Abstract
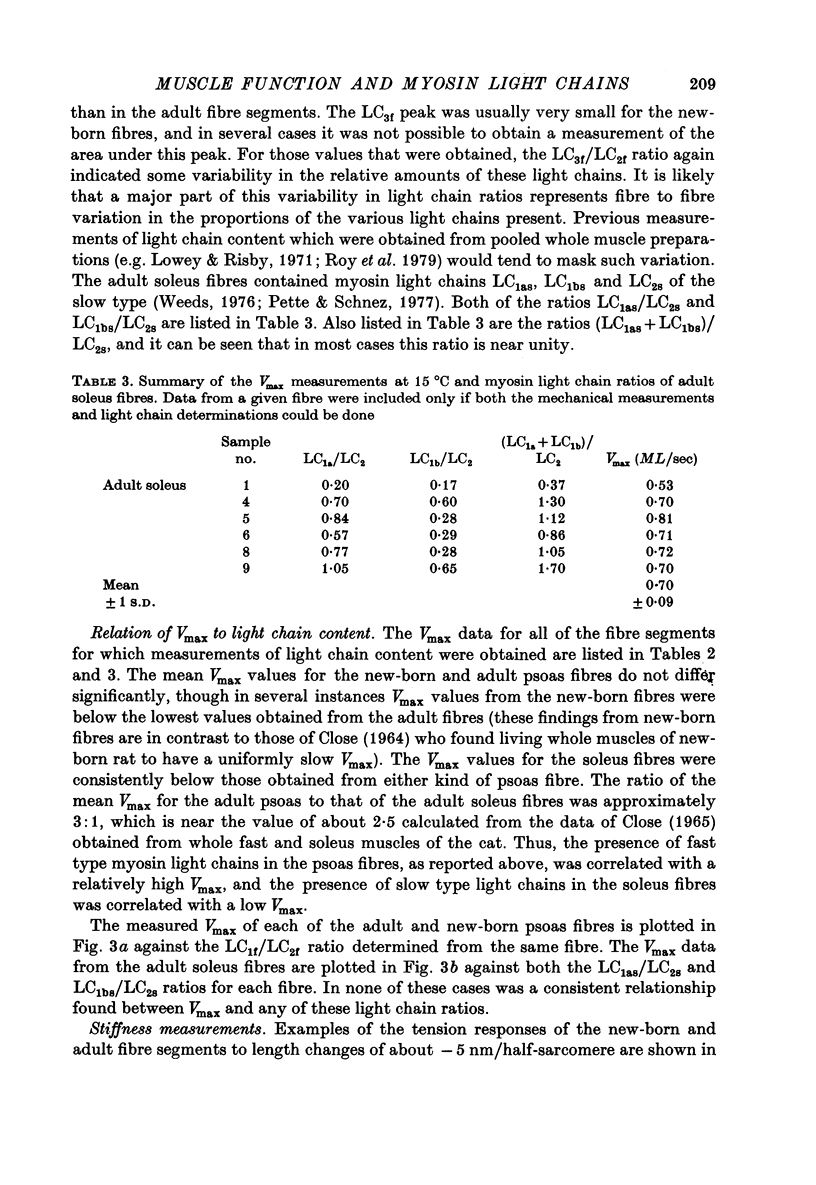
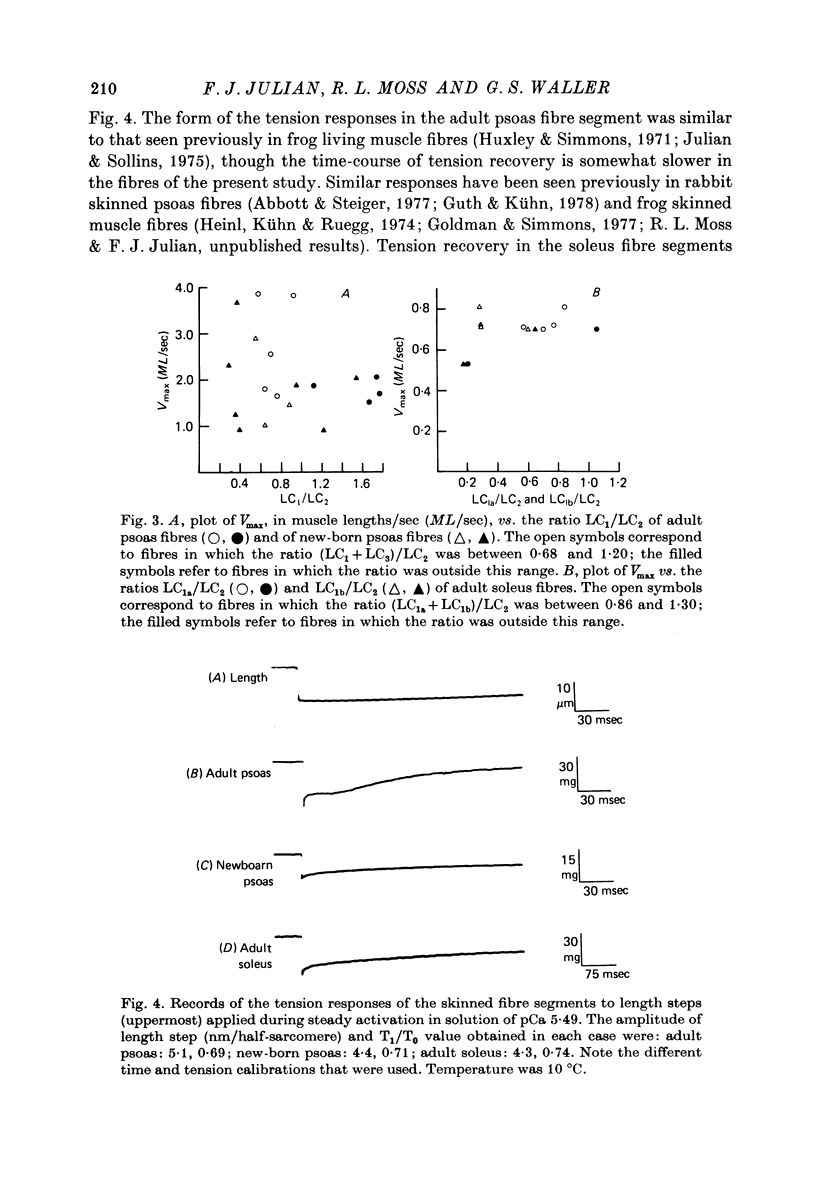
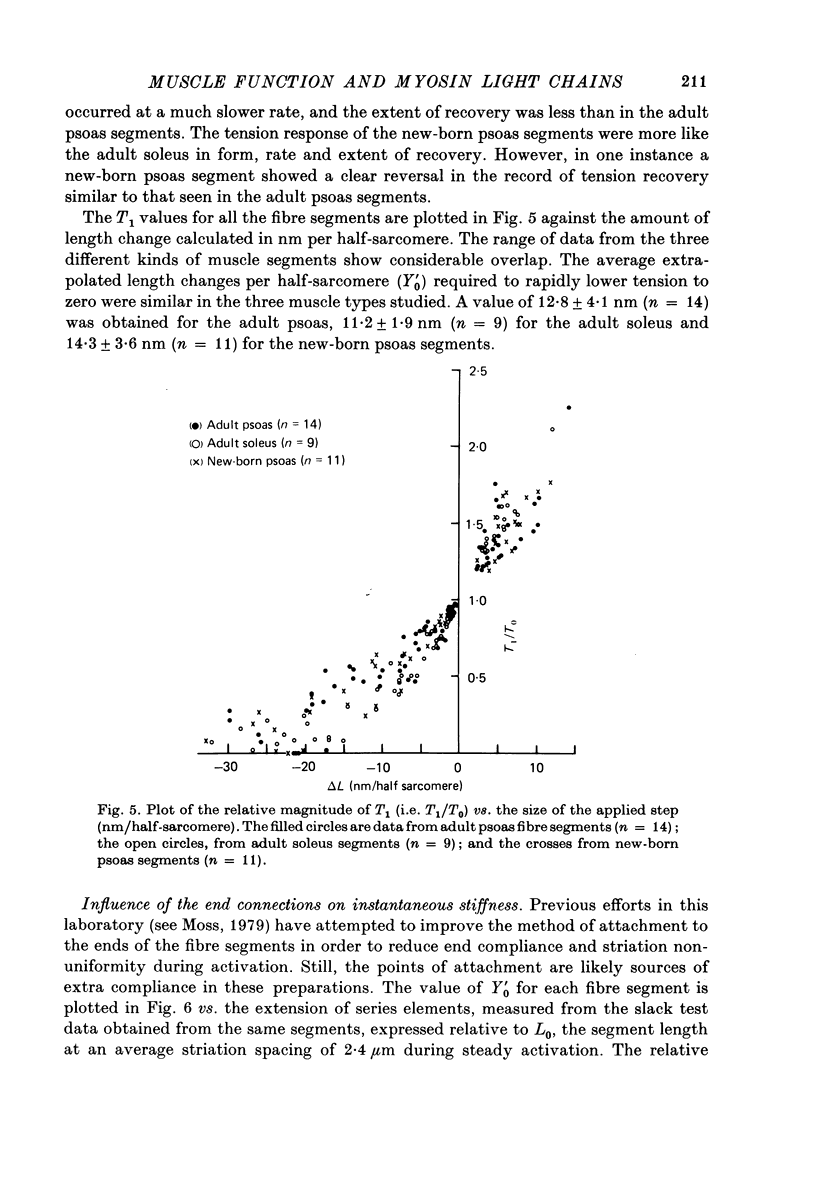
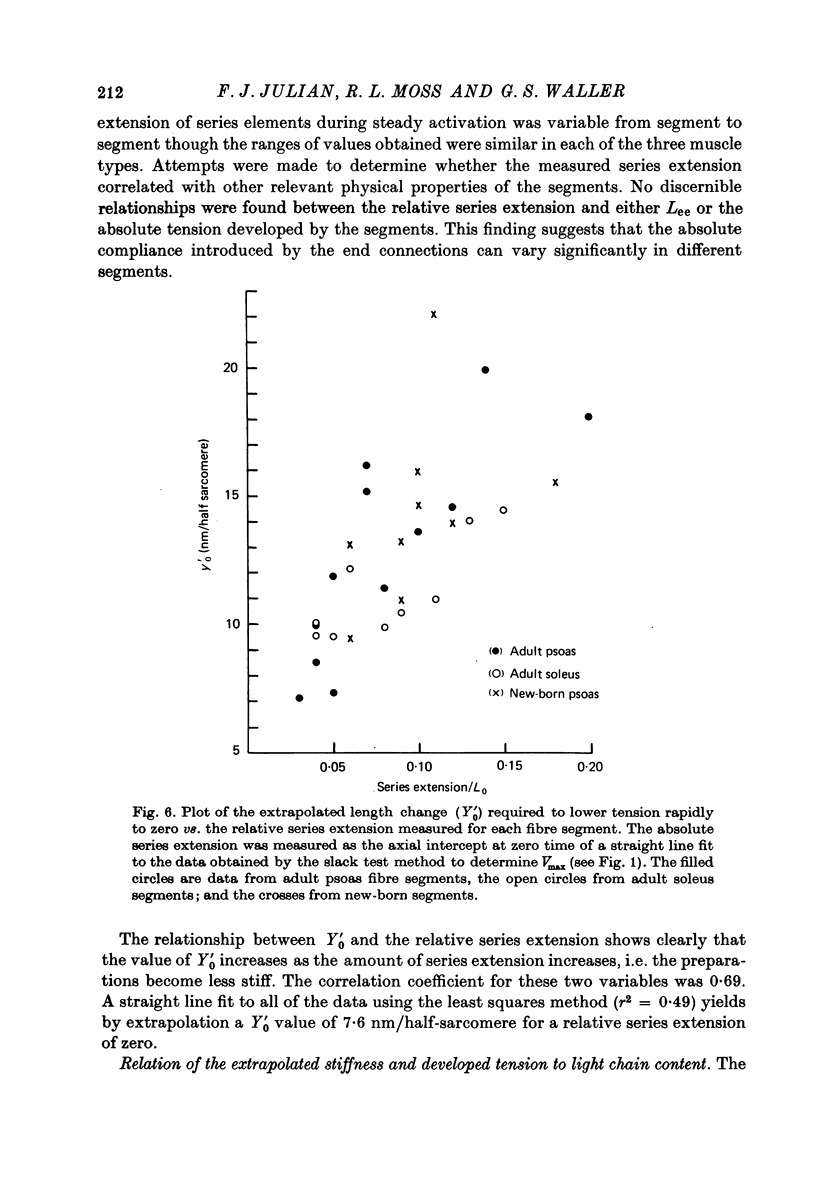
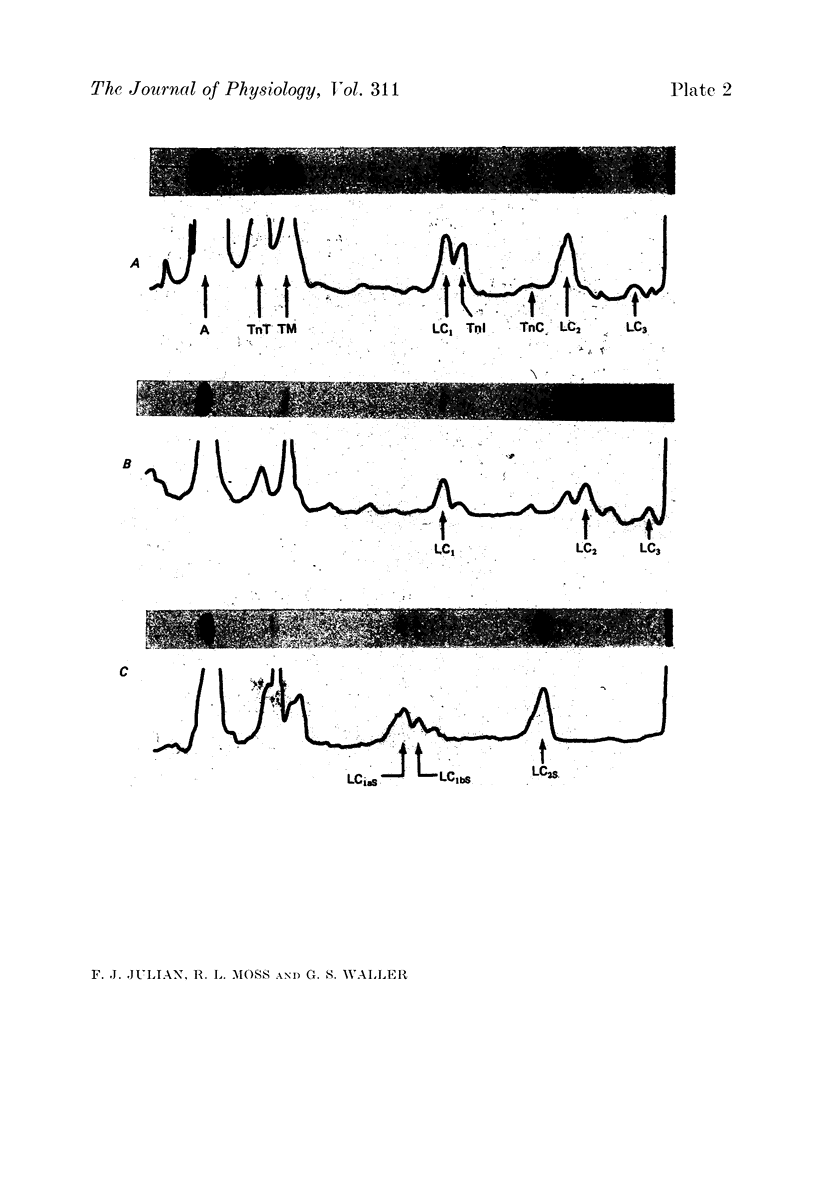
1. The maximum velocity of shortening, Vmax, and stiffness were measured in skinned single fibre segments from psoas and soleus muscles of adult rabbits and psoas muscles of new-born rabbits, and the myosin light chain composition was also determined in the same segments used in the mechanical studies. 2. Vmax was obtained at 15 degrees C during maximal activation at pCa 5.49 using a method involving measurement of the time required to take up various amounts of slack imposed on the segments. Stiffness was measured during activation at 10 degrees C by application of length steps complete in 0.6 msec. The myosin light chain composition of the segments was then determined by SDS-polyacrylamide gel electrophoresis. 3. Only fast type light chains were found to be present in the psoas fibre segments, though the relative amounts of myosin LC1f, LC2f and LC3f in these segments was somewhat variable. In most instances, the sum of the amounts of LC1f and LC3f present was equivalent to the amount of LC2f. Only slow type light chains were found in the soleus segments and the sum of the amounts of LC1as and LC1bs was about equal to the amount of LC2s. 4. The results indicate that there are no consistent relationships between Vmax, tension development or