Abstract
1. Micro-electrode recordings were made from single Ia afferents in the intact nerve to the soleus muscle in the decerebrate cat while the muscle was developing a tonic vibration reflex. This was done in order to test how effectively the afferents were excited by the vibration, and to see if any insecurity in driving might be related to tremor.
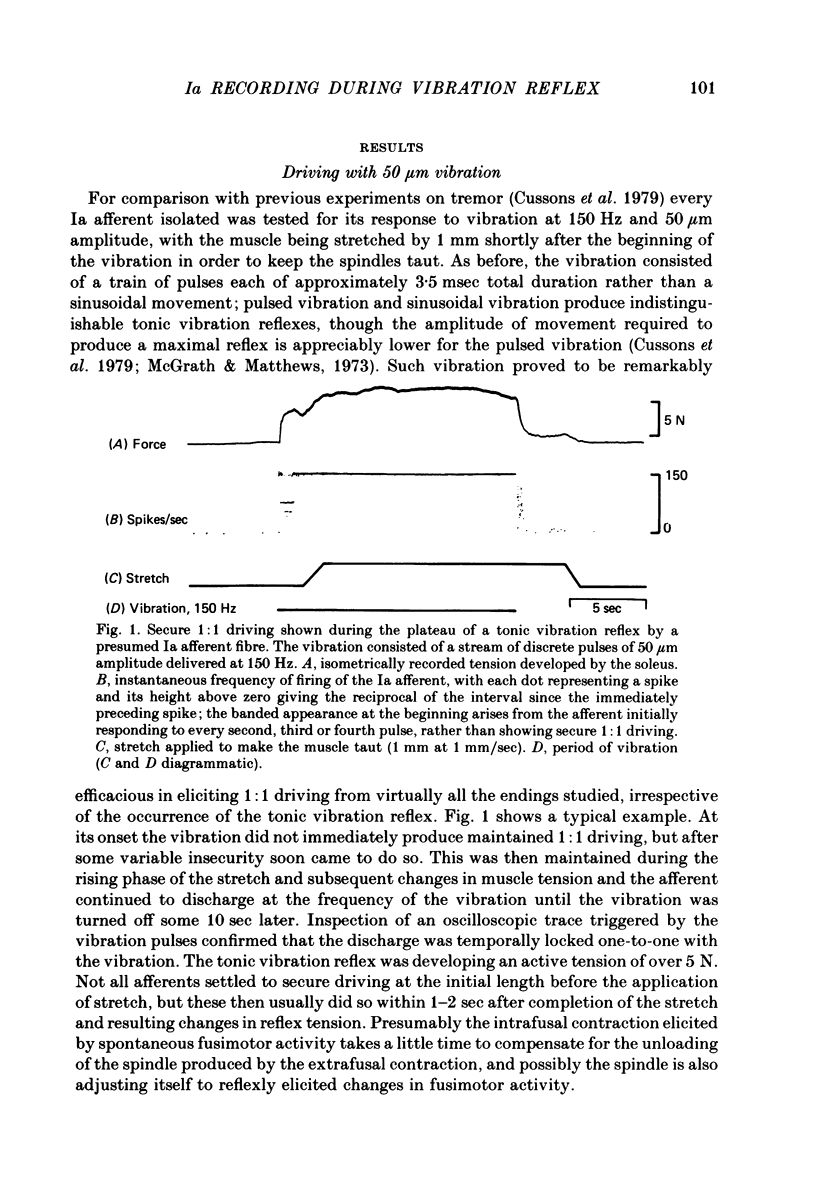
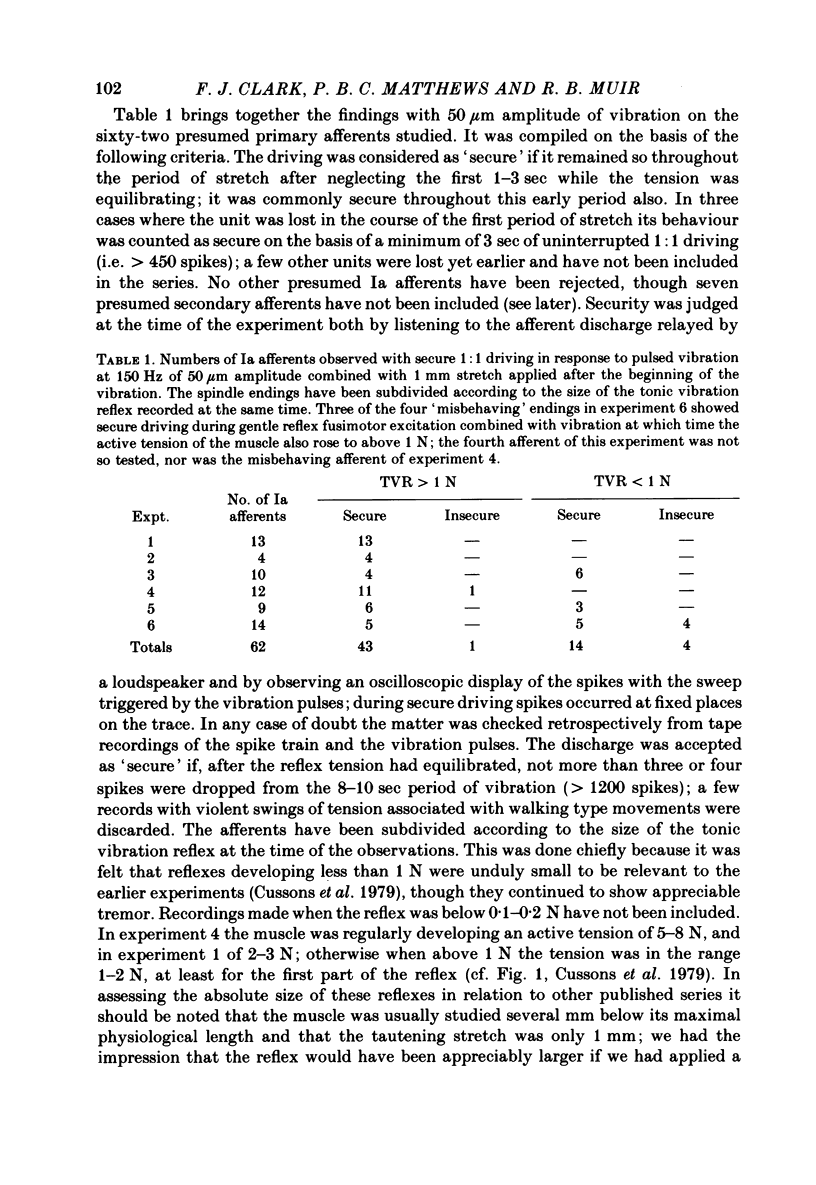
2. When the amplitude of vibration was 50 μm, and the tonic vibration reflex was reasonably well developed (> 1 N of active tension) all but one of forty-four Ia afferents were driven 1:1 by the vibration. Most were still driven by 30 μm vibration. The vibration, consisting of a train of discrete pulses at 150 Hz, was applied longitudinally in combination with a stretch of 1 mm to make the muscle taut.
3. If the reflex was poorly developed (active tension < 1 N) the driving was on average less secure. However, fourteen of eighteen afferents then studied were still driven 1:1 by 50 μm vibration. The lower level of excitation by vibration was thought to be due to a deficiency of spontaneous fusimotor activity, because stroking the cat's tail or other similar gentle manipulation led each of the three misbehaving afferents so tested to be driven securely by 50 μm vibration; at the same time the reflex tension increased.
