Abstract
Antibodies are known to affect the morphology, growth, and metabolism of mollicutes and thus may serve as candidate molecules for a plantibody-based control strategy for plant-pathogenic spiroplasmas and phytoplasmas. Recombinant single-chain variable fragment (scFv) antibodies are easy to engineer and express in plants, but their inhibitory effects on mollicutes have never been evaluated and compared with those of polyclonal and monoclonal antibodies. We describe the morphology, growth, and glucose metabolism of Spiroplasma citri in the presence of polyclonal, monoclonal, and recombinant antibodies directed against the immunodominant membrane protein spiralin. We showed that the scFv antibodies had no effect on S. citri glucose metabolism but were as efficient as polyclonal antibodies in inhibiting S. citri growth in liquid medium. Inhibition of motility was also observed.
Plant-pathogenic mollicutes include the genus Spiroplasma, comprising organisms culturable in complex artificial media and showing a helical morphology, and the Candidatus genus phytoplasma, containing large numbers of pleiomorphic organisms which, until now, have resisted in vitro cultivation (4, 9). Hence, spiroplasmas are the most-studied phytopathogenic mollicutes, and S. citri (23) is the model organism for this important group of plant pathogens.
Mollicutes are eubacteria without a cell wall and thus delimited only by a cytoplasmic membrane. This characteristic has been linked with the fact that metabolism and growth of mollicutes were strongly inhibited by antibodies directed against membrane proteins (3, 13, 14, 19, 36, 37). This is why expression of antibodies in plants is an attractive strategy to control phytopathogenic mollicutes, especially since at this time, there is no other curative method. In 1998, we produced tobacco plants expressing single-chain variable fragment (scFv) recombinant antibodies against the stolbur phytoplasma, via the secretory pathway (apoplastic route), and some resistance to infection could be observed (20). Further analysis of the plants revealed that the resistance was not complete and that phytoplasmas could invade the tobacco plant. Chen and Chen (5) successfully expressed an scFv recombinant antibody directed against Spiroplasma kunkelii, the agent of corn stunt disease, in the cytoplasm (symplasmic route) of maize, but no resistance to the corn stunt spiroplasma was obtained.
Difficulty in targeting functional antibodies into the phloem sieve tubes, where phytoplasmas reside, is probably one reason for failure, but it cannot be ruled out that scFv recombinant antibodies are not or are less efficient than polyclonal or monoclonal antibodies in inhibiting metabolism and growth of mollicutes. Indeed, Chen and Chen (5) showed that monoclonal antibodies were less efficient than polyclonal antibodies in inhibiting growth and metabolism of S. kunkelii.
For the study presented here, we have constructed an S. citri-specific scFv recombinant antibody from a hybridoma cell line producing a monoclonal antibody against spiralin, the major and immunodominant protein of the S. citri cytoplasmic membrane (15). The effect of the scFv recombinant antibody on the morphology, growth, and metabolism of S. citri was compared with that of polyclonal and monoclonal antibodies.
MATERIALS AND METHODS
Strains and antibodies. (i) Escherichia coli strains.
Escherichia coli strain XL1-Blue (Stratagene, La Jolla, Calif.) was used for scFv cloning and plasmid multiplication, and E. coli strain JM109 (Promega, Madison, Wis.) was used for scFv expression. Culture conditions were as described by Sambrook et al. (24).
(ii) S. citri strain.
The triply cloned Morocco S. citri strain GII3 (33) was multiplied at 32°C on solid or liquid SP4 culture medium as described by Tully et al. (32).
Hybridoma cell lines.
Hybridomas producing monoclonal antibodies 12G9 (immunoglobulin G1 [IgG1]) and 12H6 (IgG2) were obtained after immunization with cultured S. citri cells, Israël strain NCPPB 2565. Both monoclonal antibodies are directed against the spiralin protein and recognize all S. citri strains tested, including strain GII3 (unpublished data). Monoclonal antibody 9E10, directed against the human c-Myc protein (12) produced by Myc-9E10-2 hybridoma cells (ATCC CRL 1729), was also used in this study to detect the c-Myc tag included in the scFv sequence. Hybridomas were multiplied as described previously (20).
S. citri antiserum.
Polyclonal serum was from a rabbit that had been immunized with S. citri strain R8A2. Complement was inactivated by heating for 1 h at 56°C.
Purification of monoclonal and recombinant antibodies.
Monoclonal antibodies were purified on protein A-Sepharose (Amersham Pharmacia Biotech) from hybridoma culture medium. scFv produced in E. coli periplasmic fractions were purified by immunoaffinity using the 9E10 anti-c-Myc antibody coupled with CNBr-activated Sepharose (Pharmacia) as described by the manufacturer. Antibodies were suspended in phosphate-buffered saline (PBS, pH 7.2). Purity of monoclonal and recombinant antibodies was checked after silver staining of sodium dodecyl sulfate (SDS)-polyacrylamide gels. Protein concentration was determined with the Bio-Rad protein assay (Bio-Rad Laboratories GmbH, Paris, France).
Deformation test.
