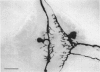
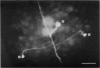
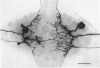
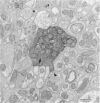
Abstract
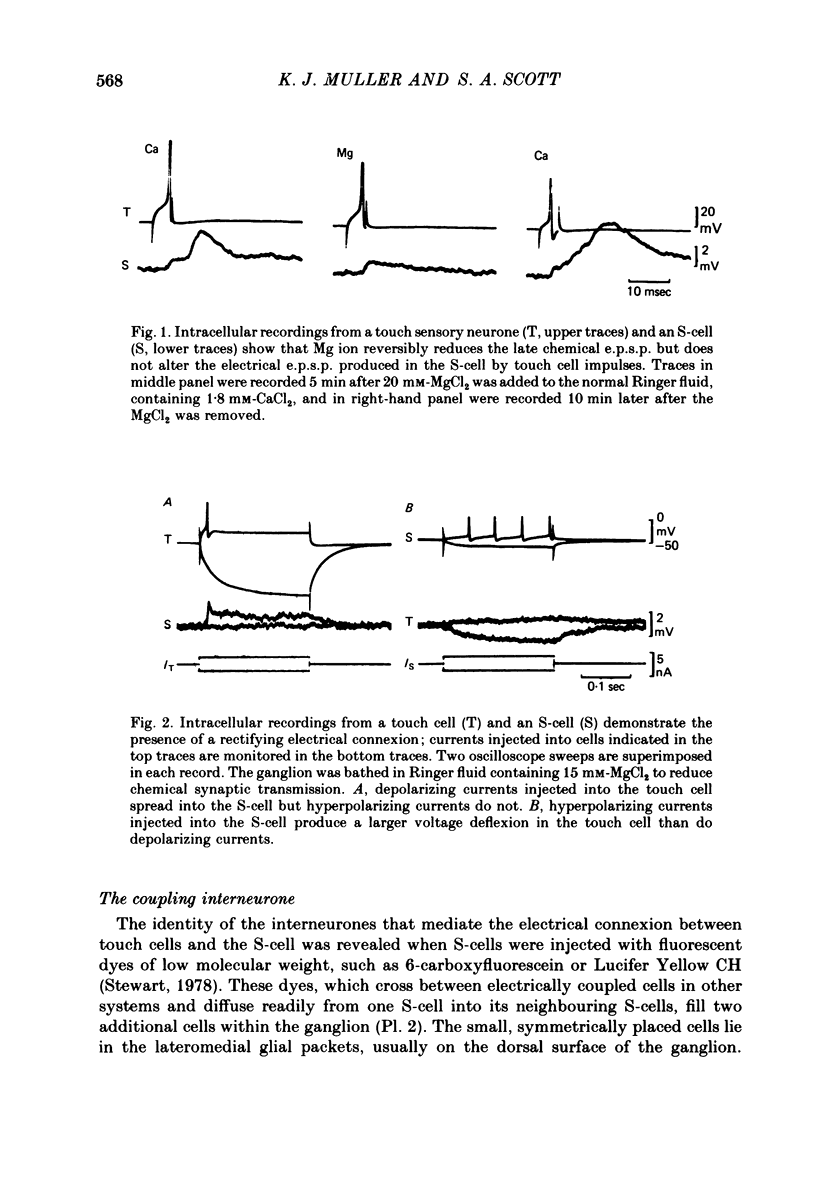
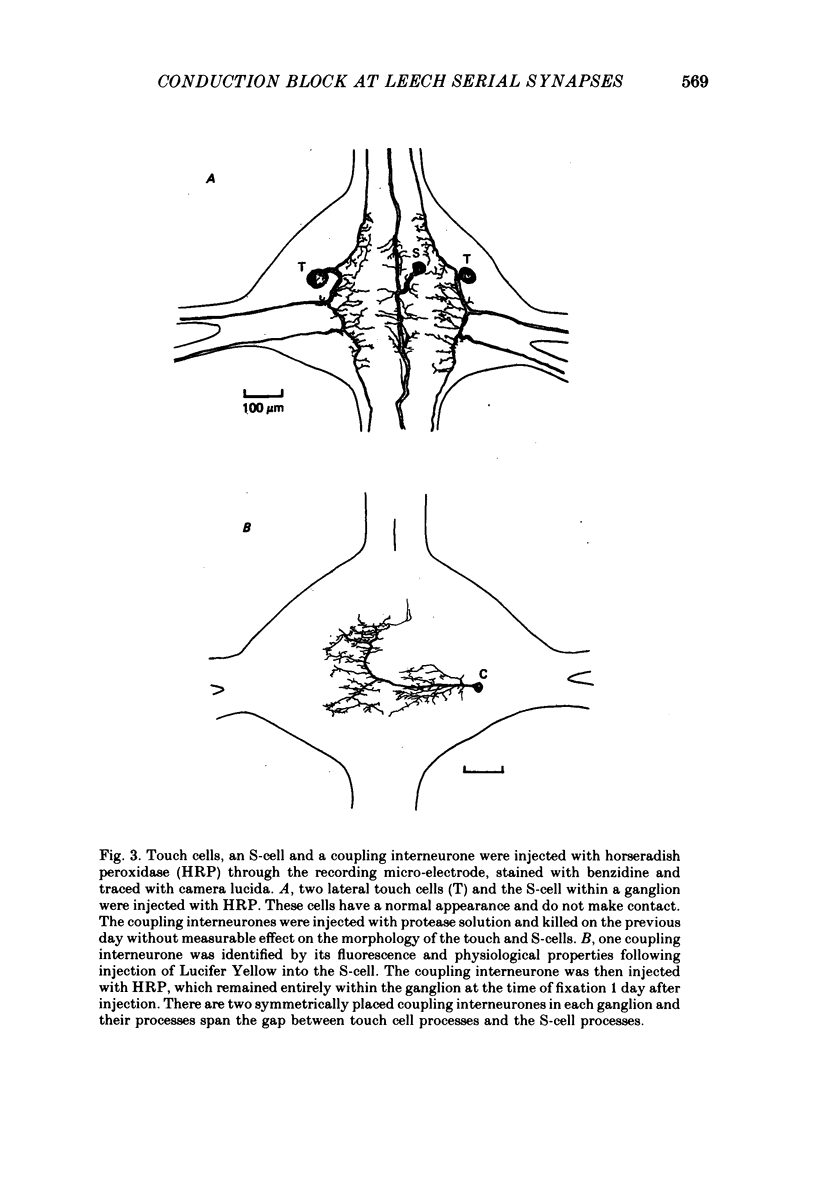
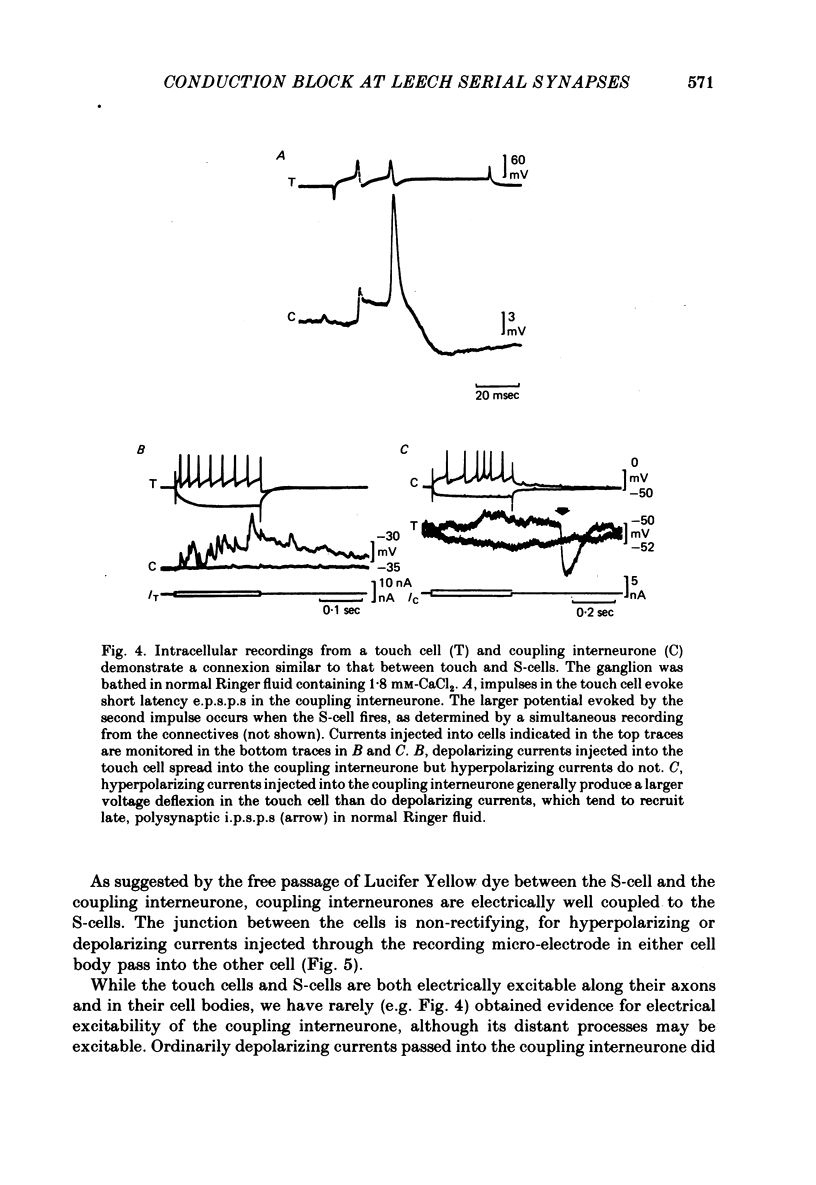