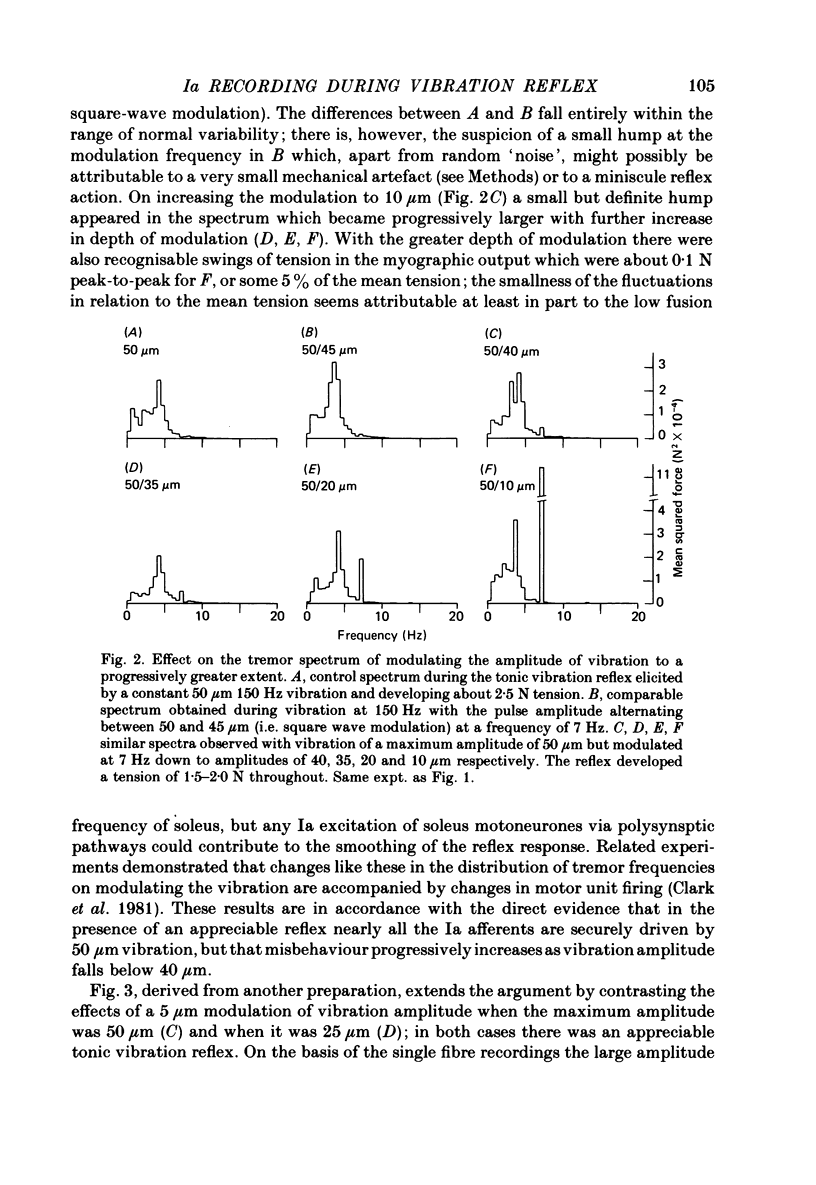
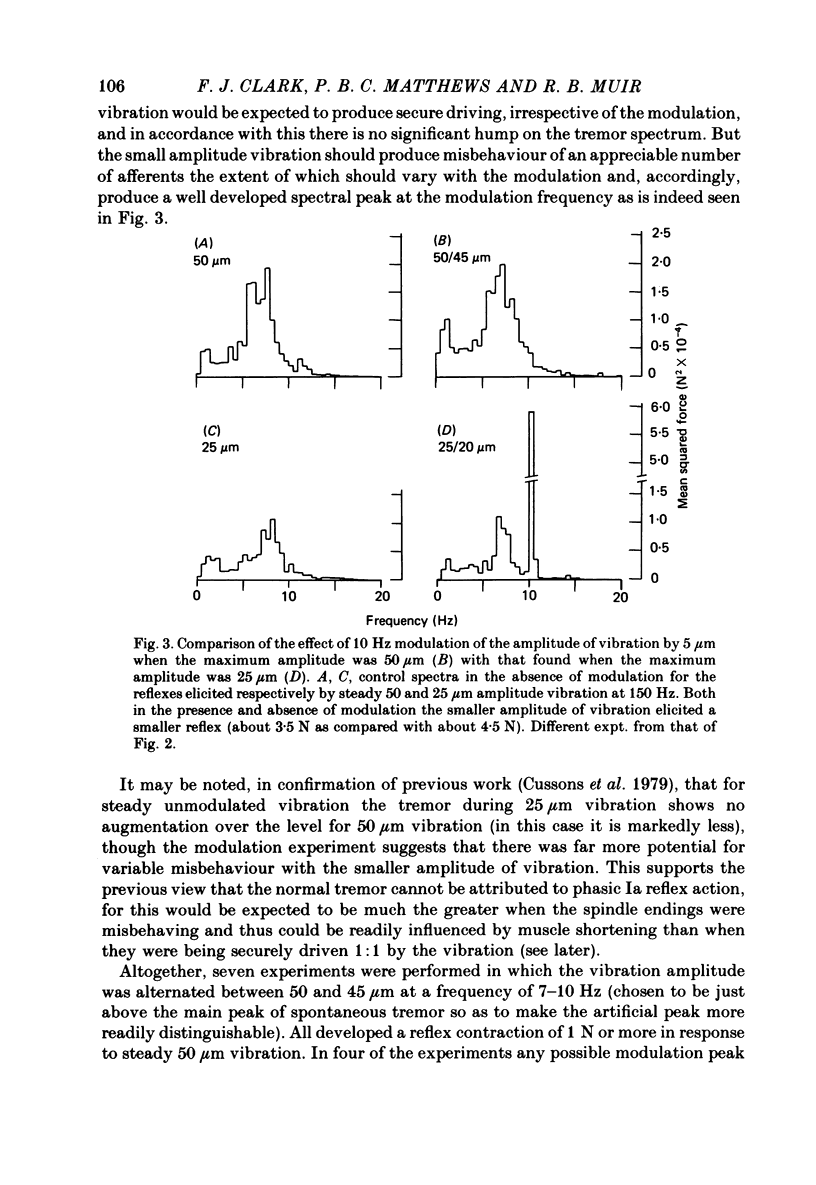
4. Additional, indirect evidence favouring widespread security of Ia driving by 50 μm vibration in the presence of the reflex was obtained by modulating the amplitude of the 150 Hz vibration with a 7-10 Hz square wave and detecting any tension fluctuations at that frequency by spectral analysis. Small degrees of modulation (e.g. < 10%) produced little if any effect, although larger depths of modulation had a powerful action.
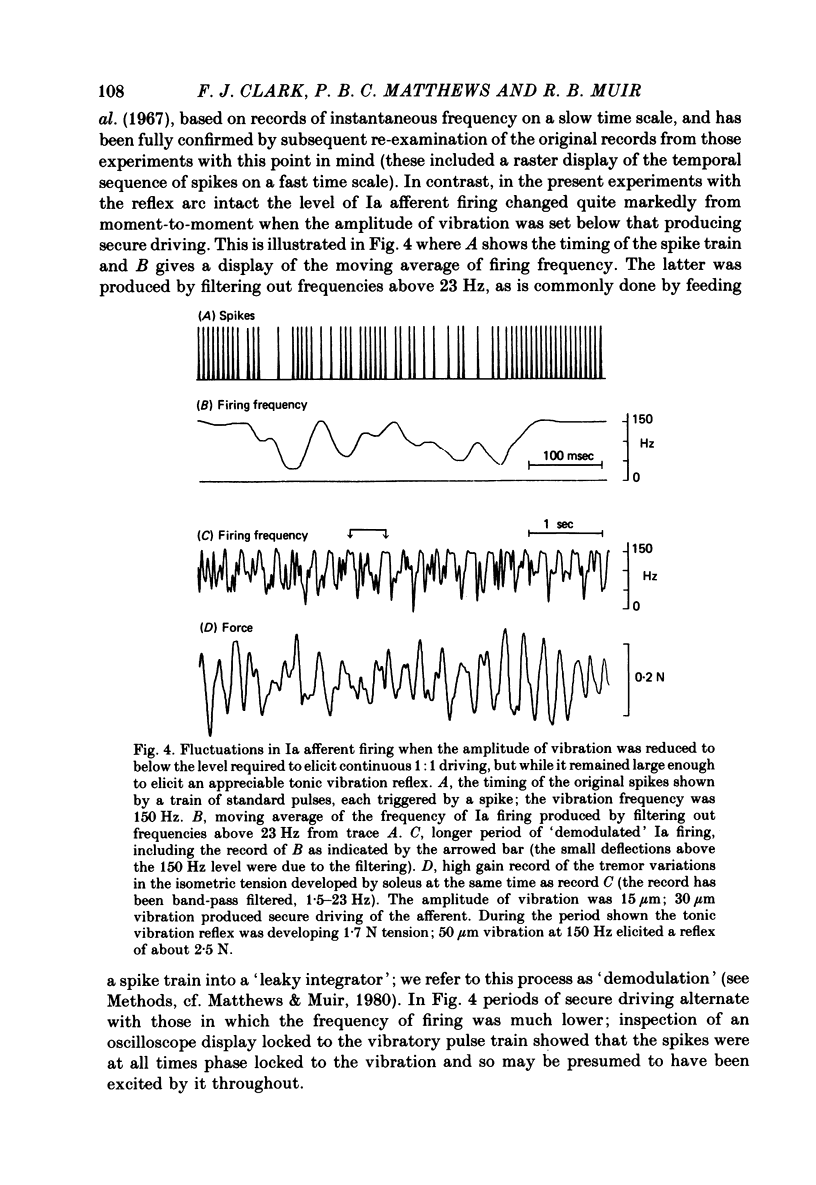
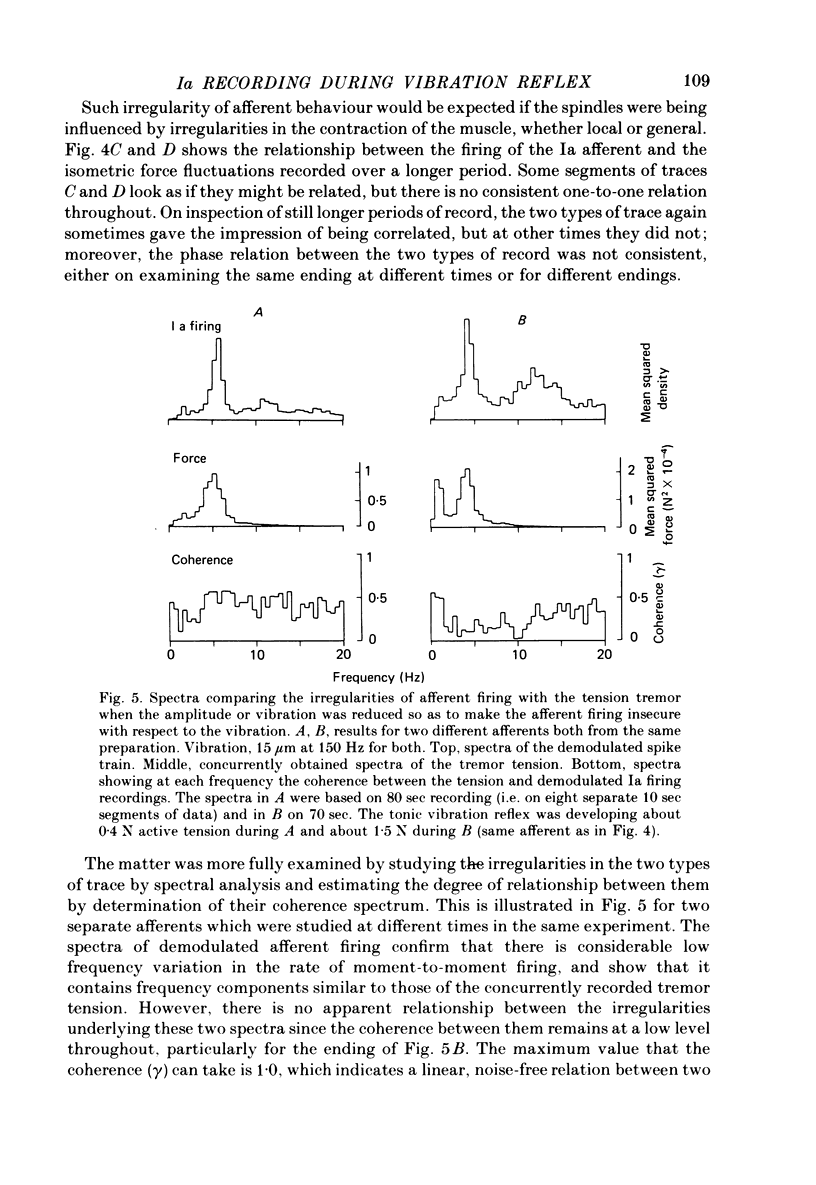
5. When the amplitude of vibration was reduced to permit insecure driving but still to elicit a reflex response, the fluctuations in Ia firing pattern were unlike those previously seen in the de-efferented muscle. Spectral analysis showed that these firing fluctuations bore a general similarity to the tremor in the same preparation, but measurement of coherence demonstrated that the tremor and Ia firing were not well related. This was probably because individual Ia afferents were primarily influenced by local factors, and provides further evidence against the tremor of this preparation being attributable to the action of the stretch reflex.