Abstract
1. Rats injected with purified acetylcholine receptors (AChR) extracted from electric organs of Torpedo marmorata showed clinical symptoms consistent with the development of experimental myasthenia gravis.
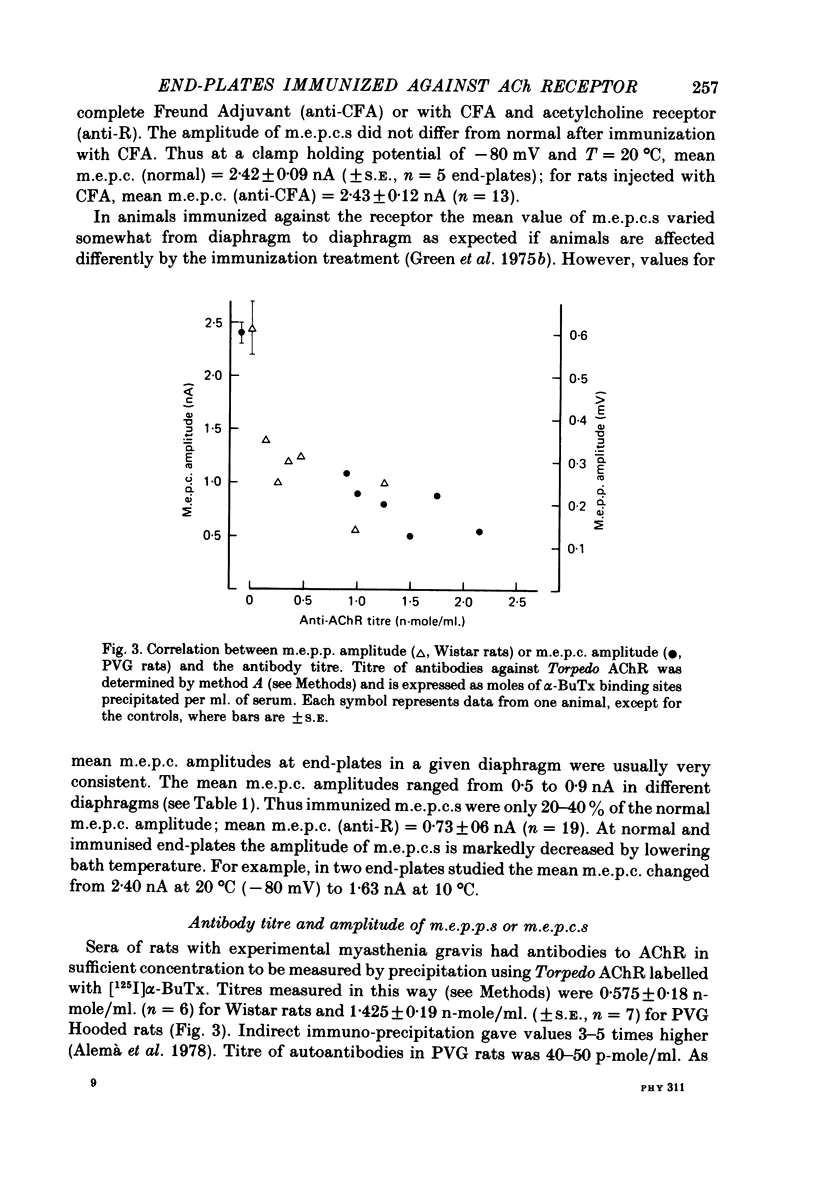
2. Sera of rats with this disease contain high levels of anti-AChR antibodies. However, no simple correlation was found between antibody titre and miniature end-plate current (m.e.p.c.) amplitude.
