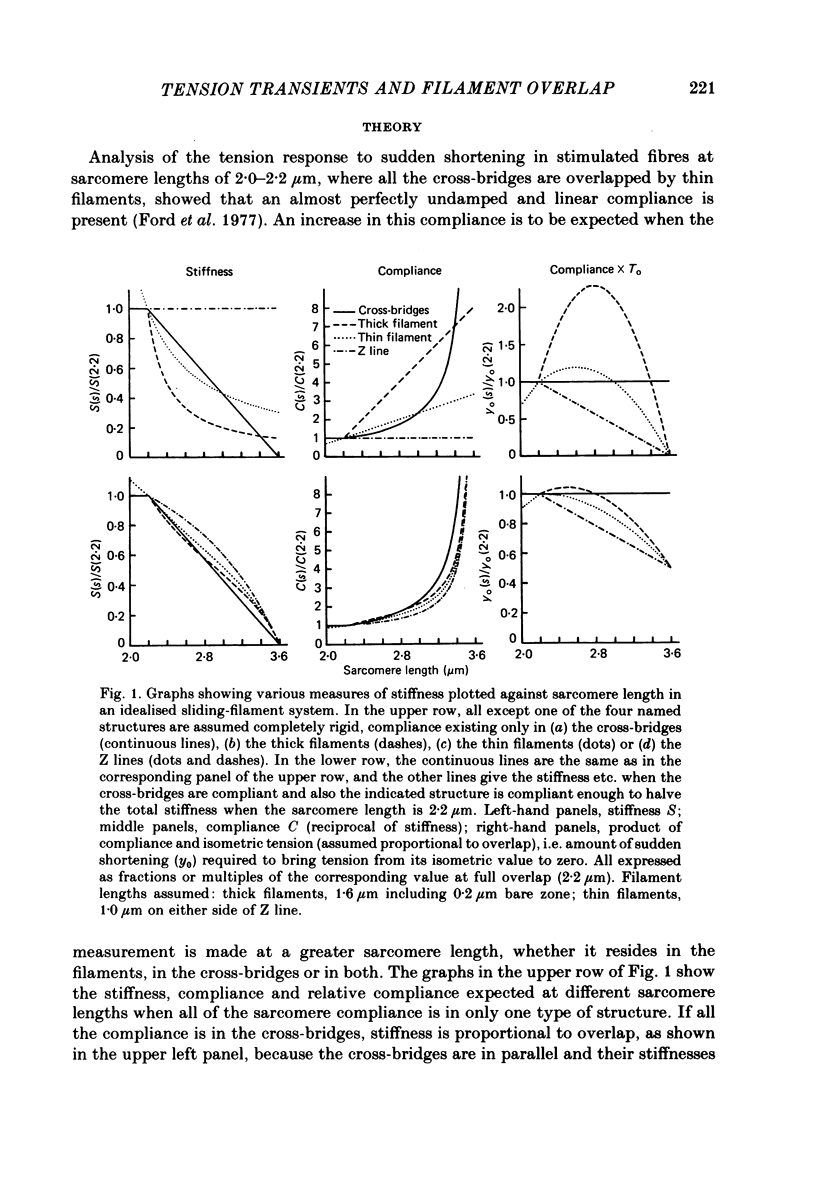
Abstract
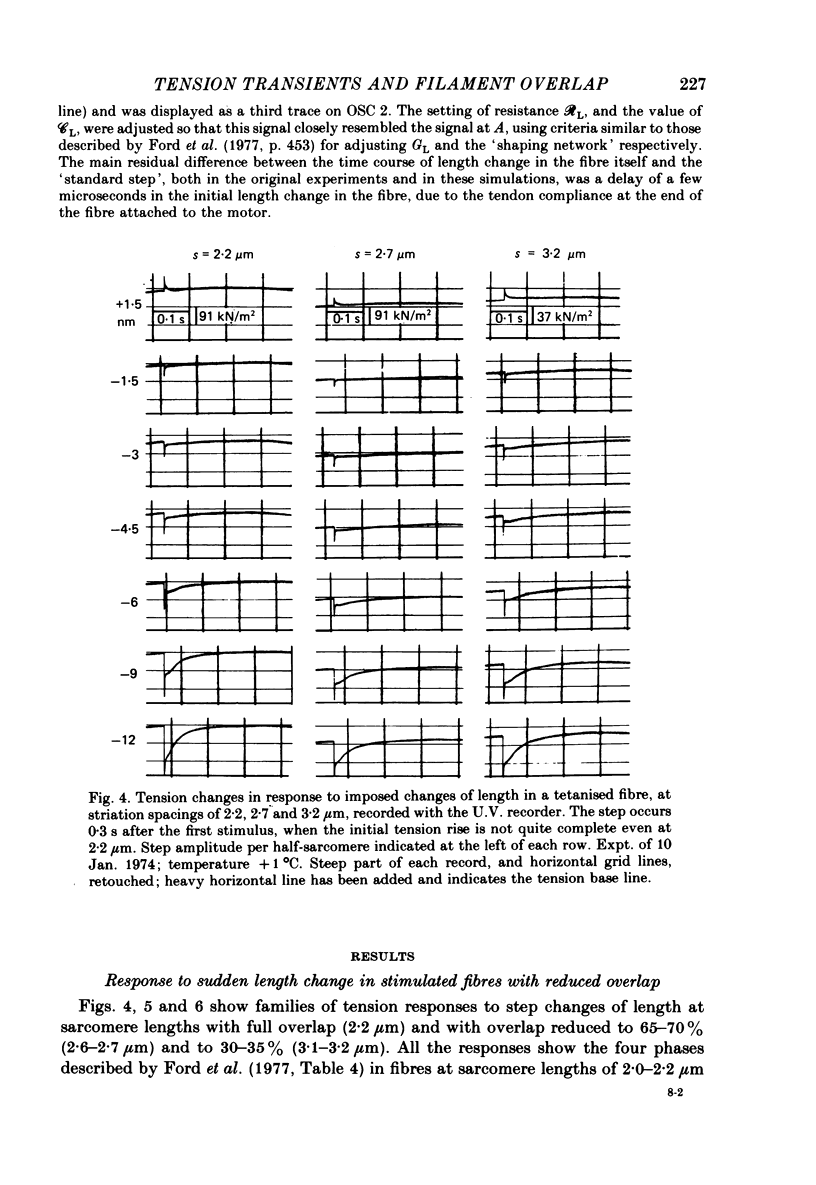
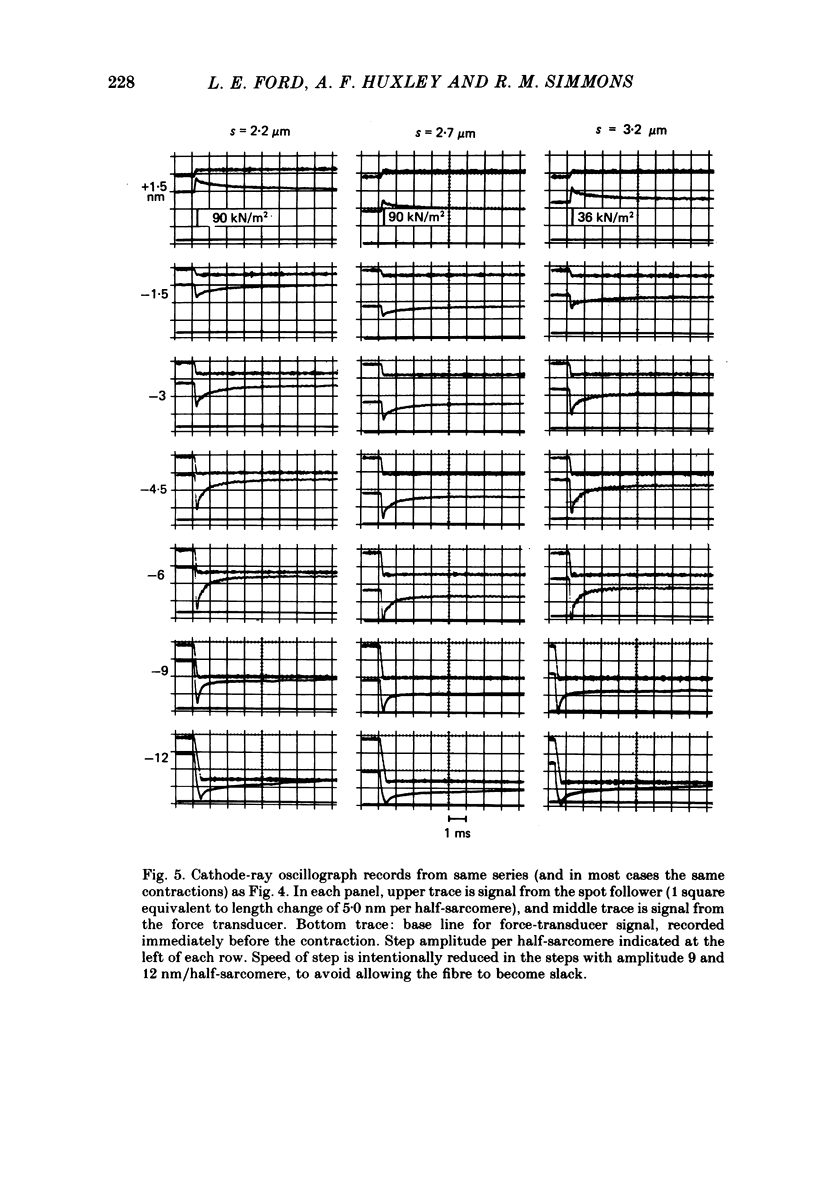
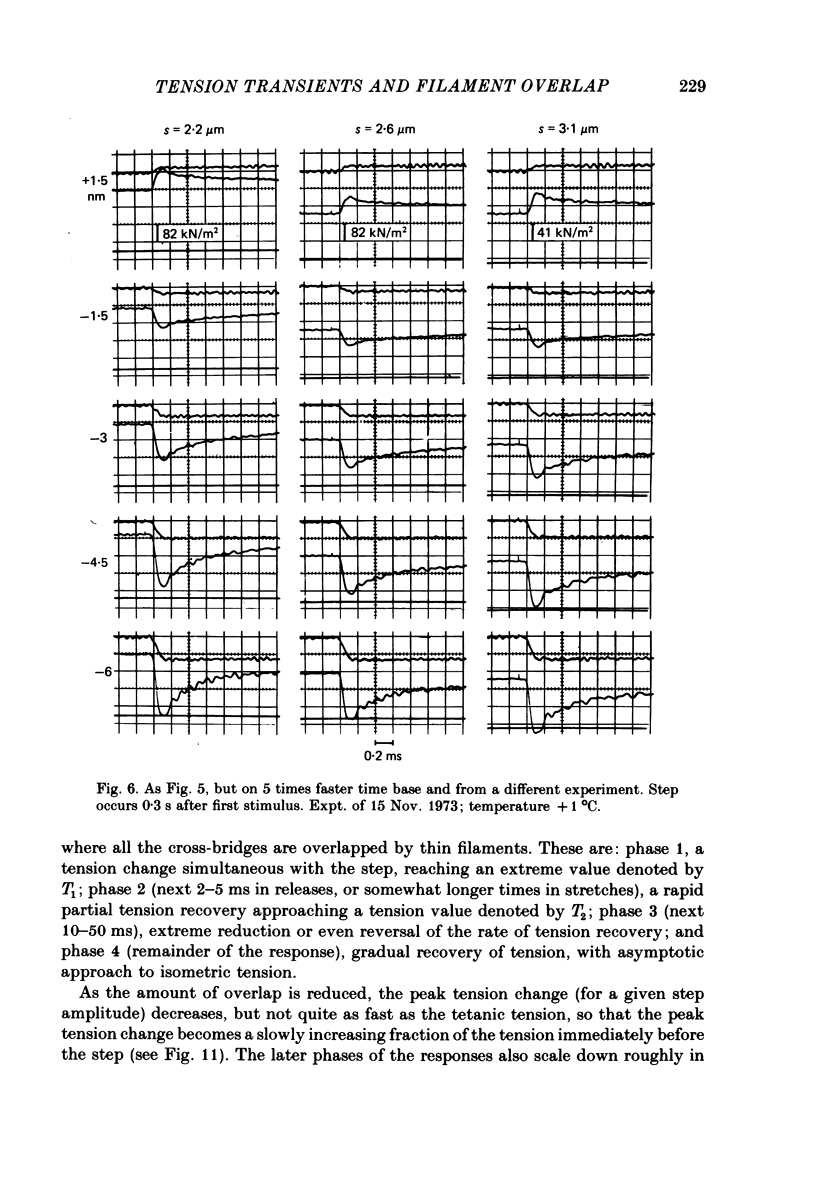
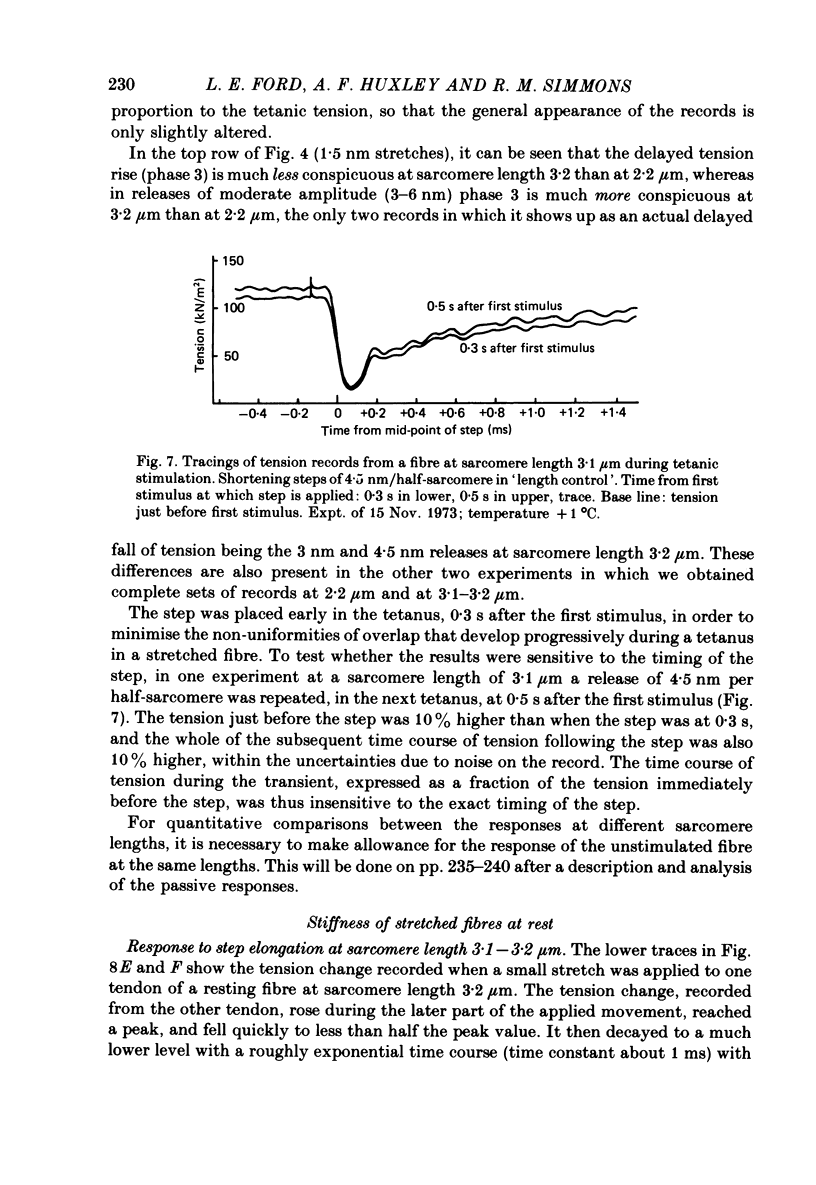
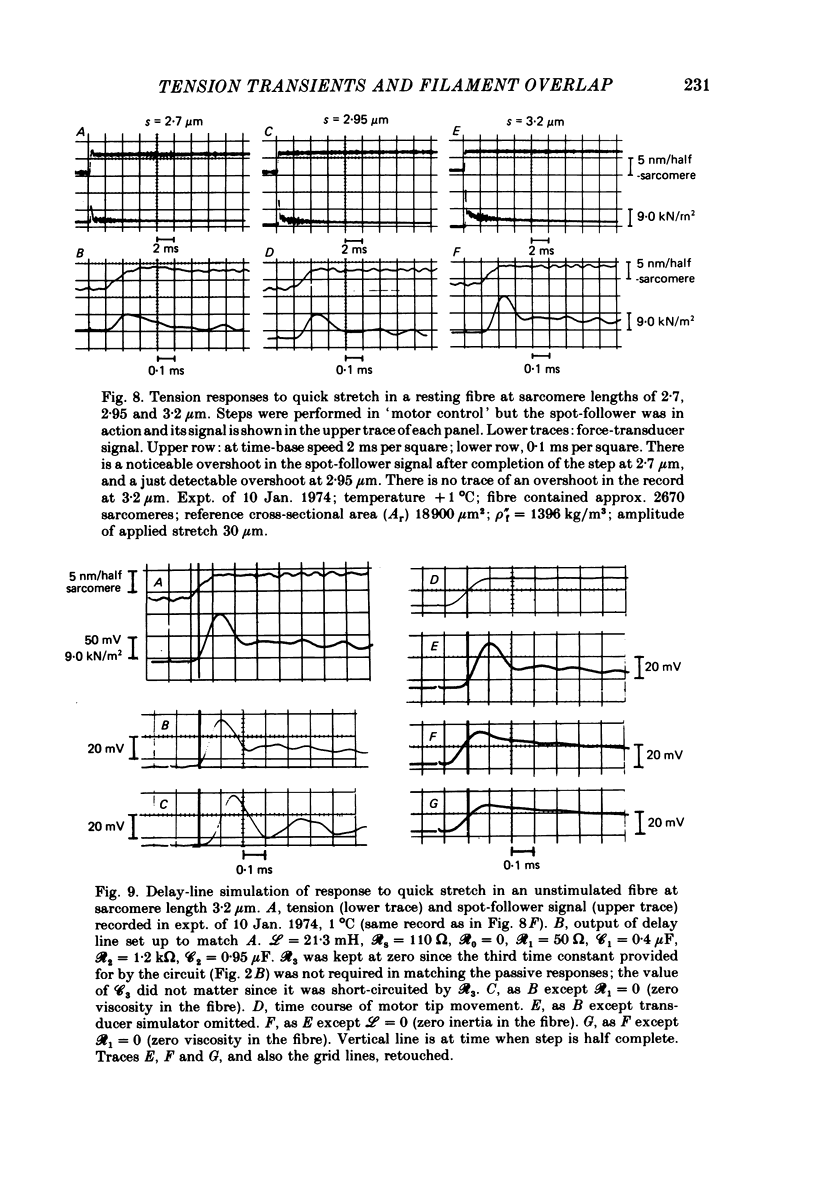
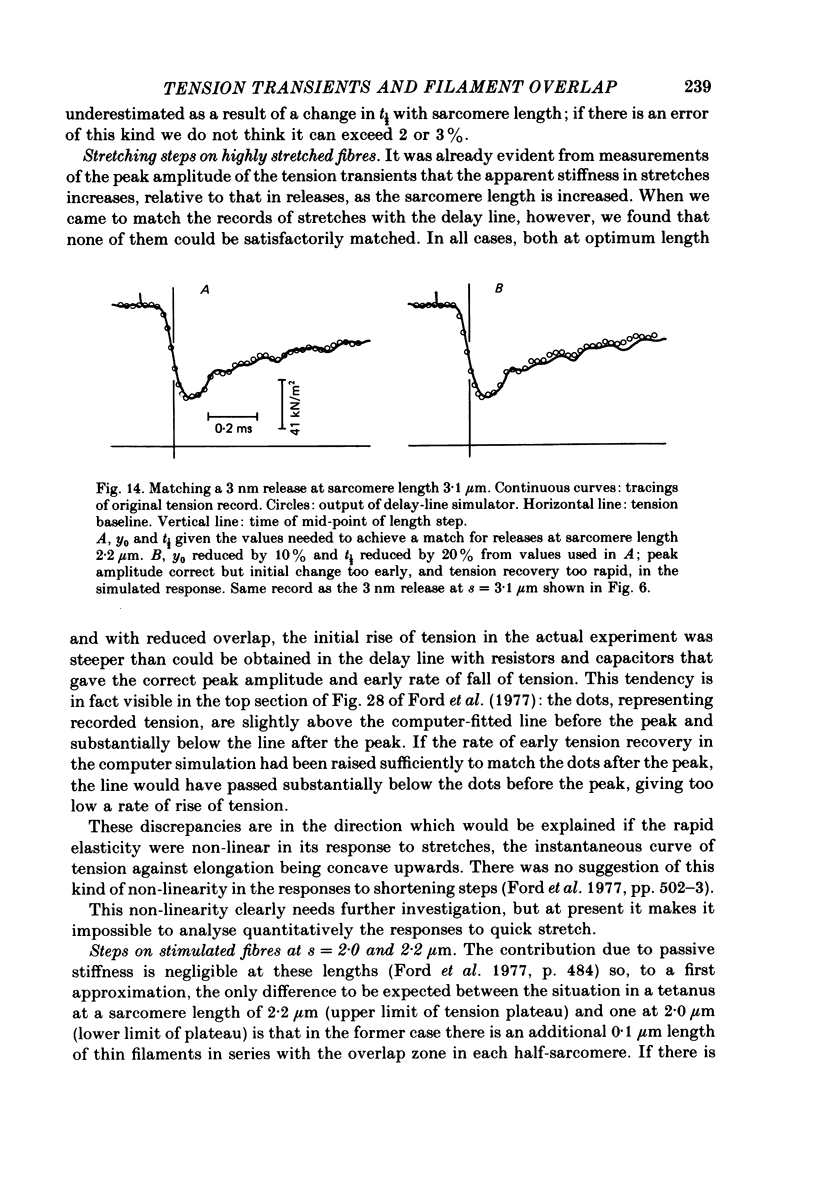
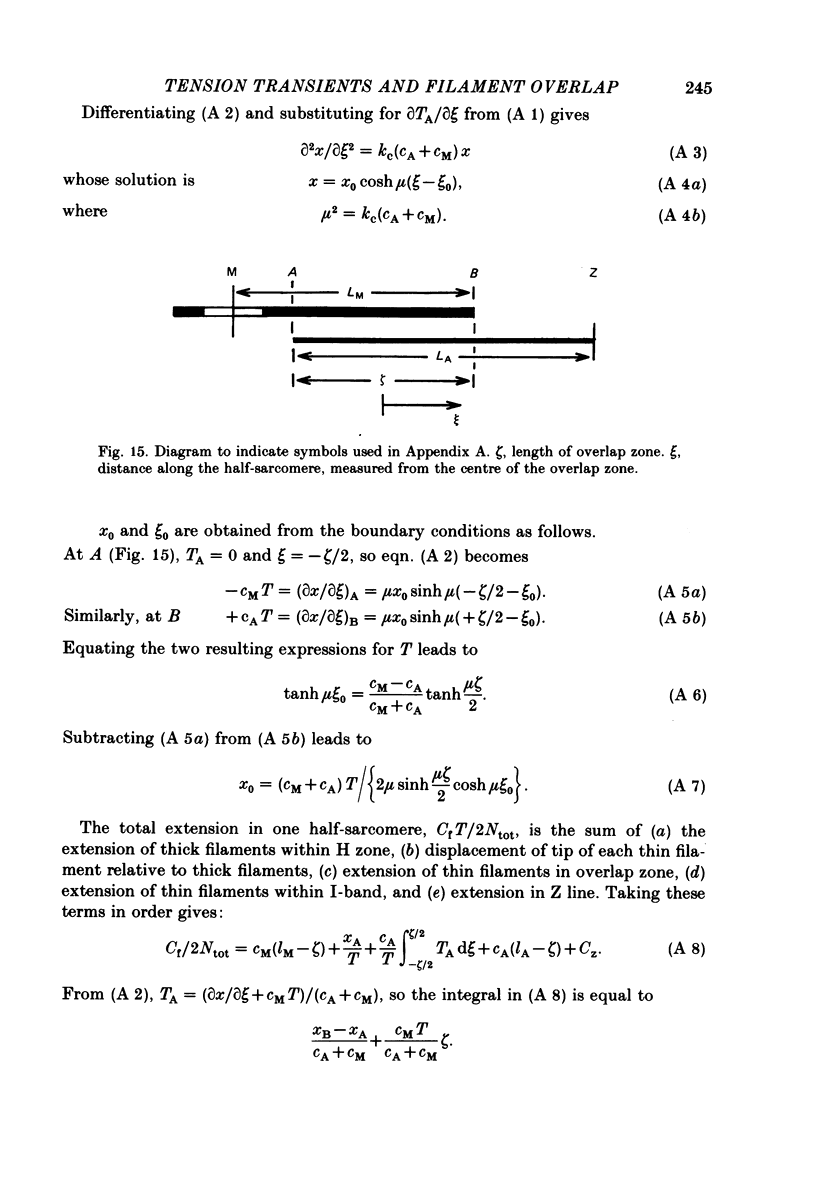
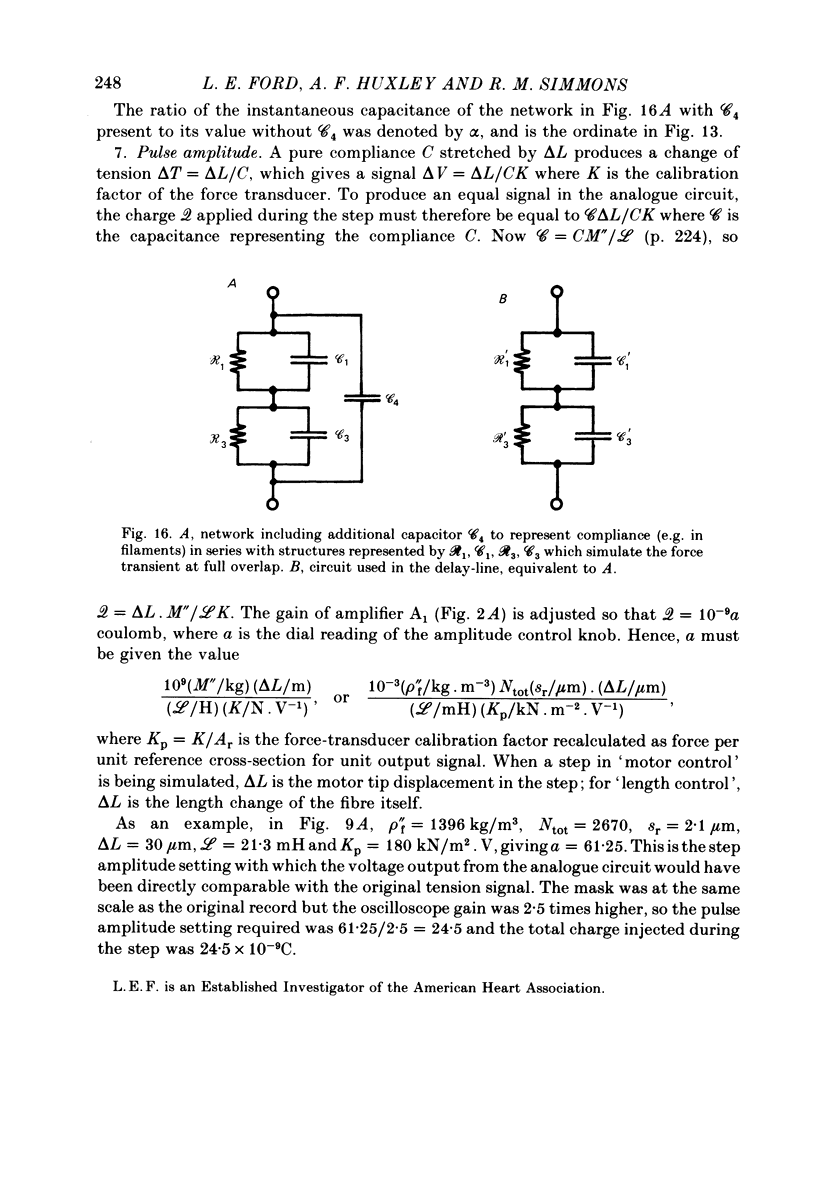
1. Tension transients were recorded at sarcomere lengths from 2.0 to 3.2 mum in isolated fibres from the tibialis anterior muscle