stiffness and LC1f/LC2f in the segments from adult and new-born psoas muscles, or between these mechanical parameters and LC1as/LC2s or LC1bs/LC2s in the adult soleus segments. However, the psoas segments, which had light chains of the fast type, had Vmax values that were consistently higher than those of the soleus segments, which had light chains of the slow type. 5. The stiffness values obtained in each of the three kinds of muscle were similar, suggesting that cross-bridge stiffness is similar in rabbit skeletal muscles of different type and age. Moreover, the results indicate that the amount of end compliance introduced by the connections to the fibre segments has a marked influence on the stiffness that is measured.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. H., Steiger G. J. Temperature and amplitude dependence of tension transients in glycerinated skeletal and insect fibrillar muscle. J Physiol. 1977 Mar;266(1):13–42. doi: 10.1113/jphysiol.1977.sp011754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Close R. The relation between intrinsic speed of shortening and duration of the active state of muscle. J Physiol. 1965 Oct;180(3):542–559. doi: 10.1113/jphysiol.1965.sp007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. C. Elasticity of soft tissues in simple elongation. Am J Physiol. 1967 Dec;213(6):1532–1544. doi: 10.1152/ajplegacy.1967.213.6.1532. [DOI] [PubMed] [Google Scholar]
- Gazith J., Himmelfarb S., Harrington W. F. Studies on the subunit structure of myosin. J Biol Chem. 1970 Jan 10;245(1):15–22. [PubMed] [Google Scholar]
- Gershman L. C., Stracher A., Dreizen P. Subunit structure of myosin. 3. A proposed model for rabbit skeletal myosin. J Biol Chem. 1969 May 25;244(10):2726–2736. [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Active and rigor muscle stiffness [proceedings]. J Physiol. 1977 Jul;269(1):55P–57P. [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J. Force-velocity characteristics for calcium-activated mammalian slow-twitch and fast-twitch skeletal fibers from the guinea pig. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4693–4697. doi: 10.1073/pnas.73.12.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güth K., Kuhn H. J. Stiffness and tension during and after sudden length changes of glycerinated rabbit psoas muscle fibres. Biophys Struct Mech. 1978 Jul 12;4(3):223–236. doi: 10.1007/BF02426087. [DOI] [PubMed] [Google Scholar]
- Heinl P., Kuhn H. J., Rüegg J. C. Tension responses to quick length changes of glycerinated skeletal muscle fibres from the frog and tortoise. J Physiol. 1974 Mar;237(2):243–258. doi: 10.1113/jphysiol.1974.sp010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R. Variation of muscle stiffness with force at increasing speeds of shortening. J Gen Physiol. 1975 Sep;66(3):287–302. doi: 10.1085/jgp.66.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloni-muller G., Ermini M., Jenny E. Myosin light chains of developing fast and slow rabbit skeletal muscle. FEBS Lett. 1976 Aug 1;67(1):68–74. doi: 10.1016/0014-5793(76)80872-1. [DOI] [PubMed] [Google Scholar]
