Abstract
1. The influence of Ca2+ concentration and ionic strength on the maximum velocity of shortening (Vmax) and the tension generating capability of frog skinned muscle fibres has been studied at temperatures between 1 and 10 degrees C. 2. Fibre segments were mounted between a force transducer and servo motor, where they could be viewed and photographed through a microscope. Segments in which the striations became non-uniform during activation were discarded. 3. Velocity was obtained as a function of load by stepping the tension to values less than the steady isometric tension. Vmax was then determined by an extrapolation technique. Vmax was also obtained using a second, independent method by measuring the times required to take up various amounts of slack imposed on the segments. 4. Vmax was significantly influenced by the Ca2+ concentration, decreasing by about one half when the Ca2+ concentration was reduced to give steady tensions less than half-maximal. 5. Vmax was not influenced by changes in ionic strength, in the range 0.09-0.18 M. Steady tension was found to increase as ionic strength was decreased in the same range. 6. These results indicate that the effect of changes in ionic strength is to alter the numbers or stiffness of attached cross-bridges, while there is no apparent influence of ionic strength on the steady-state kinetics of the actin-myosin interaction during unloaded shortening. The mechanism responsible for the influence of Ca2+ on Vmax is unknown, though possible sites of action for Ca2+ are discussed.
Full text