The S. citri deformation test (35, 36) was performed in 96-well microtiter plates. Twofold dilution series of crude antiserum, monoclonal antibodies (initial concentration, 4 mg/ml), or recombinant antibodies (initial concentration, 300 μg/ml) were done in 25 μl of SP4 medium. Then, 25 μl of SP4 medium containing 5 × 108 CFU of spiroplasmas per ml was added to each dilution. As a negative control, the same procedure was carried out with either PBS or periplasmic fraction of nontransformed E. coli cells in place of antibodies. The microtiter plate was incubated at room temperature for 30 min, and 6-μl samples from each well were observed under a dark-field microscope to determine spiroplasma morphology and motility.
Metabolism inhibition test.
The S. citri metabolism inhibition test (31) was performed in 96-well microtiter plates. Horizontally, a twofold dilution series of crude antiserum, monoclonal antibody (800 μg/ml), or recombinant antibody (80 μg/ml) suspensions were distributed in a final volume of 50 μl of SP4 medium. Vertically, 200 μl of 1:10 dilution series of a 106 CFU/ml spiroplasma culture in SP4 medium was added to each well. Negative controls were done with PBS or the periplasmic fraction of nontransformed E. coli cells in place of antibodies. Glucose fermentation by S. citri leads to the production of lactic acid in SP4 medium, resulting in color change of the phenol red pH indicator from red to yellow. After incubation for 5 days at 32°C, color changes were recorded for each well.
Growth inhibition test on solid medium.
Fifty microliters of a spiroplasma culture at 105 CFU/ml were spread onto SP4 medium solidified with 1% agar in 60-mm-diameter petri dishes. Twofold dilution series of crude antiserum, monoclonal antibodies (4 mg/ml), or recombinant antibodies (300 μg/ml) in a final SP4 volume of 25 μl were blotted onto sterile 5-mm-diameter paper disks laid on agar in the center of the dishes. Negative controls were PBS and the periplasmic fraction of nontransformed E. coli cells in place of antibodies. After 5 to 7 days at 32°C, the growth inhibition areas were measured from the edges of the disks.
Growth inhibition in liquid medium as determined by [32P]phosphate incorporation into spiroplasma nucleic acids.
Incorporation of [32P]phosphate into spiroplasma nucleic acids was determined from the radioactivity of a trichloroacetic acid (TCA)-insoluble precipitate of pelleted spiroplasma cells as described before (16). At time zero (t0), 5 μCi of [32P]phosphoric acid per ml was added to an S. citri culture in SP4 medium buffered with 60 mM HEPES. At t0 and at 24-h intervals during spiroplasma growth, different concentrations of antiserum or monoclonal or recombinant antibodies were added to the culture. Every 8 h, 300 μl of culture was collected and divided into three 100-μl fractions. Each fraction was TCA precipitated, and the radioactivity was counted in a scintillation spectrometer (Packard Tri-Carb 2100TR).
Engineering and cloning of scFv 12G9.
Double-stranded cDNAs coding for variable regions of the IgG 12G9 heavy (VH) and light (VL) chains were synthesized by reverse transcription followed by PCR from 100 ng of mRNA isolated from 12G9 hybridoma cells as previously described (20). A mixture of the following primers, VH160FOR [5′-ACGGTGACCTG(C/A)(G/A)AGAC(T/G/A)GTGA(G/C) TGA(G/A)G-3′], VH161FOR [5′-ACGGTGACCTG(C/A)(G/A)AGAC(T/G/A)GTGA(G/C) (A/C)GT(G/A)G-3′], VH162FOR [5′-ACGGTGACCTG(C/A)(G/A)AGAC(T/G/A)GTGA(G/C) CAG(G/A)G-3′], and VH158BACK [5′-GGCTGCAGCCCCAGGTGAAGCT(T/G)(C/G)T(C/G)GA(A/G)TC-3′] was used for VH regions. They derived from those described by Stinson et al. (28). Primers VK2FOR (5′-TTTGATCTCGAGCTTGGTCCC-3′) and VK2BACK (5′-GACATCGAGCTCACTCAGTCTCCA-3′) (29) were used for VL regions. The DNA sequences of VH and VL coding fragments were determined and compared to those contained in the GenBank database with the Blast program (1).
The synthetic gene coding for scFv 12G9 was engineered by overlap extension using the High Expand Fidelity PCR System (Roche Diagnostics, Meylan, France). The genes encoding the variable domains were independently modified in an initial PCR amplification at the 3′ VH end with primers VH158BACK/VH-Link and at the 5′ VL end with primers VK-link and VK2FOR. Primers VH-Link (5′-AGAGCCACCTCCGCCTGAACCGCCTCCACCTGAGGAGACGGTGACCTGAGAGACGGTC-3′) and VK-link (5′-TCAGGCGGAGGTGGCTCTGGGGGTGGCGGATCGGACATCGAGCTCACTCAGTC-3′) carry the overlapping sequence coding for the linker peptide (Gly4Ser)3. Twenty PCR cycles of 45 s at 94°C, 45 s at 59°C, and 45 s at 72°C were performed in 50 μl of 1× Expand HF buffer containing 200 μM each of the deoxynucleoside triphosphates, 1.3 U of Expand HF enzyme mix (Roche), 1 μM each primer, and 1 μl of the variable domain matrix.