of frogs during tetanic stimulation at 0-1 degrees C. 2. The length of a selected portion of the fibre was controlled by feed-back from a spot-follower device. The step was complete in 0.2 ms and the natural frequency of the force transducer was 10.8 kHz. 3. The transients were analysed by comparing the tension record with the output of an analogue circuit (delay line) which contained components representing (a) force transducer response, (b) fibre inertia, (c) viscosity and inertia of surrounding fluid, (d) passive stiffness and viscosity of the fibre, (e) tendon compliance and (f) stiffness and early tension recovery of the contractile apparatus. 4. In releases at different sarcomere lengths, the instantaneous stiffness and the early tension recovery attributed to the contractile apparatus varied almost exactly in proportion to the developed tension. In the later phases of the transient there were minor deviations from proportionality. 5. The results confirm that the entire transient represents events in the cross-bridges. 6. At full overlap, the compliance attributable to the cross-bridges is at least 80%, and probably well over 90% of the measured instantaneous compliance of the fibre. Stiffness can therefore be used as a measure of the number of attached cross-bridges. 7. The amount of instantaneous sliding movement of thick relative to thin filaments required to bring tension in a cross-bridge from the isometric value to zero is about 3.9 nm if filament and Z-line compliance are negligible, as suggested by the results. It is not however excluded that filament compliance, though small, may be sufficient to reduce this figure to 3.5 nm or possibly 3.1 nm. 8. The responses to quick stretch, unlike those to release, could not be satisfactorily matched with the delay line. The deviations suggest that the instantaneous elasticity is non-linear in stretches. 9. In resting fibres at all sarcomere lengths, the first peak of the tension response was determined chiefly by fibre inertia and viscosity, rather than elasticity.