PDF




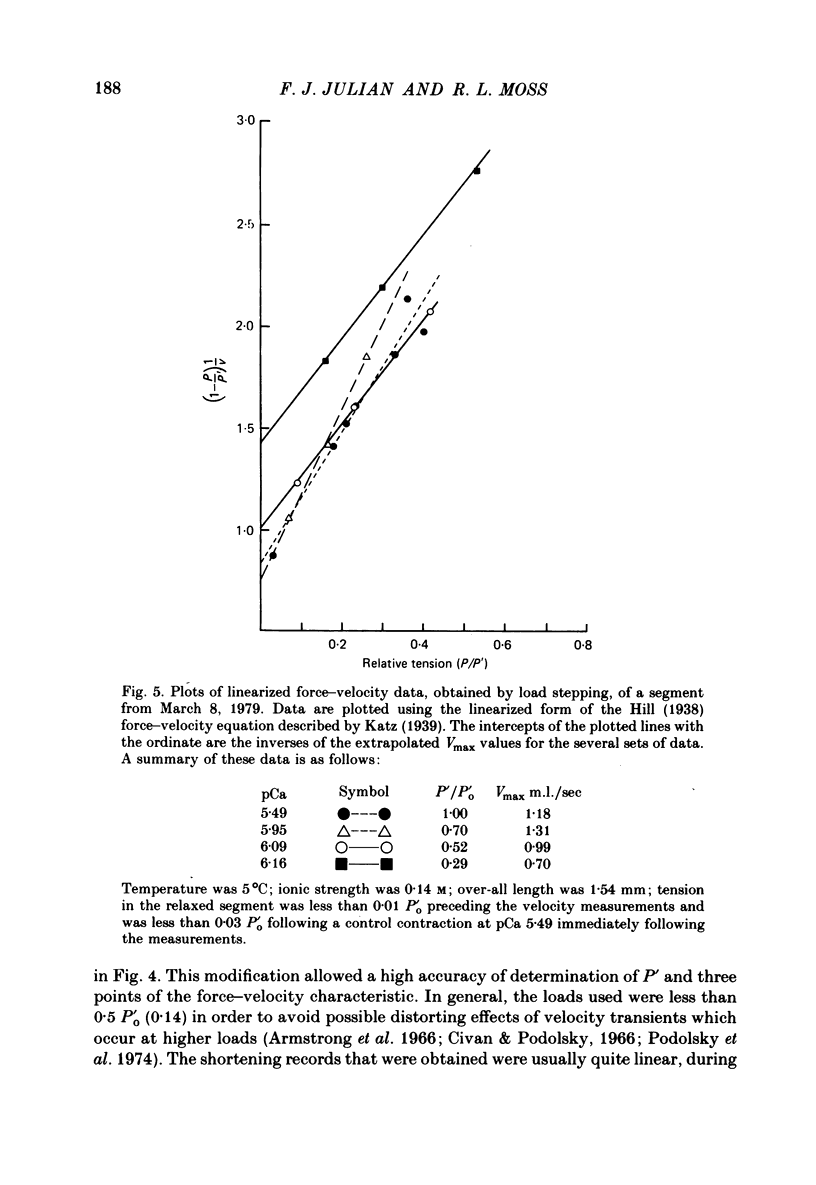
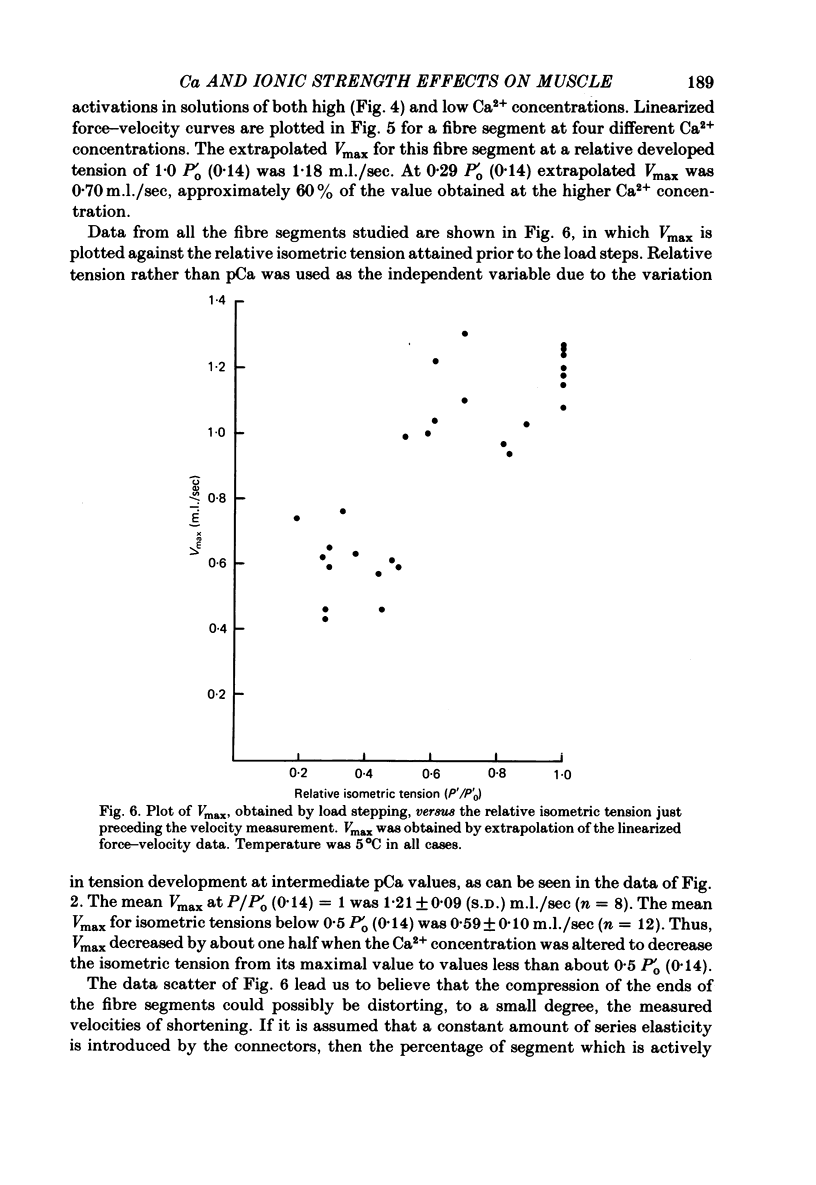
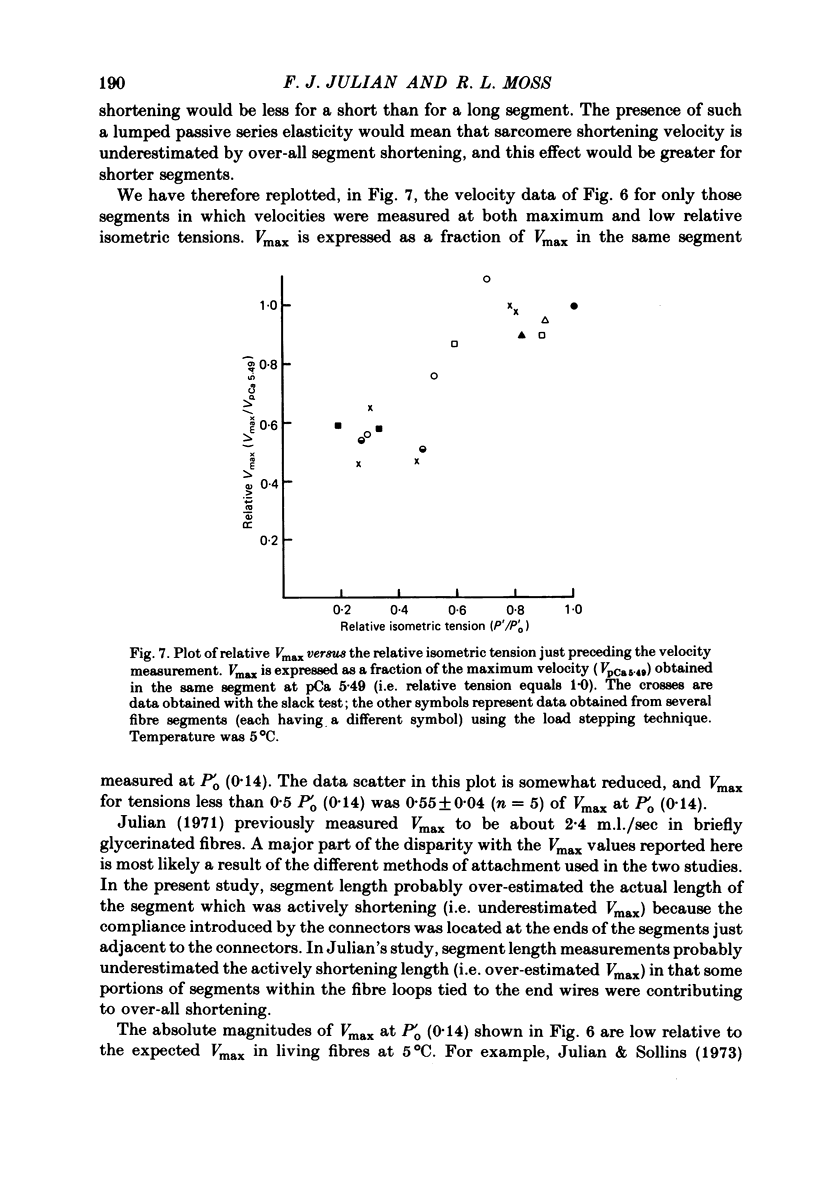
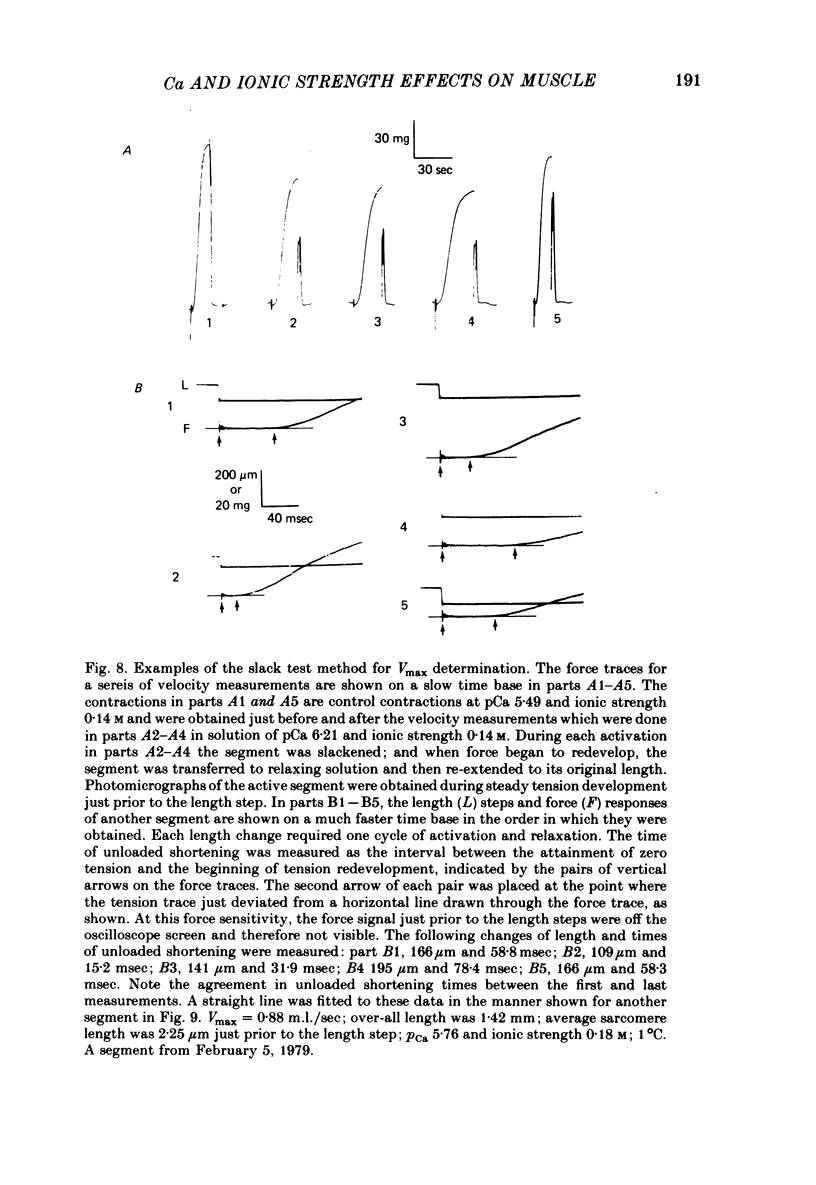





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M., Reisler E., Himmelfarb S., Harrington W. F. Myosin adenosine triphosphatase. Convergence of activation by actin and by SH1 modification at physiological ionic strength. J Biol Chem. 1974 Oct 10;249(19):6361–6363. [PubMed] [Google Scholar]
- Civan M. M., Podolsky R. J. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol. 1966 Jun;184(3):511–534. doi: 10.1113/jphysiol.1966.sp007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clerck N. M., Claes V. A., Brutsaert D. L. Force velocity relations of single cardiac muscle cells: calcium dependency. J Gen Physiol. 1977 Feb;69(2):221–241. doi: 10.1085/jgp.69.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Hwang J. C. The force-velocity relationship in vertebrate muscle fibres at varied tonicity of the extracellular medium. J Physiol. 1977 Jul;269(2):255–272. doi: 10.1113/jphysiol.1977.sp011901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Thorens S. Release of calcium from the sarcoplasmic reticulum induced by hypotonic solutions. Nihon Seirigaku Zasshi. 1975;37(12):422–424. [PubMed] [Google Scholar]
- Goodno C. C., Wall C. M., Perry S. V. Kinetics and regulation of the myofibrillar adenosine triphosphatase. Biochem J. 1978 Dec 1;175(3):813–821. doi: 10.1042/bj1750813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E., Donaldson S. K., Harris C. E. Tension in skinned frog muscle fibers in solutions of varying ionic strength and neutral salt composition. J Gen Physiol. 