The modified VH and VL were mixed together and reamplified in a second round of PCR with the external primers VH158BACK and VK2FOR with the following cycles: 45 s at 93°C, 45 s at 56°C, and 1 min at 72°C six times, then 1 min at 94°C, 45 s at 60°C, and 1 min at 72°C 25 times. The resulting PCR product was cleaved with EcoRI and HindIII restriction enzymes (MBI Fermentas, St. Leon, Germany) and inserted in frame with the pelB leader sequence, into the pUC19::scFv-Secr[2A10] vector (20) linearized with the same enzymes. The recombinant vector pUC19::pel-scFv[12G9] was electroporated into competent XL1-Blue cells, and the DNA sequence of the cloned scFv was determined.
Expression of scFv 12G9 in E. coli.
E. coli JM109 cells were transformed with pUC19-pel-scFv[12G9] or with pUC19::scFv-Secr[2A10] vectors. Nontransformed cells were used as negative controls. Induction was carried out overnight at 30°C in 1 volume of Luria-Bertani (LB) medium containing 100 μM ampicillin and 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). E. coli periplasmic fraction was obtained as described before (19), filtered, dialyzed extensively against PBS, and concentrated on Centricon concentrators with a 10-kDa cutoff (Amicon, Inc., Beverly, Mass.). The fraction was stored at 4°C until used in growth inhibition experiments or scFv purification.
Western blot and immunofluorescence.
scFv 12G9 detection in E. coli periplasmic fractions was done by Western blot and immunofluorescence with the 9E10 anti-c-Myc antibody as described previously (20). Binding of scFv 12G9 onto S. citri total soluble proteins was verified by Western blot incubated with periplasmic fractions from transformed and nontransformed E. coli cells and revealed with monoclonal antibody 9E10 as described above.
RESULTS
Morphology and motility of S. citri cells in the presence of polyclonal and monoclonal antibodies.
When spiroplasmas were incubated with the polyclonal serum, folding of the helices was observed and led to round bodies with or without a protruding filament as described by Williamson et al. (35). Such deformed spiroplasmas were nonmotile.
Deformation of 50% of spiroplasma helices (deformation titer) was obtained with a 1,000-fold dilution of the crude serum. With a 500-fold dilution, all helices were deformed and motionless. Monoclonal antibody 12G9 (IgG1) produced deformations of helices similar to those obtained with the serum, and the deformation titer was reached with a concentration of 15 μg of purified IgGs per ml. Ninety-five percent of the helices were deformed with IgGs at 30 μg/ml. However, with the highest concentration of IgG tested, i.e., 2 mg/ml, 0.5 to 1% of the organisms still remained undeformed and motile. With monoclonal antibody 12H6 (IgG2b), the characteristic deformation of helices into round bodies was not observed; only nonhelical, nonmotile filaments were seen sticking to the microscope slide. To get 50% such filaments, an IgG concentration of 300 μg/ml was required. When the two monoclonal antibodies were mixed each at its respective deformation titer, 60% of the spiroplasmas were nonmotile and nonhelical filaments, 20% had the characteristic deformation morphology and were motionless, and 20% retained a normal phenotype. When added to the medium, PBS alone did not alter the morphology and motility of spiroplasmas.
Metabolism inhibition with polyclonal and monoclonal antibodies.
Metabolism inhibition was tested by growing dilutions of S. citri cells in the presence of dilutions of the respective polyclonal or monoclonal antibodies. The metabolism inhibition titer (dilution of antibodies at which no color change of the medium was observed) of polyclonal antibodies was 1:8, 1:16, 1:32, and 1:64 with, respectively, 106 105, 104, and 103 S. citri cells per ml. In contrast, the ability of S. citri to ferment sugars was not abolished by any of the concentrations of 12G9 and 12H6 monoclonal antibodies tested (20 to 400 μg/ml). Observation of the spiroplasmas for each culture dilution after 48 h revealed that the number of aggregated deformed spiroplasmas increased with the concentration of 12G9 monoclonal antibody and was paralleled by absence of motility. Motility was also prevented with monoclonal antibody 12H6, but no aggregates were observed; however, after 5 days, all spiroplasmas were helical and motile again.
Growth inhibition with polyclonal and monoclonal antibodies.
In order to calibrate the dilutions of antibodies and S. citri cultures necessary to prevent spiroplasma growth, inhibition tests were first performed on solid media. A large growth inhibition zone was observed with polyclonal serum for dilutions up to 1:64 (Table 1). No inhibition was observed with the two monoclonal antibodies even at the highest concentration tested, i.e., 4 mg/ml. Growth inhibition by the polyclonal serum was then evaluated with various titers of S. citri cultures (Table 1). The inhibition was optimum for low S. citri titers and high concentrations of antibodies. However, a growth inhibition zone of 2.5 mm was still obtained when a disk with a tenfold dilution of serum was placed on a culture plate containing 108 spiroplasmas per ml.
TABLE 1.