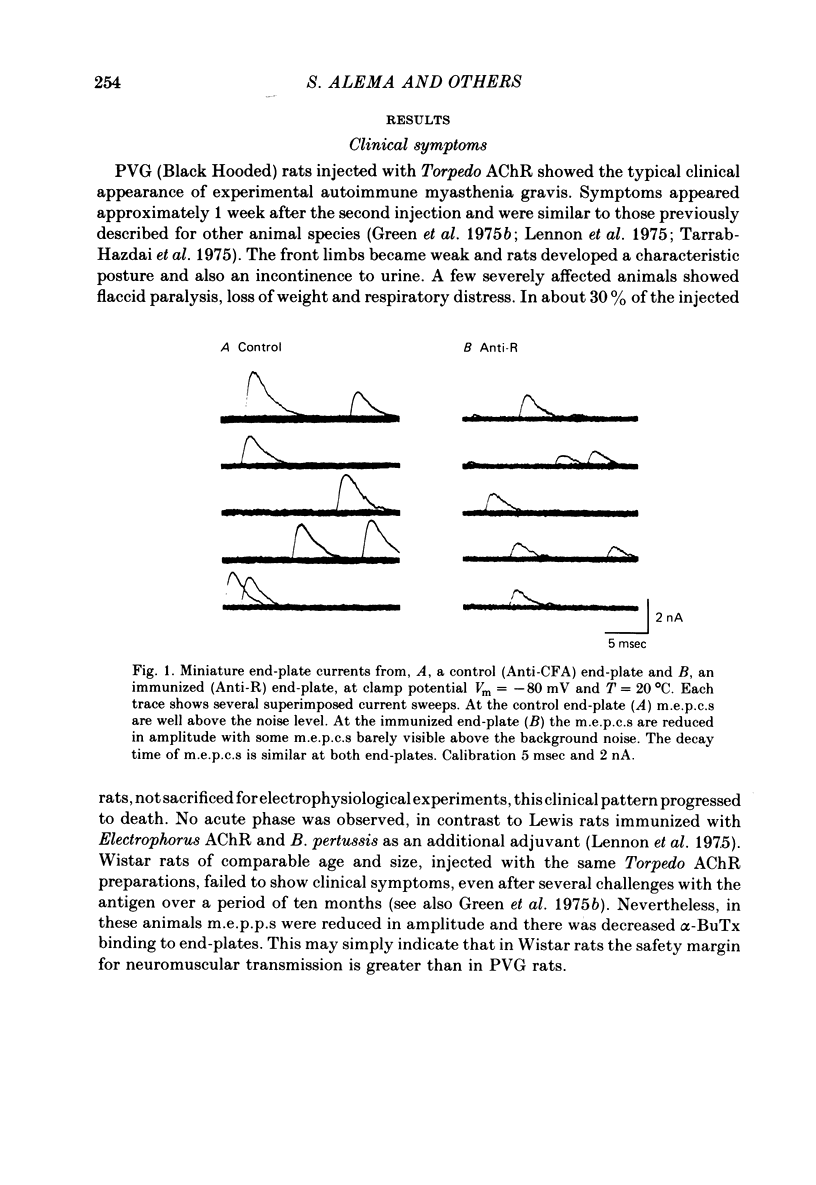
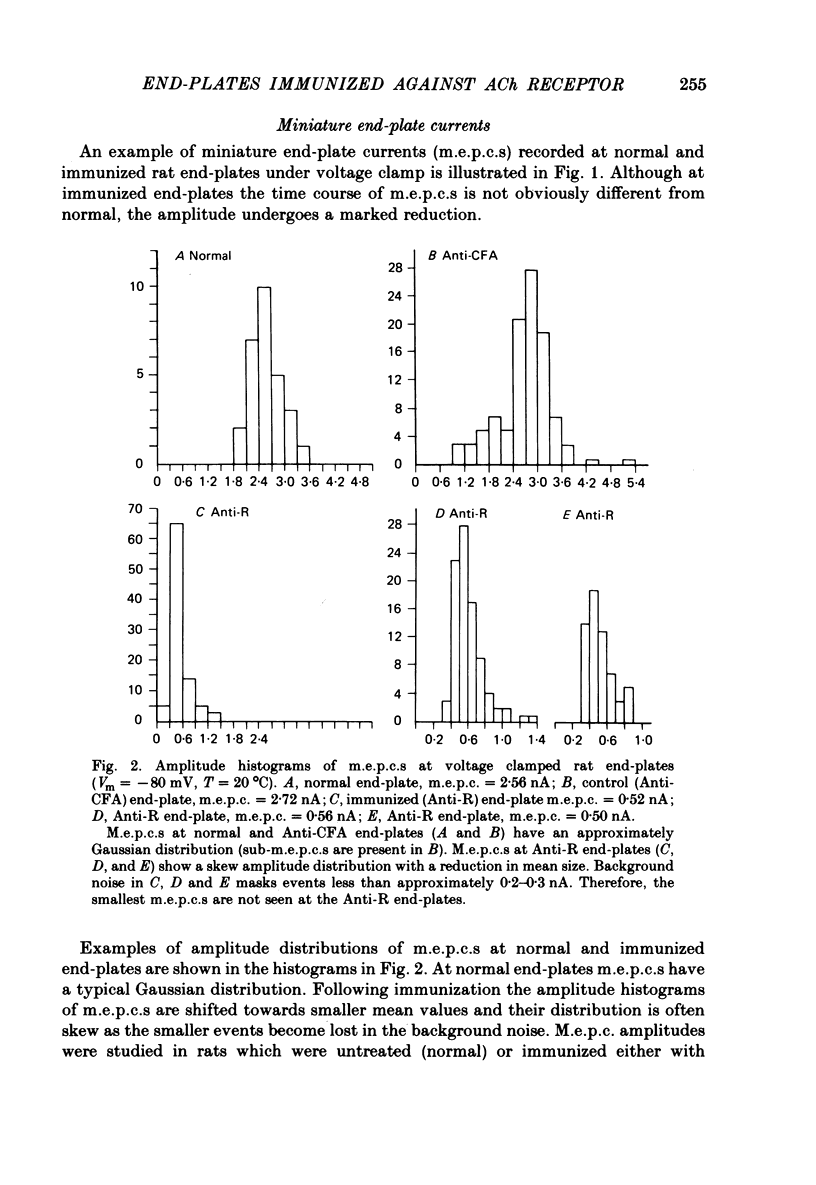
3. M.e.p.c.s. at the end-plates of rats injected with AChR (Anti-R), emulsified in complete Freund Adjuvant (CFA), were reduced to about one third the size of controls taken from rats injected only with CFA (Anti-CFA). Mean m.e.p.c. (Anti-R) = 0.73 ± 0.06 nA; mean m.e.p.c. (Anti-CFA) = 2.43 ± 0.12 nA (Vm = -80 mV, T = 20 °C).
4. The m.e.p.c. decay time constant, τm.e.p.c., is similar at immunized and control rat end-plates. τm.e.p.c. (Anti-R) = 1.32 ± 0.06 msec; τm.e.p.c. (Anti-CFA) = 1.31 ± 0.06 msec (Vm = -80 mV, T = 20 °C).
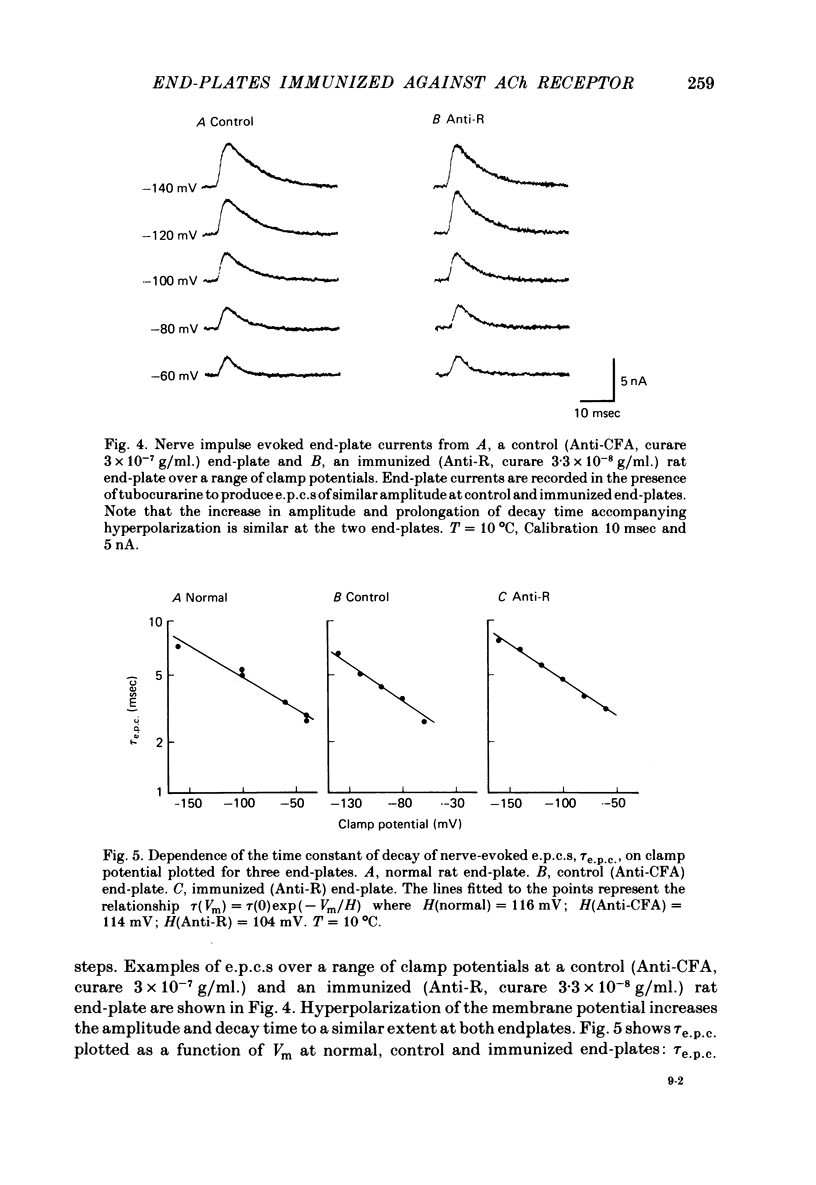
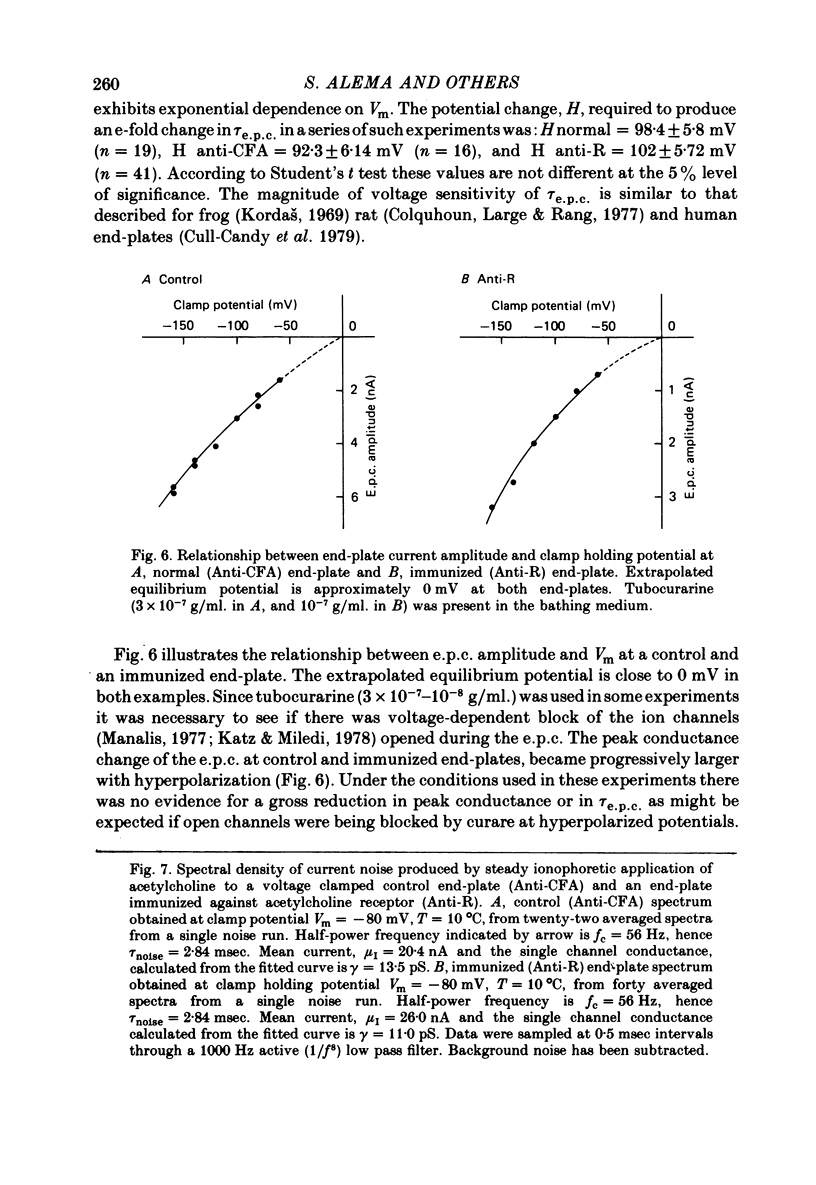
5. The end-plate current decay time constant, τe.p.c., is similar at immunized and control end-plates and in both cases depends exponentially on membrane potential. The change in membrane potential required to produce an e-fold change in τe.p.c. is 102.0 ± 5.72 mV at immunized (Anti-R) end-plates and 92.3 ± 6.14 mV at control (Anti-CFA) end-plates at T = 10 °C.
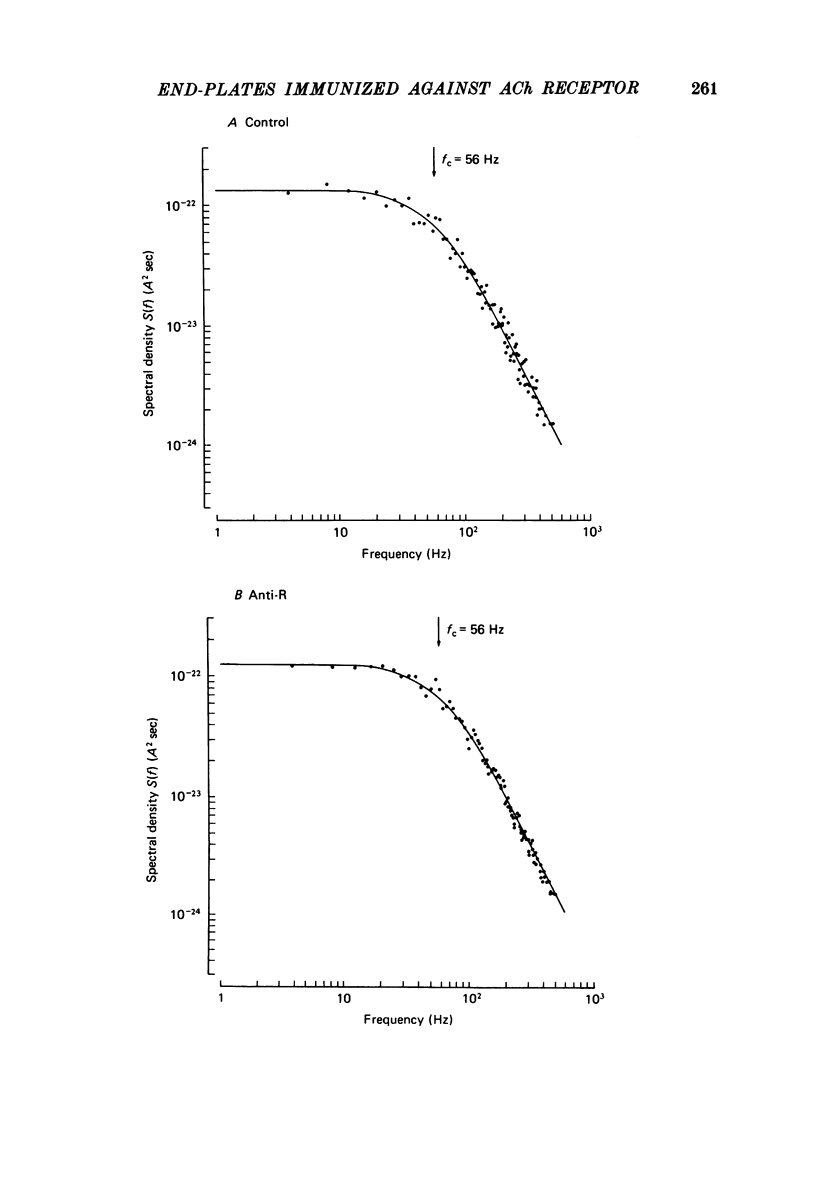
6. Acetylcholine noise was examined at immunized and control rat end-plates at 10 °C. Analysis of noise indicates that the single channel conductance, γ, and mean channel life-time, τnoise, are essentially unchanged by immunization against AChR. γ (Anti-R) = 13.15 ± 0.53 pS; γ (Anti-CFA) = 12.50 ± 0.50 pS; τnoise (Anti-R) = 2.9 ± 0.18 msec; τnoise (Anti-CFA) = 2.68 ± 0.14 msec (Vm = -80 mV, T = 10 °C).
