Abstract
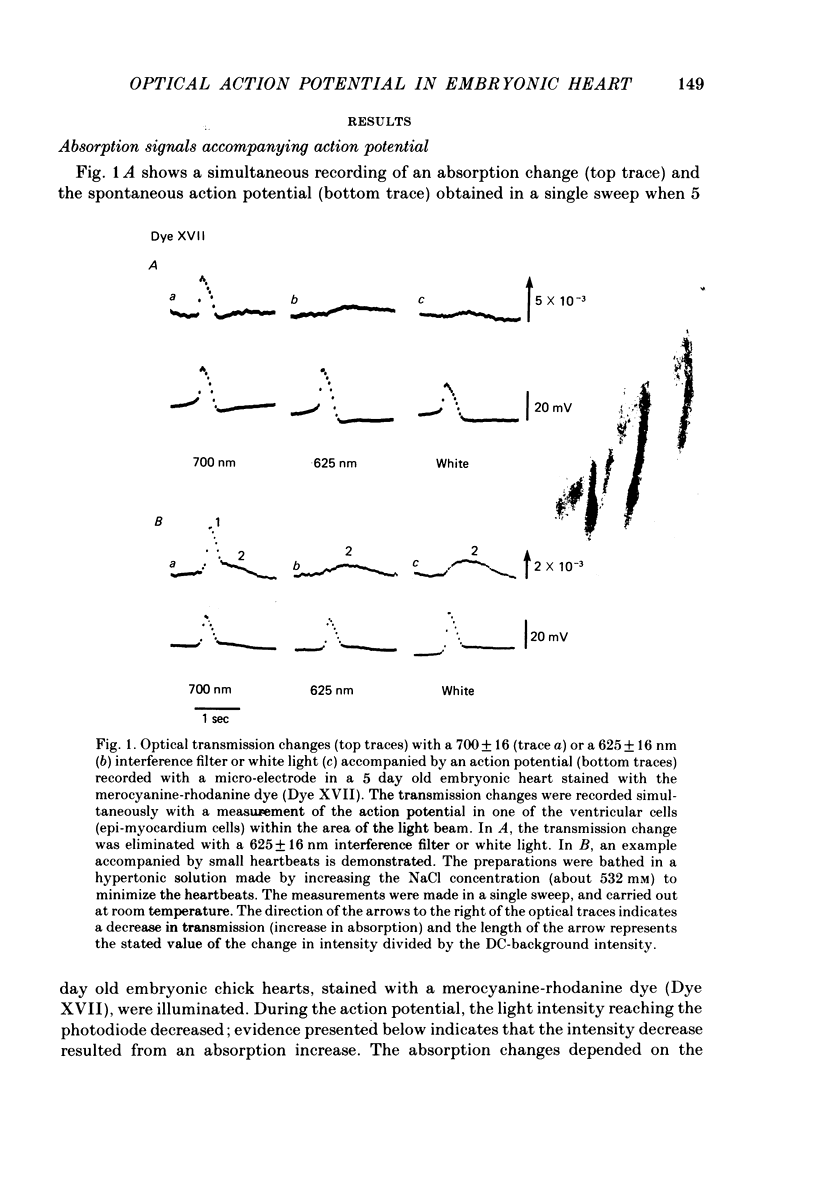
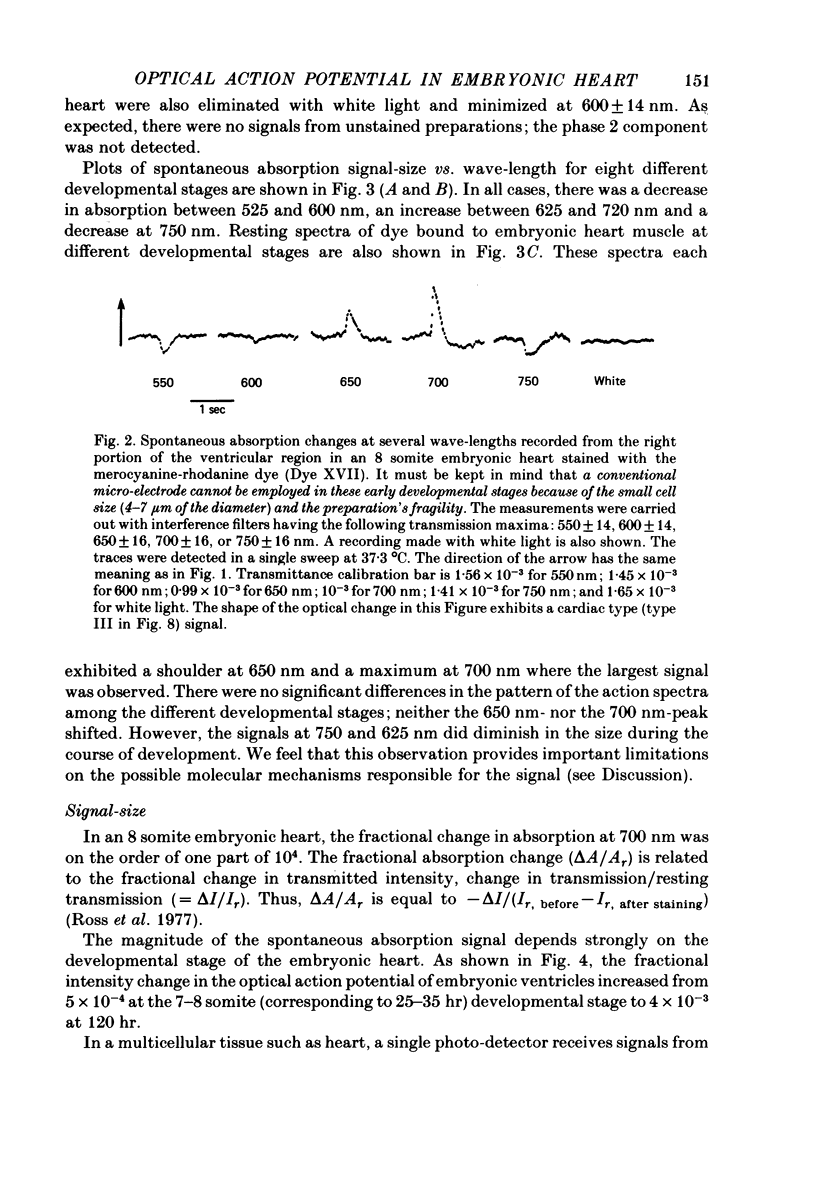
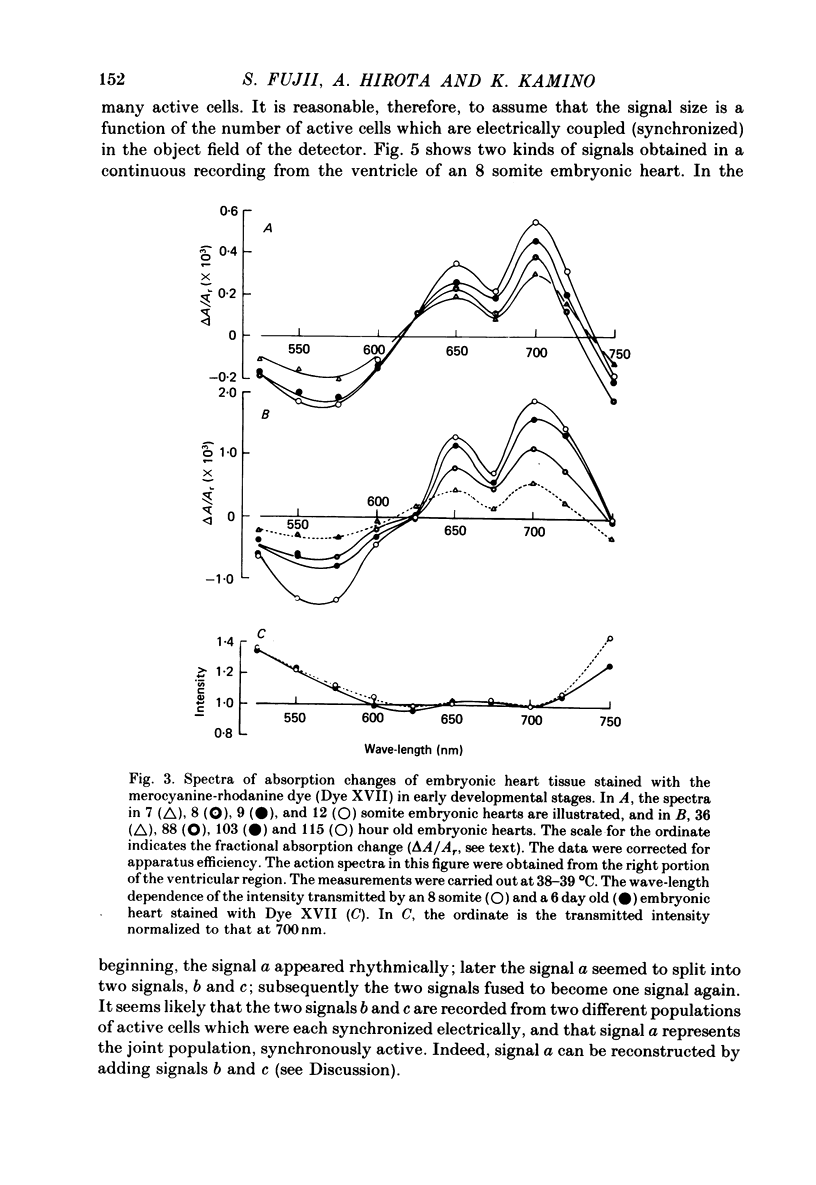
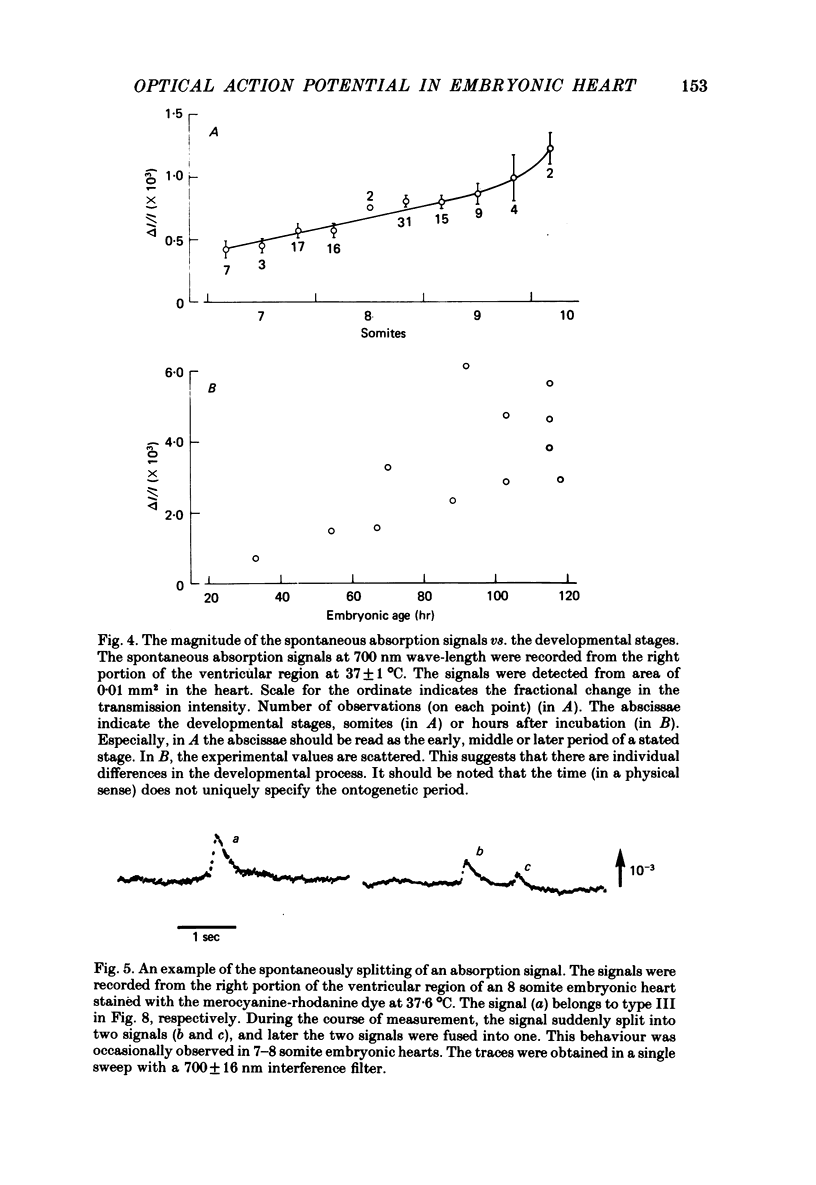
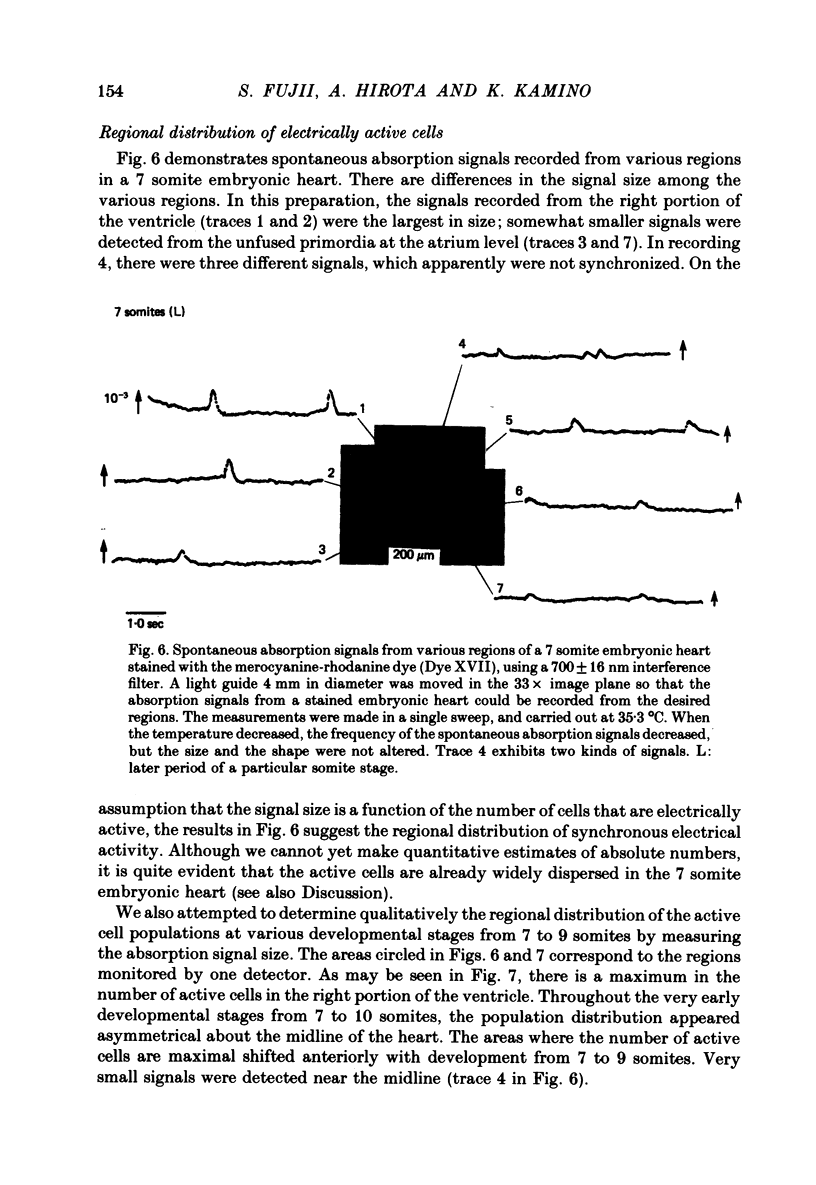
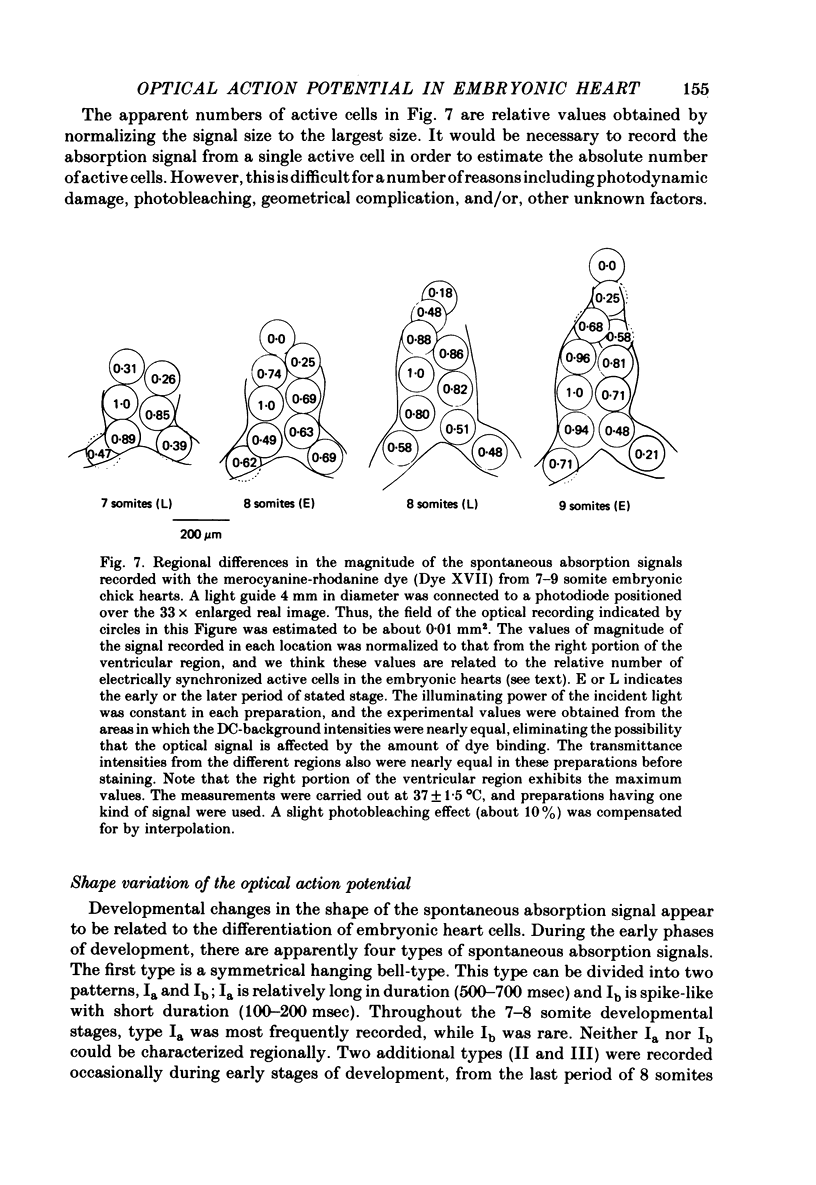
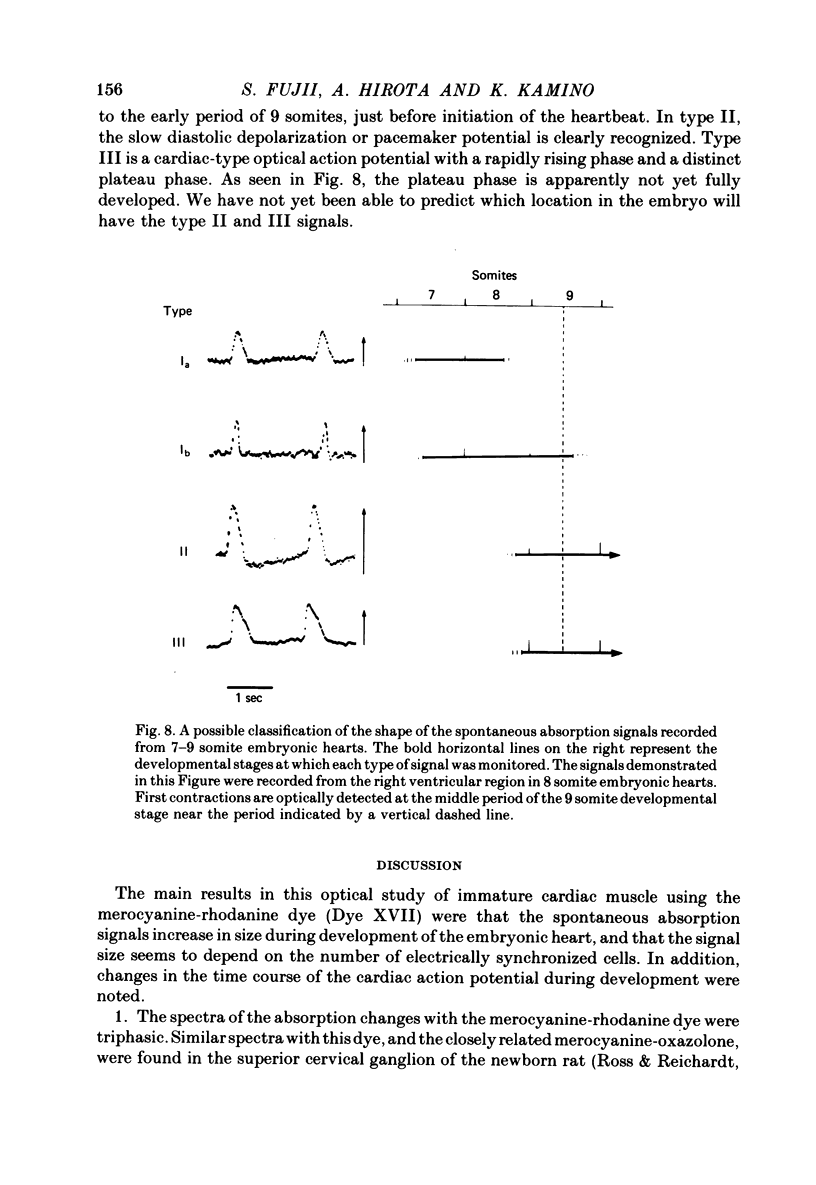
1. Developmental changes in the spontaneous action potential were measured using optical signals from early embryonic chick heart stained with a potential sensitive merocyanine-rhodanine dye. 2. The wave-length dependence of the dye signal is triphasic in early embryonic chick heart with a decrease in absorption from 525 to 600 nm, an increase from 625 to 720 nm, and a decrease at 750 nm. The signal was largest at 700 nm. 3. The