Growth inhibition induced by polyclonal antibodiesa
| Antiserum dilution | Inhibition zone (mm) | S. citri titer (CFU/ml) | Inhibition zone (mm) at antiserum dilution
|
|
|---|---|---|---|---|
| 0 | 1:10 | |||
| — | 11 | 102 | 14 | 10 |
| 1:4 | 9 | 103 | 12.5 | 8 |
| 1:8 | 8.2 | 104 | 12 | 7 |
| 1:16 | 7 | 105 | 11 | 6 |
| 1:32 | 5.5 | 106 | 10 | 5.5 |
| 1:64 | 4.5 | 107 | 8 | 3.5 |
| 1:128 | 0 | 108 | 5.5 | 2.5 |
| 1:256 | 0 | |||
| 1:512 | 0 | |||
| PBScontrol | 0 | |||
S. citri was grown on solid medium, and growth inhibition was determined as a function of antiserum dilution with S. citri at 105 CFU/ml and as a function of S. citri titer with undiluted and 1:10 diluted antiserum.
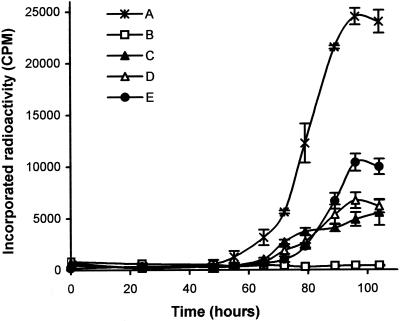
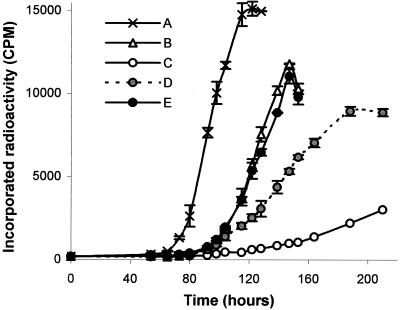
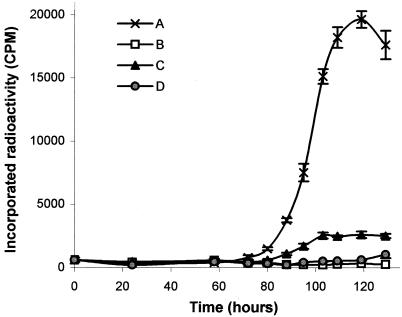
The ability of monoclonal antibodies to inhibit growth was tested by incorporation of radioactivity in liquid medium. We first compared monoclonal antibody 12G9 with the polyclonal serum on cultures inoculated with 103, 104, or 105 S. citri cells per ml. In the absence of antibodies, the various cultures reached a similar titer and differed only by the length of the lag phase preceding exponential growth (results not shown). Growth inhibition was observed with either polyclonal or monoclonal antibodies at a dilution of 1:400. Complete inhibition was obtained when polyclonal antibodies were added to the cultures (Fig. 1, curve B), although some limited residual growth was observed with monoclonal antibody 12G9 (Fig. 1, curve C). We then compared the effect of monoclonal antibodies 12G9 and 12H6 singly and in combination on S. citri in a 103 CFU/ml culture. The presence of monoclonal antibody 12H6 had no effect on S. citri multiplication when used at 40 μg/ml (results not shown), and it was necessary to use MAb12H6 at a concentration of 300 μg/ml (Fig. 1, curve D) to observe a growth inhibition similar to that with monoclonal antibody 12G9 (Fig. 1, curve C). When used together, there was no synergistic but antagonist effects of the two monoclonal antibodies, with decreased inhibition (Fig. 1, curve E).
FIG. 1.
32P-labeled phosphate incorporation during S. citri growth in SP4 liquid medium in the presence of PBS (A), polyclonal serum diluted 1:400 (B), monoclonal antibody 12G9 at 40 μg/ml (C), monoclonal antibody 12H6 at 300 μg/ml (D), and a mixture of monoclonal antibody 12H6 and monoclonal antibody 12G9 at 300 μg/ml and 40 μg/ml, respectively (E).
Engineering and expression of scFv 12G9.
Because the two monoclonal antibodies did not have a synergistic effect in deformation and growth inhibition tests, and because 12G9 proved to be more efficient than 12H6 on deformation and motility as well as in inhibiting S. citri growth in liquid medium at concentrations similar to that of a polyclonal antibody, we engineered 12G9 scFv by amplifying the VH and VL regions from hybridoma 12G9 mRNAs, as described in Materials and Methods.
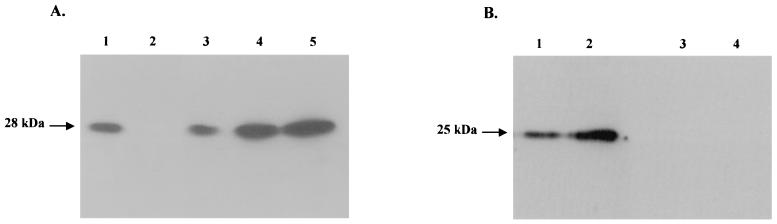
Figure 2 shows the sequence of the scFv construct cloned in plasmid pUC19:scFv-secr2A10 (20) for expression in E. coli. In order to quantify the amount of scFv produced by E. coli, Western blots were performed, and the intensity of the signals was compared with that given by known amounts of a previously purified scFv (20) (Fig. 3A, lane 1). Figure 3A, lane 3, indicated that 2 μl of E. coli periplasmic fraction contained around 50 ng of 12G9 scFv. The scFv was shown to be functional toward S. citri cells by immunofluorescence on sections of healthy or infected leaf midveins (results not shown) and to recognize spiralin specifically in Western blots of total S. citri proteins (Fig. 3B, lanes 1 and 2), while no reaction was observed when the periplasmic fraction from nontransformed E. coli cells was tested (Fig. 3B, lanes 3 and 4).