- Pette D., Schnez U. Myosin light chain patterns of individual fast and slow-twitch fibres of rabbit muscles. Histochemistry. 1977 Oct 22;54(2):97–107. doi: 10.1007/BF00489668. [DOI] [PubMed] [Google Scholar]
- Pette D., Vrbová G., Whalen R. C. Independent development of contractile properties and myosin light chains in embryonic chick fast and slow muscle. Pflugers Arch. 1979 Jan 31;378(3):251–257. doi: 10.1007/BF00592743. [DOI] [PubMed] [Google Scholar]
- Pinset-Härström I., Whalen R. G. Effect of ageing of myosin on its ability to form synthetic filaments and on proteolysis of the LC2 light chain. J Mol Biol. 1979 Oct 15;134(1):189–197. doi: 10.1016/0022-2836(79)90420-0. [DOI] [PubMed] [Google Scholar]
- Roy R. K., Sreter F. A., Sarkar S. Changes in tropomyosin subunits and myosin light chains during development of chicken and rabbit striated muscles. Dev Biol. 1979 Mar;69(1):15–30. doi: 10.1016/0012-1606(79)90271-9. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Sreter F. A., Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971 May;68(5):946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreter F. A., Elzinga M., Mabuchi K. The Ntau-methylhistidine content of myosin in stimulated and cross-reinnervated skeletal muscles of the rabbit. FEBS Lett. 1975 Sep 1;57(1):107–111. doi: 10.1016/0014-5793(75)80163-3. [DOI] [PubMed] [Google Scholar]
