
- 16.Gross, D. C., and A. K. Vidaver. 1979. A selective medium for isolation of Corynebacterium nebraskense from soil and plant parts. Phytopathology 69:82-87. [Google Scholar]
- 17.Hallmann, J., A. Quadt-Hallmann, W. F. Mahaffee, and J. W. Kloepper. 1997. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 43:895-914. [Google Scholar]
- 18.Hallmann, J., R. Rodriguez-Kabana, and J. W. Kloepper. 1999. Chitin-mediated changes in bacterial communities of the soil, rhizosphere and within roots of cotton in relation to nematode control. Soil Biol. Biochem. 31:551-560. [Google Scholar]
- 19.Jacobs, M. J., W. M. Bugbee, and D. A. Gabrielson. 1985. Enumeration, location, and characterization of endophytic bacteria within sugar beet roots. Can. J. Bot. 63:1262-1265. [Google Scholar]
- 20.Kado, C. I. 1992. Plant pathogenic bacteria, p. 659-674. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, vol. I. Springer-Verlag, New York, N.Y. [Google Scholar]
- 21.Kobayashi, D. Y., and J. D. Palumbo. 2000. Bacterial endophytes and their effects on plants and uses in agriculture, p. 199-233. In C. W. Bacon and J. F. White (ed.), Microbial endophytes. Marcel Dekker, Inc., New York, N.Y.
- 22.Kole, M. M., and I. Altosaar. 1985. Increased chitinase production by a non-pigmented mutant of Serratia marcescens. FEMS Microbiol. Lett. 26:265-269. [Google Scholar]
- 23.Komagata, K., and H. Iizuka. 1964. New species of Brevibacterium isolated from rice. J. Agric. Chem. Soc. Jpn. 38:496-502. [Google Scholar]
- 24.Lamb, T. G., D. W. Tonkyn, and D. A. Kluepfel. 1996. Movement of Pseudomonas aureofaciens from the rhizosphere to aerial plant tissue. Can. J. Microbiol. 42:1112-1120. [Google Scholar]
- 25.Leben, C., G. C. Daft, and A. F. Schmitthenner. 1968. Bacterial blight of soybeans: population levels of Pseudomonas glycinea in relation to symptom development. Phytopathology 58:1143-1146. [Google Scholar]
- 26.Legard, D. E., M. P. McQuilken, J. M. Whipps, J. S. Fenlon, T. R. Fermor, I. P. Thompson, M. J. Bailey, and J. M. Lynch. 1994. Studies of seasonal changes in the microbial populations on the phyllosphere of spring wheat as a prelude to the release of a genetically modified microorganism. Agric. Environ. 50:87-101. [Google Scholar]
- 27.McInroy, J. A., and J. W. Kloepper. 1995. Population dynamics of endophytic bacteria in field-grown sweet corn and cotton. Can. J. Microbiol. 41:895-901. [Google Scholar]
- 28.McInroy, J. A., and J. W. Kloepper. 1995. Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant Soil 173:337-342. [Google Scholar]
- 29.Meynell, G. G., and E. Meynell. 1970. Theory and practice in experimental bacteriology. Cambridge University Press, Cambridge, United Kingdom.
- 30.Murty, M. G., and J. K. Ladha. 1988. Influence of Azospirillum inoculation on the mineral uptake and growth of rice under hydroponic conditions. Plant Soil 108:281-285. [Google Scholar]
- 31.Patriquin, D. G., and J. D&oring;bereiner. 1978. Light microscopy observations of tetrazolium-reducing bacteria in the endorhizosphere of maize and other grasses in Brazil. Can. J. Microbiol. 24:734-742. [DOI] [PubMed] [Google Scholar]
- 32.Rainey, F., N. Weiss, H. Prauser, and E. Stackebrandt. 1994. Further evidence for the phylogenetic coherence of actinoycetes with group B-peptidoglycan and evidence for the phylogenetic intermixing of the genera Microbacterium and Aureobacterium as determined by 16S rDNA analysis. FEMS Microbiol. Lett. 118:135-140. [Google Scholar]
- 33.Raupach, G. S., and J. W. Kloepper. 2000. Biocontrol of cucumber diseases in the field by plant growth-promoting rhizobacteria with and without methyl bromide fumigation. Plant Dis. 84:1073-1075. [DOI] [PubMed] [Google Scholar]
- 34.Relman, D. A., J. S. Loutit, T. M. Schmidt, S. Falkow, and L. S. Tompkins. 1990. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N. Engl. J. Med. 323:1573-1580. [DOI] [PubMed] [Google Scholar]
- 35.Relman, D. A., T. M. Schmidt, R. P. MacDermott, and S. Falkow. 1992. Identification of the uncultured bacillus of Whipple's disease. N. Engl. J. Med. 327:293-301. [DOI] [PubMed] [Google Scholar]
- 36.Roos, I. M. M., and M. J. Hattingh. 1983. Scanning electron microscopy of Pseudomonas syringae pv. morsprunorum on sweet cherry leaves. Phytopathol. Z. 108:18-25. [Google Scholar]
- 37.Ryder, M. H., C. E. Pankhurst, A. D. Rovira, R. L. Correll, and K. M. Ophel-Keller. 1994. Detection of introduced bacteria in the rhizosphere using marker genes and DNA probes, p. 29-47. In F. O'Gara, D. N. Dowling, and B. Boesten (ed.), Molecular ecology of rhizosphere microorganisms: biotechnology and the release of GMOs. VCH Verlagsgesellschaft mbH, Weinheim, Germany.