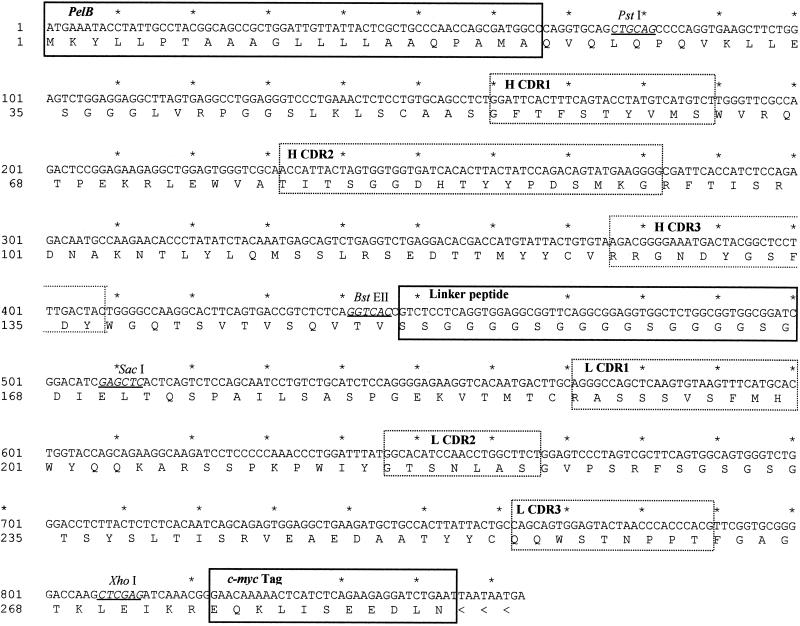
FIG. 2.
Nucleotide and predicted amino acid sequences of pel-scFv12G9. pelB, linker peptide, and c-Myc peptide tag are in solid-line boxes. Sequences of the deduced complementary determining regions are in dotted-line boxes. Restriction sites are underlined. The GenBank accession number is AY049714.
FIG. 3.
(A) Western blots of 50 ng of purified scFv 2A10 (lane 1), 8 μl of periplasmic fraction from nontransformed E. coli cells (lane 2), and 2, 4, and 8 μl of periplasmic fraction from E. coli transformed with pUC::pel-scFv[12G9] (lanes 3, 4, and 5, respectively) after incubation with 9E10 anti-Myc peptide antibodies. (B) Western blots of 1.2 μg (lanes 1 and 3) or 2.4 μg (lanes 2 and 4) of total soluble proteins from S. citri cells after incubation with periplasmic fractions of transformed (lanes 1 and 2) or nontransformed (lanes 3 and 4) E. coli cells followed by anti-Myc peptide monoclonal antibody 9E10.
Effect of 12G9 scFv on S. citri morphology, motility, metabolism, and growth.
When a deformation test was performed with a pure or a 20-fold dilution of the E. coli periplasmic fraction containing recombinant scFv 12G9 (about 300 μg/ml), no deformation was observed, but 100% of the filaments were nonmotile and similar to those obtained with 12H6 monoclonal antibody. This proportion decreased to 25% with a 40-fold dilution and reached 4% with an 80-fold dilution, i.e., 4 μg of scFv per ml. No modification of the morphology and motility of spiroplasma helices was observed with dilutions of the periplasmic fraction prepared from nontransformed bacteria.
As with the corresponding 12G9 monoclonal antibody, the scFv had no effect on spiroplasma metabolism when used at concentrations from 2 μg/ml to 40 μg/ml, and growth was not prevented on solid media with scFv at 50 to 300 μg/ml. However, when various concentrations of scFv were added to 103 S. citri liquid cultures (Fig. 4), strong growth inhibition was observed for a concentration of about 10 μg/ml (Fig. 4, curve C). A control curve made with the periplasmic fraction from nontransformed E. coli cells showed that it also had a faint inhibitory effect (Fig. 4, curve B). Lower concentrations of scFv (Fig. 4, curves D and E) failed to prevent S. citri growth.
FIG. 4.
32P-labeled phosphate incorporation during S. citri growth in liquid medium in the presence of PBS (A), periplasmic fraction of nontransformed (B) or transformed (C, D, and E) E. coli cells. C, D, and E correspond to 10, 2.5, and 0.5 μg of scFv 12G9 per ml, respectively.
To better compare the action of scFv with that of polyclonal and monoclonal IgGs, an experiment was performed by adding to an initial 103 S. citri culture either polyclonal serum (1:500), monoclonal antibody 12G9 (25 μg/ml), or periplasmic fraction (10 μg of scFv per ml). As control, the periplasmic fraction from nontransformed E. coli cells was used. Results shown on Fig. 5 indicated that scFv, even at a low concentration, has a growth-inhibitory effect stronger than that obtained with the monoclonal antibody and similar to that of the polyclonal serum.