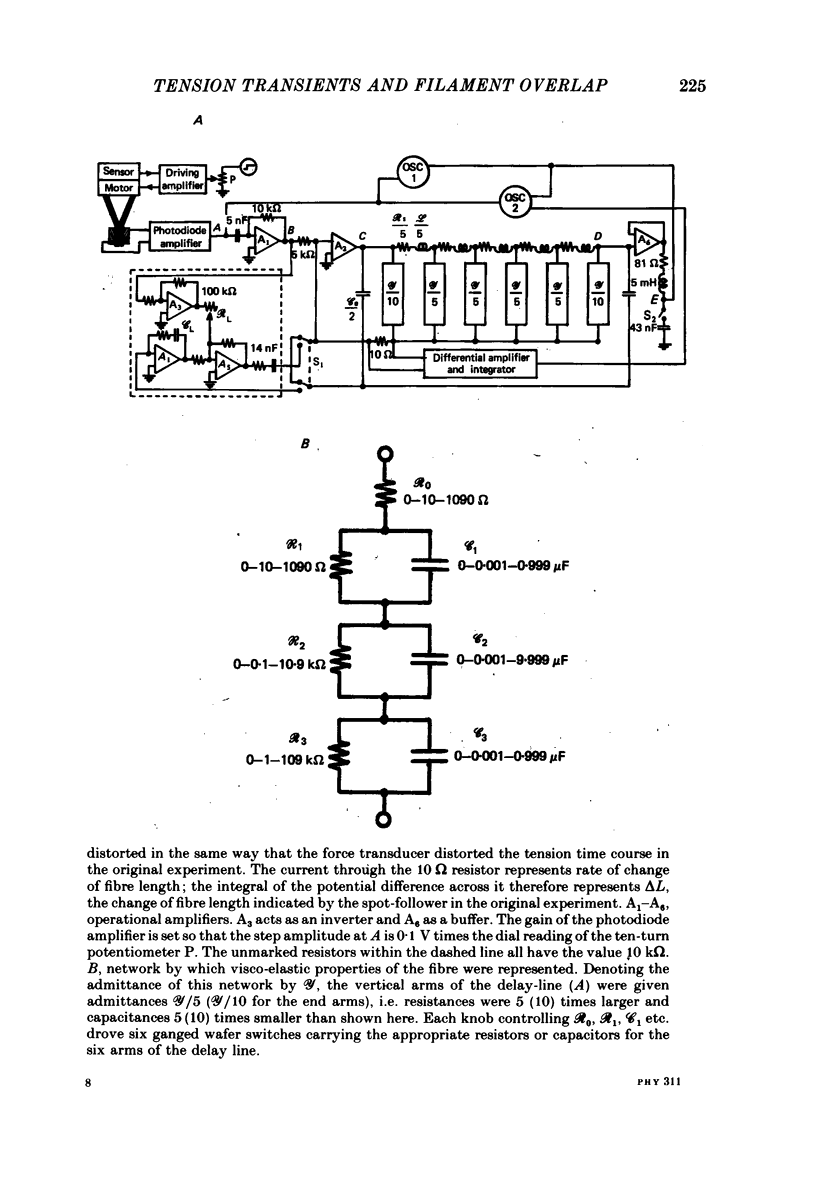
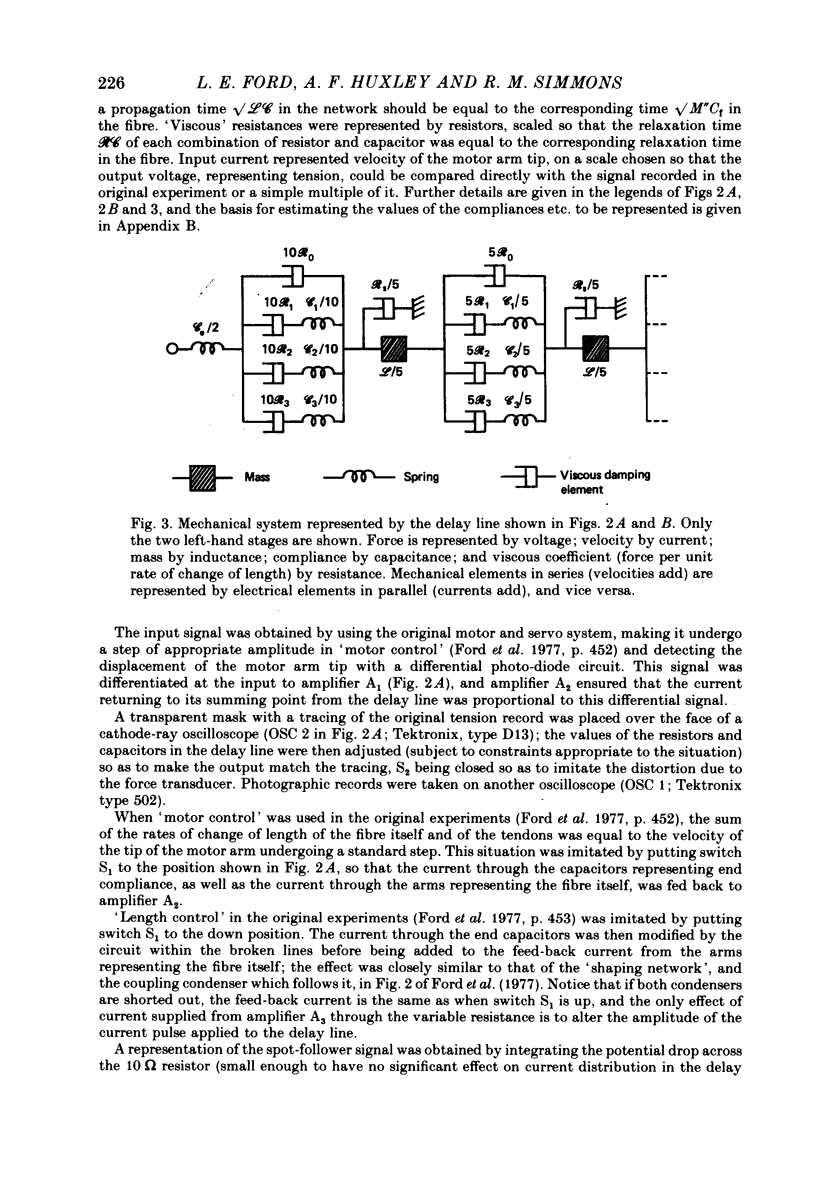
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Active and rigor muscle stiffness [proceedings]. J Physiol. 1977 Jul;269(1):55P–57P. [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley R., James D., Miller A., White J. W. Phonons and the elastic moduli of collagen and muscle. Nature. 1977 May 19;267(5608):285–287. doi: 10.1038/267285a0. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975 Feb 15;92(1):113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Chen Y. D., Podolsky R. J. Some self-consistent two-state sliding filament models of muscle contraction. Biophys J. 1975 Apr;15(4):335–372. doi: 10.1016/S0006-3495(75)85823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. A quick phase in the series-elastic component of striated muscle, demonstrated in isolated fibres from the frog. J Physiol. 1970 Jun;208(2):52P–53P. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Elliott G. F. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- Thorson J., White D. C. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J. 1969 Mar;9(3):360–390. doi: 10.1016/S0006-3495(69)86392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S., Umazume Y., Natori R., Fujime S., Chiba S. Optical diffraction study of muscle fibers. II. Electro-optical properties of muscle fibers. Biophys Chem. 1978 Sep;8(4):317–326. doi: 10.1016/0301-4622(78)80014-3. [DOI] [PubMed] [Google Scholar]