7. Mean quantal content and Ca2+ dependence of the end-plate potential are unchanged at immunized end-plates.
8. It is concluded that at immunized end-plates the number of activated receptor-channel complexes is reduced without modification of single channel properties. In this respect the immunized rat end-plate is a good model for myasthenia gravis affected human end-plates.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti F., Clementi F., Conti-Tronconi B., Folco G. C. A cholinoceptor antiserum: its pharmacological properties. Br J Pharmacol. 1976 May;57(1):17–22. doi: 10.1111/j.1476-5381.1976.tb07651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Heinemann S., Lennon V. A., Lindstrom J. Reduced muscle acetylcholine sensitivity in rats immunised with acetylcholine receptor. Nature. 1976 Apr 1;260(5550):438–439. doi: 10.1038/260438a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D., Reich E. Neurotoxin from venom of the cobra, Naja naja siamensis. Purification and radioactive labeling. J Biol Chem. 1972 May 25;247(10):3008–3013. [PubMed] [Google Scholar]
- Cull-Candy S. G., Lundh H., Thesleff S. Effects of botulinum toxin on neuromuscular transmission in the rat. J Physiol. 1976 Aug;260(1):177–203. doi: 10.1113/jphysiol.1976.sp011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A. Acetylcholine-induced channels and transmitter release at human endplates. Nature. 1978 Jan 5;271(5640):74–75. doi: 10.1038/271074a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A. End-plate currents and acetylcholine noise at normal and myasthenic human end-plates. J Physiol. 1979 Feb;287:247–265. doi: 10.1113/jphysiol.1979.sp012657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A., Uchitel O. D. On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiol. 1980 Feb;299:621–638. doi: 10.1113/jphysiol.1980.sp013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Müller K. D., Peper K., Sterz R. The M. omohyoideus of the mouse as a convenient mammalian muscle preparation. A study of junctional and extrajunctional acetylcholine receptors by noise analysis and cooperativity. Pflugers Arch. 1976 Dec 28;367(2):115–122. doi: 10.1007/BF00585146. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E. Experimental autoimmune myasthenia gravis: the rabbit as an animal model. Fed Proc. 1978 Dec;37(14):2823–2827. [PubMed] [Google Scholar]
- Fambrough D. M., Drachman D. B., Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973 Oct 19;182(4109):293–295. doi: 10.1126/science.182.4109.293. [DOI] [PubMed] [Google Scholar]