FIG. 5.
32P-labeled phosphate incorporation during S. citri growth in liquid medium in the presence of periplasmic fraction of nontransformed E. coli cells (A), polyclonal serum diluted 1:500 (B), monoclonal antibody 12G9 at 25 μg/ml (C), and scFv 12G9 at 10 μg/ml (D).
DISCUSSION
In vertebrate animals, the immune system plays an important role in the control of pathogens. Plants lack an immune system, but with the development of genetic engineering it is now possible to express entire or recombinant antibodies in planta (2, 7, 8, 18, 22, 25, 30). This approach has been used with variable success for the control of several pathogens (5, 19, 26, 29, 34, 38) and seems particularly well suited for the control of phytopathogenic mollicutes. Indeed, the growth of these wall-less bacteria is strongly inhibited by antibodies in vitro. In addition, plant-pathogenic mollicutes being naturally transmitted by leafhoppers or psyllids, only very small amounts of cells (<103) are inoculated by the insect upon feeding on the phloem sieve tube sap. Thus, constitutive expression of specific antibodies in this cell compartment should be adequate to control a given mollicute.
Proteins present in the phloem sap are synthesized in the cytoplasm of companion cells and move into sieve tubes via plasmodesms (21). Expression of functional, full-size immunoglobulins in plants has been achieved, provided that a signal peptide allowing proper assembly in the endoplasmic reticulum is included (18, 25, 34). This results in excretion of the antibodies into the extracellular spaces. Expression of full-size IgGs could not be obtained in the cytoplasm, probably because of its reducing environment, preventing the formation of disulfide bonds (8, 18). On the contrary, several, but not all, recombinant antibody (scFv) molecules have been functionally expressed in the cytoplasm (2, 5, 7, 25, 27, 30), and because of their ease of construction, their relatively small size, which facilitates diffusion, and no requirement for assembly, they have become the best candidates for plantibody production. However, the effect of scFv molecules on morphology, growth, and metabolism of mollicutes has not been evaluated.
In the study reported here, we have shown that scFv 12G9 has some properties that are different from its monoclonal antibody parent, while other properties are conserved. It did not deform spiroplasma helices, probably because it has only one antigen binding site; however, helicity and motility were lost, even though these two properties are not always related (11). The scFv as well as the monoclonal antibodies used in this study did not inhibit metabolism. This is different from results obtained by others (5, 13), who reported metabolism inhibition with monoclonal antibodies that are able to agglutinate mycoplasma or spiroplasma cells. This is why agglutination was thought to play a role in metabolism inhibition. Our results indicate that, even though monoclonal antibody 12G9 induced agglutination, metabolism was not affected, and prediction of the effect of monoclonal antibodies or scFv on metabolism cannot be inferred from their agglutination properties.
In the initial description of the test, fresh complement was added to the medium and was reported to be required for metabolism inhibition (36), but Fletcher and Wijetunga obtained equivalent S. citri metabolism inhibitions using polyclonal antibodies with and without complement (14). As complement is not produced in plants, all our experiments were done in the absence of complement, and we did obtain metabolism inhibition with the polyclonal serum but not with monoclonal antibodies and scFv. Whether or not metabolism inhibition can be obtained by adding complement to monoclonal antibodies or scFv remains to be tested.
The effect of antibodies on spiroplasma growth in liquid medium was very strong with all types of molecules, particularly with polyclonal and recombinant scFv antibodies, while monoclonal antibodies and scFv were inefficient when tested on solid medium. Monoclonal antibodies have been shown to produce no or only faint zones of growth inhibition, with fewer colonies on solid medium (5). This is probably because monoclonal antibodies are less stable, especially when acidification of the medium occurs during mollicute growth. Also, when inhibition was tested in liquid medium, antibodies were added every 24 h to mimic the conditions that would occur in transgenic plants with a constitutive expression of the transgene.
Because recombinant antibodies are capable of strongly inhibiting spiroplasma growth, they are appropriate molecules for plantibody-based control strategies for mollicutes. However, this may require engineering of bi- or trispecific antibodies for more efficient effects, since our study suggests that some spiroplasma cells are not affected by the action of a single monoclonal antibody. Currently, the potential inhibitory effects of monoclonal antibodies or scFv on mollicute growth can be assessed only by in vitro testing. Unfortunately, this cannot be achieved with the uncultured phytoplasmas, which are economically the most important group of plant-pathogenic mollicutes. Panels of different recombinant antibodies have to be tested by the long-term process of plant transformation and subsequent challenge with the pathogen. In addition, conditions for cytoplasmic expression of recombinant antibodies and their delivery into the phloem sap are not yet fully mastered.
An alternative approach to the delivery of antibodies to the phloem sap, in which antibodies against phytoplasmas would be delivered directly by a nonphytopathogenic mutant of S. citri (17), is currently being pursued in our laboratory.