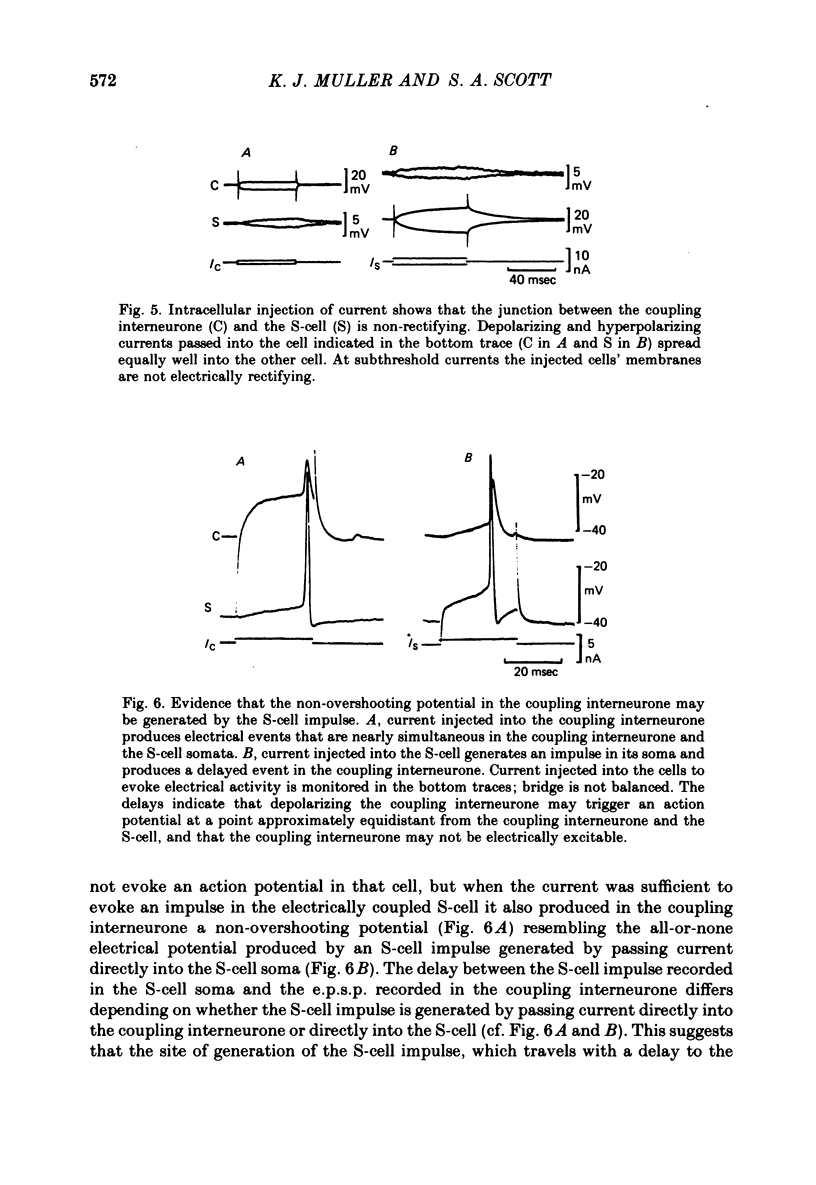
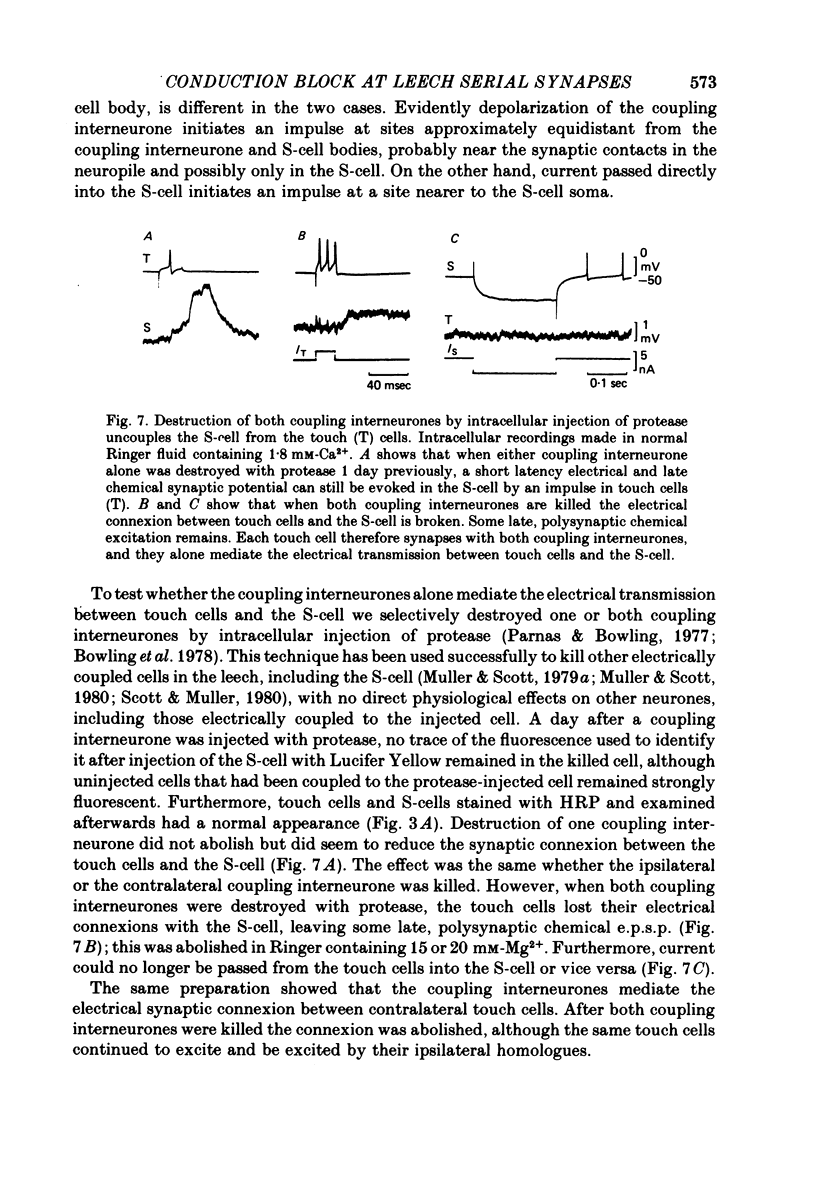