- Granato D. A., Fulpius B. W., Moody J. F. Experimental myasthenia in Balb/c mice immunized with rat acetylcholine receptor from rat denervated muscle. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2872–2876. doi: 10.1073/pnas.73.8.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. P., Miledi R., Perez de la Mora M., Vincent A. Acetylcholine receptors. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):551–559. doi: 10.1098/rstb.1975.0031. [DOI] [PubMed] [Google Scholar]
- Green D. P., Miledi R., Vincent A. Neuromuscular transmission after immunization against acetylcholine receptors. Proc R Soc Lond B Biol Sci. 1975 Apr 29;189(1094):57–68. doi: 10.1098/rspb.1975.0041. [DOI] [PubMed] [Google Scholar]
- Heinemann S., Bevan S., Kullberg R., Lindstrom J., Rice J. Modulation of acetylcholine receptor by antibody against the receptor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3090–3094. doi: 10.1073/pnas.74.7.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Miledi R., Vincent A., Newsom-Davis J. Acetylcholine receptors and end-plate electrophysiology in myasthenia gravis. Brain. 1978 Jun;101(2):345–368. doi: 10.1093/brain/101.2.345. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert E. H., Lindstrom J. M., Lennon V. A. End-plate potentials in experimental autoimmune myasthenia gravis in rats. Ann N Y Acad Sci. 1976;274:300–318. doi: 10.1111/j.1749-6632.1976.tb47694.x. [DOI] [PubMed] [Google Scholar]