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Schumann, P., F. A. Rainey, J. Burghardt, E. Stackebrandt, and N. Weiss. 1999. Reclassification of Brevibacterium oxydans (Chatelain and Second 1966) as Microbacterium oxydans comb. nov. Int. J. Syst. Bacteriol. 49:175-177. [DOI] [PubMed] [Google Scholar]
- 40.Sturz, A. V., B. R. Christie, B. G. Matheson, and J. Nowak. 1997. Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. Biol. Fertil. Soils 25:13-19. [Google Scholar]
- 41.Suslow, T. V., M. N. Schroth, and M. Isaka. 1982. Application of a rapid method for Gram differentiation of plant pathogenic and saprophytic bacteria without staining. Phytopathology 72:917-918. [Google Scholar]
- 42.Takeuchi, M., and K. Hatano. 1998. Proposal of six new species in the genus Microbacterium and transfer of Flavobacterium marinotypicum ZoBell and Upham to the genus Microbacterium as Microbacterium maritypicum comb. nov. Int. J. Syst. Bacteriol. 48:973-982. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi, M., and K. Hatano. 1998. Union of the genera Microbacterium Orla-Jensen and Aureobacterium Collins et al. in a redefined genus Microbacterium. Int. J. Syst. Bacteriol. 48:739-747. [DOI] [PubMed] [Google Scholar]
- 44.Tang, Y. W., N. M. Ellis, M. K. Hopkins, D. H. Smith, D. E. Dodge, and D. H. Persing. 1998. Comparison of phenotypic and genotypic techniques for identification of unusual aerobic pathogenic gram-negative bacilli. J. Clin. Microbiol. 36:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsiantos, J., and W. A. Stevens. 1986. The population dynamics of Corynebacterium michiganense pv. michiganensis and other selected bacteria in tomato leaves. Phytopathol. Mediterr. 25:160-162. [Google Scholar]
- 46.Vargas-Ayala, R., R. Rodriguez-Kabana, G. Morgan-Jones, J. A. McInroy, and J. W. Kloepper. 2000. Shifts in soil microflora induced by velvetbean (Mucuna deeringiana) in cropping systems to control root-knot nematodes. Biol. Control 17:11-22. [Google Scholar]
- 47.Vidaver, A. K. 1982. The plant pathogenic corynebacteria. Annu. Rev. Microbiol. 36:495-517. [DOI] [PubMed] [Google Scholar]
- 48.Vidaver, A. K. 1967. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl. Microbiol. 15:1523-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webster, G., C. Gough, J. Vasse, C. A. Batchelor, K. J. O'Callaghan, S. L. Kothari, M. R. Davey, J. Dénarié, and E. C. Cocking. 1997. Interactions of rhizobia with rice and wheat. Plant Soil 194:115-122. [Google Scholar]
- 50.White, D. G. 1999. Compendium of corn diseases, 3rd ed. American Phytopathological Society Press, St. Paul, Minn.
- 51.Xu, H., M. Griffith, C. L. Patten, and B. R. Glick. 1998. Isolation and characterization of an antifreeze protein with ice nucleation activity from the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Can. J. Microbiol. 44:64-73. [Google Scholar]
- 52.Yokota, A., M. Takeuchi, T. Sakane, and N. Weiss. 1993. Proposal of six new species in the genus Aureobacterium and transfer of Flavobacterium esteraromaticum Omelianski to the genus Aureobacterium as Aureobacterium esteraromaticum comb. nov. Int. J. Syst. Bacteriol. 43:555-564. [DOI] [PubMed] [Google Scholar]
- 53.Zupan, J. R., and P. Zambryski. 1995. Transfer of T-DNA from Agrobacterium to the plant cell. Plant Physiol. 107:1041-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]