Acknowledgments
This work was supported by a grant from the French Ministry for Education Research and Technology (MENRT). Sylvie Malembic was supported by the Centre Interprofessionnel des Vins de Bordeaux (CIVB).
We thank D. L. Williamson, SUNY Health Science Center, Stony Brook, N.Y., for critical review of the manuscript.
REFERENCES
- 1.Altshul, R. W., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Artsaenko, O., M. Peisker, U. zur Nieden, U. Fiedler, E. W. Weiler, K. Müntz, and U. Conrad. 1995. Expression of a single-chain Fv antibody against abscisic acid creates a wilty phenotype in transgenic tobacco. Plant J. 8:745-750. [DOI] [PubMed] [Google Scholar]
- 3.Avakian, A. P., and D. H. Ley. 1993. Inhibition of Mycoplasma gallisepticum growth and attachment to chick tracheal rings by antibodies to a 64-kilodalton membrane protein of M. gallisepticum. Avian Dis. 37:706-714. [PubMed] [Google Scholar]
- 4.Bové, J. M., and M. Garnier. 1998. Walled and wall-less eubacteria from plants: sieve-tube-restricted plant pathogens and plant surface contaminants. Plant Cell Tissue Organ Culture 52:7-16. [Google Scholar]
- 5.Chen, Y. D., and T. A. Chen. 1998. Expression of engineered antibodies in plants: a possible tool for spiroplasma and phytoplasma disease control. Phytopathology 88:1367-1371. [DOI] [PubMed] [Google Scholar]
- 6.Chirgwin, J. M., A. E. Przybyla, R. J. MacDonald, and W. J. Rutter. 1979. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochem. J. 18:5294-5299. [DOI] [PubMed] [Google Scholar]
- 7.De Jaeger, G., E. Buys, D. Eeckhout, C. DeWilde, A. Jacobs, J. Kapila, G. Angenon, M. Van Montagu, T. Gerats, and A. Depicker. 1999. High level accumulation of single-chain variable fragment in the cytosol of transgenic Petunia hybrida. Eur. J. Biochem. 259:426-434. [DOI] [PubMed] [Google Scholar]
- 8.De Wilde, C., R. De Rycke, T. Beeckman, M. De Neve, M. Van Montagu, G. Engler, and A. Depicker. 1998. Accumulation pattern of IgG antibodies and Fab fragments in transgenic Arabidopsis thaliana plants. Plant Cell Physiol. 39:639-646. [DOI] [PubMed] [Google Scholar]
- 9.Doi, Y., M. Teranaka, K. Yora, and H. Asuyama. 1967. Mycoplasma or PLT group-like microorganisms found in the phloem elements of plants infected with mulberry dwarf and potato witches' broom. Ann. Phytopathol. Soc. Jpn. 33:259-266. [Google Scholar]
- 10.Dübel, S., F. Breitling, R. Kontermann, T. Schmidt, A. Skerra, and M. Little. 1995. Bifunctional and multimeric complexes of streptavidin fused to single chain antibodies (scFv). J. Immunol. Methods. 178:201-209. [DOI] [PubMed] [Google Scholar]
- 11.Duret, S., J. L. Danet, M. Garnier, and J. Renaudin. 1999. Gene disruption through homologous recombination in Spiroplasma citri: an scm1 disrupted motility mutant is pathogenic. J. Bacteriol. 181:7449-7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evan, G. J., G. K. Lewis, G. Ramsay, and J. M. Bishop. 1985. Isolation of monoclonal antibodies specific for human c-Myc proto-oncogene product. Mol. Cell. Biol. 5:3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmann, R. C., B. Henrich, V. Kolb-Bachofen, and U. Hadding. 1992. Decreased metabolism and viability of Mycoplasma hominis induced by monoclonal antibody-mediated agglutination. Infect. Immun. 60:166-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher, J., and C. Wijetunga. 1990. Serological characterisation of surface proteins of Spiroplasma citri. Can. J. Microbiol. 37:28-33. [Google Scholar]
- 15.Foissac, X., C. Saillard, J. Gandar, L. Zreik, and J. M. Bové. 1996. Spiralin polymorphysm in strains of Spiroplasma citri is not due to posttranslational palmitoylation. J. Bacteriol. 178:2934-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garnier, M., M. Clerc, and J. M. Bové. 1981. Growth and division of spiroplasmas: morphology of Spiroplasma citri during growth in liquid medium. J. Bacteriol. 147:642-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaurivaud, P., J. L. Danet, F. Laigret, M. Garnier, and, J. M. Bové. 2000. Fructose utilization and pathogenicity of Spiroplasma citri. Mol. Plant-Microbe Interact. 13:1145-1155. [DOI] [PubMed] [Google Scholar]
- 18.Hiatt, A., R. Cafferkey, and K. Bowdish. 1989. Production of antibodies in transgenic plants. Nature 342:76-78. [DOI] [PubMed] [Google Scholar]