- Lennon V. A., Lindstrom J. M., Seybold M. E. Experimental autoimmune myasthenia: A model of myasthenia gravis in rats and guinea pigs. J Exp Med. 1975 Jun 1;141(6):1365–1375. doi: 10.1084/jem.141.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J., Einarson B., Merlie J. Immunization of rats with polypeptide chains from torpedo acetylcholine receptor causes an autoimmune response to receptors in rat muscle. Proc Natl Acad Sci U S A. 1978 Feb;75(2):769–773. doi: 10.1073/pnas.75.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. Immunological studies of acetylcholine receptors. J Supramol Struct. 1976;4(3):389–403. doi: 10.1002/jss.400040310. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebs D., Narita K., Iwanaga S., Samejima Y., Lee C. Y. Purification, properties and amino acid sequence of -bungarotoxin from the venom of Bungarus multicinctus. Hoppe Seylers Z Physiol Chem. 1972 Feb;353(2):243–262. doi: 10.1515/bchm2.1972.353.1.243. [DOI] [PubMed] [Google Scholar]
- Patrick J., Lindstrom J., Culp B., McMillan J. Studies on purified eel acetylcholine receptor and anti-acetylcholine receptor antibody. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3334–3338. doi: 10.1073/pnas.70.12.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiness C. G., Weinberg C. B., Hall Z. W. Antibody to acetylcholine receptor increases degradation of junctional and extrajunctional receptors in adult muscle. Nature. 1978 Jul 6;274(5666):68–70. doi: 10.1038/274068a0. [DOI] [PubMed] [Google Scholar]
- Sanders D. B., Schleifer L. S., Eldefrawi M. E., Norcross N. L., Cobb E. E. An immunologically induced defect of neuromuscular transmission in rats and rabbits. Ann N Y Acad Sci. 1976;274:319–336. doi: 10.1111/j.1749-6632.1976.tb47695.x. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Benda P., Meunier J. C., Changeux J. P. Immunological characterisation of the cholinergic receptor protein from Electrophorus electricus. FEBS Lett. 1973 Sep 1;35(1):124–128. doi: 10.1016/0014-5793(73)80592-7. [DOI] [PubMed] [Google Scholar]
- Tarrab-Hazdai R., Aharonov A., Silman I., Fuchs S., Abramsky O. Experimental autoimmune myasthenia induced in monkeys by purified acetylcholine receptor. Nature. 1975 Jul 10;256(5513):128–130. doi: 10.1038/256128a0. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurn A. D., Fulpius B. W. Study of two different subpopulations of anti-acetylcholine receptor antibodies in a rabbit with experimental auto-immune myasthenia gravis. Eur J Immunol. 1977 Aug;7(8):529–532. doi: 10.1002/eji.1830070807. [DOI] [PubMed] [Google Scholar]