magnitude of the spontaneous absorption signal increased as the development of the embryonic heart proceeded. In addition, the magnitude of the absorption signal differed among the various regions of an early embryonic chick heart and the number of electrically active cells increased dramatically throughout the 7-9 somite developmental stages. 4. The number of the electrically active cells was largest in the right portion of the ventricle at the 7-9 somite developmental stages. 5. The shape of spontaneous absorption signals could be classified into four types in the developmental stages between 7 and 9 somites: Signals resembling the cardiac action potential and the pace-maker potential were not recorded until the 8-9 somite developmental stage.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Harary H. H., Waggoner A. Isopotentiality and an optical determination of series resistance in Limulus ventral photoreceptors. J Physiol. 1979 Nov;296:357–372. doi: 10.1113/jphysiol.1979.sp013010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Davila H. V., Ross W. N., Landowne D., Waggoner A. S., Wang C. H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19(1):1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Fujii S., Hirota A., Kamino K. Optical signals from early embryonic chick heart stained with potential sensitive dyes: evidence for electrical activity. J Physiol. 1980 Jul;304:503–518. doi: 10.1113/jphysiol.1980.sp013339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A., Fujii S., Kamino K. Optical monitoring of spontaneous electrical activity of 8-somite embryonic chick heart. Jpn J Physiol. 1979;29(5):635–639. doi: 10.2170/jjphysiol.29.635. [DOI] [PubMed] [Google Scholar]
- Ishima Y., Waku K. Phospholipid analysis of the chick ventricles in the early stages of development when their excitability changes in tetrodotoxin or in sodium substitute media. Comp Biochem Physiol C. 1978;61 100(2):283–286. doi: 10.1016/0306-4492(78)90055-2. [DOI] [PubMed] [Google Scholar]
- Manasek F. J. Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J Morphol. 1968 Jul;125(3):329–365. doi: 10.1002/jmor.1051250306. [DOI] [PubMed] [Google Scholar]