1973 Nov;62(5):550–574. doi: 10.1085/jgp.62.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E. Some effects of hypertonic solutions on contraction and excitation-contraction coupling in frog skeletal muscles. J Gen Physiol. 1970 Feb;55(2):254–275. doi: 10.1085/jgp.55.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Podolsky R. J. Contraction transients of skinned muscle fibers: effects of calcium and ionic strength. J Gen Physiol. 1978 Nov;72(5):701–715. doi: 10.1085/jgp.72.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellam D. C., Podolsky R. J. Force measurements in skinned muscle fibres. J Physiol. 1969 Feb;200(3):807–819. doi: 10.1113/jphysiol.1969.sp008723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig C. R., Takauji M. Effect of Ca++ on Vmax measured in absence of external or internal load. Eur J Cardiol. 1976 May;4 (Suppl):5–11. [PubMed] [Google Scholar]
- IWANAMI M., OSIBA S., YAMADA T., YOSHIMURA H. Seasonal variations in serum inorganic phosphate and calcium with special reference to parathyroid activity. J Physiol. 1959 Dec;149:23–33. doi: 10.1113/jphysiol.1959.sp006322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. The effect on tension of non-uniform distribution of length changes applied to frog muscle fibres. J Physiol. 1979 Aug;293:379–392. doi: 10.1113/jphysiol.1979.sp012895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai M., Miyamoto H. Depolarization-induced calcium release from sarcoplasmic reticulum fragments. I. Release of calcium taken up upon using ATP. J Biochem. 