- 19.Lapidot, Z., R. Siman-Tov, and Y. Naot. 1995. Monoclonal antibody that inhibits mitogenic activity of Mycoplasma pulmonis. Infect. Immun. 63:134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gall, F., J. M. Bové, and M. Garnier. 1998. Engineering of a single-chain variable fragment (scFv) antibody specific for the stolbur phytoplasma (Mollicute) and its expression in Escherichia coli and tobacco plants. Appl. Environ. Microbiol. 64:4566-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oparka, K. J., and S. Santa Cruz. 2000. The great escape: phloem transport and unloading of macromolecules. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51:323-347. [DOI] [PubMed] [Google Scholar]
- 22.Owen, M., A. Gandecha, B. Cockburn, and G. Whitelam. 1992. Synthesis of a functional anti-phytochrome single-chain Fv protein in transgenic tobacco. Bio/Technology 10:790-794. [DOI] [PubMed] [Google Scholar]
- 23.Saglio, P., M. Lhospital, D. Laflèche, G. Dupont, J. M. Bové, J. G. Tully, and E. A. Freundt. 1973. Spiroplasma citri gen. and sp. nov.: a mycoplasma-like organism associated with Stubborn disease of citrus. Int. J. Syst. Bacteriol. 23:191-204. [Google Scholar]
- 24.Sambrook, J., F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Shillberg, S., S. Zimmermann, A. Voss, and R. Fisher. 1999. Apoplastic and cytosolic expression of full size antibodies and antibodies fragments in Nicotiana tabacum. Transgenic Res. 8:255-263. [DOI] [PubMed] [Google Scholar]
- 26.Schillberg, S., S. Zimmermann, M. Y. Zhang, and R. Fischer. 2001. Antibody-based resistance to plant pathogens. Transgenic Res. 10:1-12. [DOI] [PubMed] [Google Scholar]
- 27.Schouten, A., J. Roossien, J. M. de Boer, A. Wilmink, M. N. Rosso, D. Bosch, W. J. Stiekema, F. J. Gommers, J. Bakker, and A. Schots. 1997. Improving scFv antibody expression levels in the plant cytosol. FEBS Lett. 415:235-241. [DOI] [PubMed] [Google Scholar]
- 28.Stinson, J. R., V. Wittman, and H. C. Wong. 1995. Generation of single-chain antibody fragments by PCR, p. 300-312. In M. A. Innis (ed.), PCR strategies. Academic Press, New York, N.Y.
- 29.Tavladoraki, P., E. Benvenuto, S. Trinca, D. De Martinis, A. Cattaneo, and P. Galeffi. 1993. Transgenic plants expressing a functional single-chain Fv antibody are specifically protected from virus attack. Nature 366:469-472. [DOI] [PubMed] [Google Scholar]
- 30.Tavladoraki, P., A. Girotti, M. Donini, F. J. Arias, C. Mancini, V. Morea, R. Chiaraluce, V. Consalvi, and E. Benvenuto. 1999. A single-chain antibody fragment is functionally expressed in the cytoplasm of both Escherichia coli and transgenic plants. Eur. J. Biochem. 262:617-624. [DOI] [PubMed] [Google Scholar]
- 31.Taylor-Robinson, D., R. H. Purcell, D. C. Wong, and R. M. Chanock. 1966. A colour test for the measurement of antibody to certain mycoplasma species based upon inhibition of acid production. J. Hyg. 6:91-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tully, J. G., R. F. Whitcomb, H. F. Clark, and D. L. Williamson. 1977. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science 195:892-894. [DOI] [PubMed] [Google Scholar]
- 33.Vignault, J. C., J. M. Bové, C. Saillard, R. Vogel, A. Faro, L. Venegas, W. Stemmer, S. Aoki, R. E. McCoy, A. S. Albeldawi, M. Larue, M. Tuzcu, M. Ozsan, A. Nhami, M. Abassi, M. Bonfils, G. Moutous, A. Fos, F. Poutier, and G. Viennot-Bougin. 1980. Mise en culture de spiroplasmes à partir de matériel végétal et d'insectes provenant des pays circum-méditerranéens et du proche-orient. C. R. Acad. Sci. Paris 290:775-778. [Google Scholar]
- 34.Voss, A., M. Niersback, R. Hain, H. J. Hirsch, Y. C. Liao, F. Kreuzaler, and R. Fischer. 1995. Reduced virus infectivity in N. tabacum secreting a TMV-specific full size antibody. Mol. Breed. 1:39-50. [Google Scholar]
- 35.Williamson, D. L., R. F. Whitcomb, and J. G. Tully. 1978. The spiroplasma deformation test, a new serological method. Curr. Microbiol. 1:203-207. [Google Scholar]
- 36.Williamson, D. L. 1983. The combined deformation-metabolism inhibition test, p. 477-483. In S. Razin and J. G. Tully (ed.), Methods in mycoplasmology, vol. I. Academic Press, New York, N.Y. [Google Scholar]
- 37.Yoshida, S., A. Fujisawa, Y. Tsusaki, and S. Saitoh. 2000. Identification and expression of a Mycoplasma gallisepticum surface antigen recognized by a monoclonal antibody capable of inhibiting both growth and metabolism. Infect. Immun. 68:3186-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmermann, S., S. Schillberg, Y. C. Liao, and R. Fischer. 1998. Intracellular expression of TMV-specific single-chain Fv fragments leads to improved virus resistance in Nicotiana tabacum. Mol. Breed. 4:369-379. [Google Scholar]