- Sréter F. A., Bálint M., Gergely J. Structural and functional changes of myosin during development: comparison with adult fast, slow and cardiac myosin. Dev Biol. 1975 Oct;46(2):317–325. doi: 10.1016/0012-1606(75)90108-6. [DOI] [PubMed] [Google Scholar]
- Sréter F. A., Luff A. R., Gergely J. Effect of cross-reinnervation on physiological parameters and on properties of myosin and sarcoplasmic reticulum of fast and slow muscles of the rabbit. J Gen Physiol. 1975 Dec;66(6):811–821. doi: 10.1085/jgp.66.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrový I. Changes in light chains of myosin during animal development. Int J Biochem. 1979;10(3):223–227. doi: 10.1016/0020-711x(79)90038-7. [DOI] [PubMed] [Google Scholar]
- Thames M. D., Teichholz L. E., Podolsky R. J. Ionic strength and the contraction kinetics of skinned muscle fibers. J Gen Physiol. 1974 Apr;63(4):509–530. doi: 10.1085/jgp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski L., Botts J., Wang A., Woodward J., Highsmith S. Time-resolved fluorescence depolarization measurements of F-actin binding to myosin subfragments-1 bearing different alkali light chains. Arch Biochem Biophys. 1979 Dec;198(2):397–402. doi: 10.1016/0003-9861(79)90512-5. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Slater C. S., Pope B., Weeds A. G. Studies on the actin activation of myosin subfragment-1 isoezymes and the role of myosin light chains. Eur J Biochem. 