1976 May;79(5):1053–1066. doi: 10.1093/oxfordjournals.jbchem.a131147. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Noth J. Tension in isolated frog muscle fibers induced by hypertonic solutions. J Gen Physiol. 1973 Feb;61(2):158–175. doi: 10.1085/jgp.61.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., Gulati J., Nolan A. C. Contraction transients of skinned muscle fibers. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1516–1519. doi: 10.1073/pnas.71.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., Teichholz L. E. The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol. 1970 Nov;211(1):19–35. doi: 10.1113/jphysiol.1970.sp009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzino A. A., Barouch W. W., Eisenberg E., Moos C. Actin-heavy meromyosin biding. Determination of binding stoichiometry from adenosine triphosphatase kinetic measurements. Biochemistry. 1970 Jun 9;9(12):2402–2408. doi: 10.1021/bi00814a003. [DOI] [PubMed] [Google Scholar]
- Thames M. D., Teichholz L. E., Podolsky R. J. Ionic strength and the contraction kinetics of skinned muscle fibers. J Gen Physiol. 1974 Apr;63(4):509–530. doi: 10.1085/jgp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. M., Rondinone J. F., Briggs F. N. Effect of calcium on force-velocity characteristics of glycerinated skeletal muscle. Am J Physiol. 1971 Oct;221(4):973–979. doi: 10.1152/ajplegacy.1971.221.4.973. [DOI] [PubMed] [Google Scholar]