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder M. D., Kroin J. S., Moore G. P., Stauffer E. K., Stuart D. G. Correlation analysis of muscle spindle responses to single motor unit contractions. J Physiol. 1976 May;257(2):325–336. doi: 10.1113/jphysiol.1976.sp011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Clark F. J. Dorsal column projection of fibres from the cat knee joint. J Physiol. 1969 Aug;203(2):301–315. doi: 10.1113/jphysiol.1969.sp008865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Perl E. R. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967 Jun;190(3):541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976 Oct;261(3):695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussons P. D., Matthews P. B., Muir R. B. Enhancement by agonist or antagonist muscle vibration of tremor at the elastically loaded human elbow. J Physiol. 1980 May;302:443–461. doi: 10.1113/jphysiol.1980.sp013255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussons P. D., Matthews P. B., Muir R. B. Tremor in the tension developed isometrically by soleus during the tonic vibration reflex in the decerebrate cat. J Physiol. 1979 Jul;292:35–57. doi: 10.1113/jphysiol.1979.sp012837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., HOLMGREN B., MERTON P. A. The two routes for excitation of muscle and their subservience to the cerebellum. J Physiol. 1955 Oct 28;130(1):213–224. doi: 10.1113/jphysiol.1955.sp005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Roberts R. C. The role of muscle spindle afferents in stretch and vibration reflexes of the soleus muscle of the decerebrate cat. Brain Res. 1978 May 12;146(2):366–372. doi: 10.1016/0006-8993(78)90981-2. [DOI] [PubMed] [Google Scholar]
- Kanda K., Rymer W. Z. An estimate of the secondary spindle receptor afferent contribution to the stretch reflex in extensor muscles of the decerebrate cat. J Physiol. 1977 Jan;264(1):63–87. doi: 10.1113/jphysiol.1977.sp011658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Evidence that the secondary as well as the primary endings of the muscle spindles may be responsible for the tonic stretch reflex of the decerebrate cat. J Physiol. 1969 Oct;204(2):365–393. doi: 10.1113/jphysiol.1969.sp008918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Muir R. B. Comparison of electromyogram spectra with force spectra during human elbow tremor. J Physiol. 1980 May;302:427–441. doi: 10.1113/jphysiol.1980.sp013254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966 May;184(2):450–472. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath G. J., Matthews P. B. Evidence from the use of vibration during procaine nerve block that the spindle group II fibres contribute excitation to the tonic stretch reflex of the decerebrate cat. J Physiol. 1973 Dec;235(2):371–408. doi: 10.1113/jphysiol.1973.sp010392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbury D. R. A study of stretch and vibration reflexes of the cat by intracellular recording from motoneurones. J Physiol. 1972 Oct;226(1):37–56. doi: 10.1113/jphysiol.1972.sp009972. [DOI] [PMC free article] [PubMed] [Google Scholar]