1979 Sep;99(2):385–394. doi: 10.1111/j.1432-1033.1979.tb13267.x. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Weeds A. G. Studies on the role of myosin alkali light chains. Recombination and hybridization of light chains and heavy chains in subfragment-1 preparations. J Mol Biol. 1977 Jan 25;109(3):455–470. doi: 10.1016/s0022-2836(77)80023-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeds A. G., Burridge K. Myosin from cross-reinnervated cat muscles. Evidence for reciprocal transformation of heavy chains. FEBS Lett. 1975 Sep 15;57(2):203–208. doi: 10.1016/0014-5793(75)80717-4. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Hall R., Spurway N. C. Characterization of myosin light chains from histochemically identified fibres of rabbit psoas muscle. FEBS Lett. 1975 Jan 1;49(3):320–324. doi: 10.1016/0014-5793(75)80776-9. [DOI] [PubMed] [Google Scholar]
- Weeds A. G. Light chains from slow-twitch muscle myosin. Eur J Biochem. 1976 Jun 15;66(1):157–173. doi: 10.1111/j.1432-1033.1976.tb10436.x. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Lowey S. Substructure of the myosin molecule. II. The light chains of myosin. J Mol Biol. 1971 Nov 14;61(3):701–725. doi: 10.1016/0022-2836(71)90074-x. [DOI] [PubMed] [Google Scholar]
- Wood D. S., Zollman J., Reuben J. P., Brandt P. W. Human skeletal muscle: properties of the "chemically skinned%" fiber. Science. 1975 Mar 21;187(4181):1075–1076. doi: 10.1126/science.187.4181.1075. [DOI] [PubMed] [Google Scholar]