- Manasek F. J. Embryonic development of the heart. II. Formation of the epicardium. J Embryol Exp Morphol. 1969 Nov;22(3):333–348. [PubMed] [Google Scholar]
- Morad M., Salama G. Optical probes of membrane potential in heart muscle. J Physiol. 1979 Jul;292:267–295. doi: 10.1113/jphysiol.1979.sp012850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTEN B. M. Initiation and early changes in the character of the heart beat in vertebrate embryos. Physiol Rev. 1949 Jan;29(1):31–47. doi: 10.1152/physrev.1949.29.1.31. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Reichardt L. F. Species-specific effects on the optical signals of voltage-sensitive dyes. J Membr Biol. 1979 Aug;48(4):343–356. doi: 10.1007/BF01869445. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Grinvald A., Cohen L. B., Davila H. V., Ross W. N. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol. 1977 Nov;40(6):1281–1291. doi: 10.1152/jn.1977.40.6.1281. [DOI] [PubMed] [Google Scholar]
- Senseman D. M., Salzberg B. M. Electrical activity in an exocrine gland: optical recording with a potentiometric dye. Science. 1980 Jun 13;208(4449):1269–1271. doi: 10.1126/science.7375937. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Tasaki K. Electrical excitability of developing cardiac muscle in chick embryos. Tohoku J Exp Med. 1966 Jan 25;88(1):49–56. doi: 10.1620/tjem.88.49. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Shigenobu K. Changes in membrane properties of chick embryonic hearts during development. J Gen Physiol. 1972 Oct;60(4):430–453. doi: 10.1085/jgp.60.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalsberg H., DeHaan R. L. The precardiac areas and formation of the tubular heart in the chick embryo. Dev Biol. 1969 Feb;19(2):128–159. doi: 10.1016/0012-1606(69)90052-9. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Grinvald A. Mechanisms of rapid optical changes of potential sensitive dyes. Ann N Y Acad Sci. 1977 Dec 30;303:217–241. [PubMed] [Google Scholar]
- Woolum J. C., Strumwasser F. Membrane-potential-sensitive dyes for optical monitoring of activity in Aplysia neurons. J Neurobiol. 1978 May;9(3):185–193. doi: 10.1002/neu.480090302. [DOI] [PubMed] [Google Scholar]