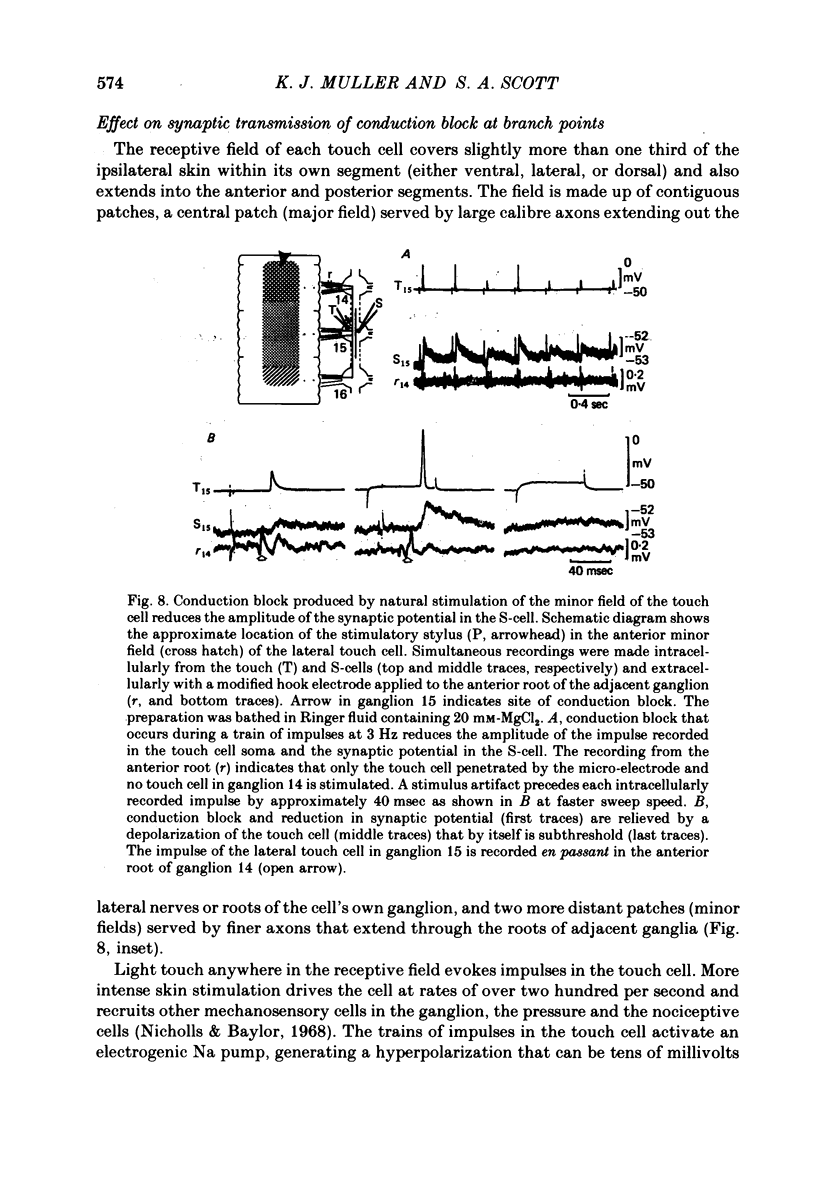
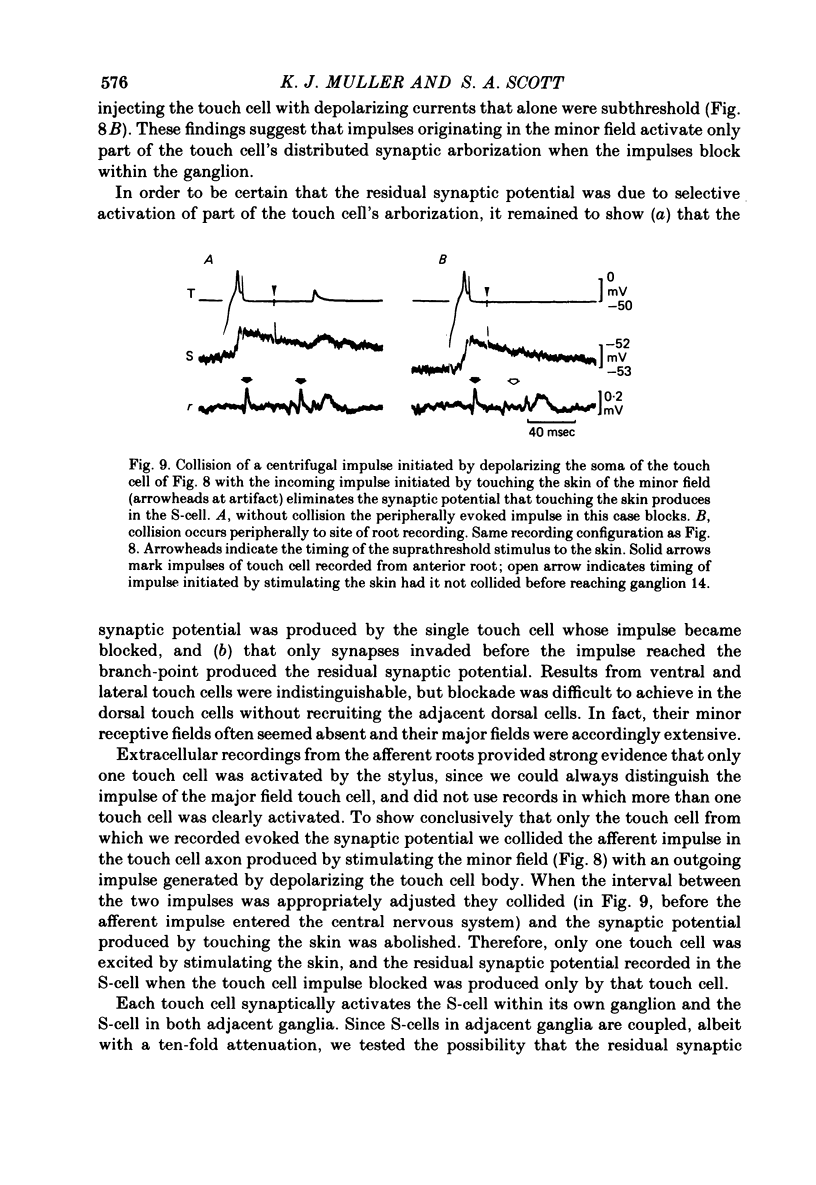
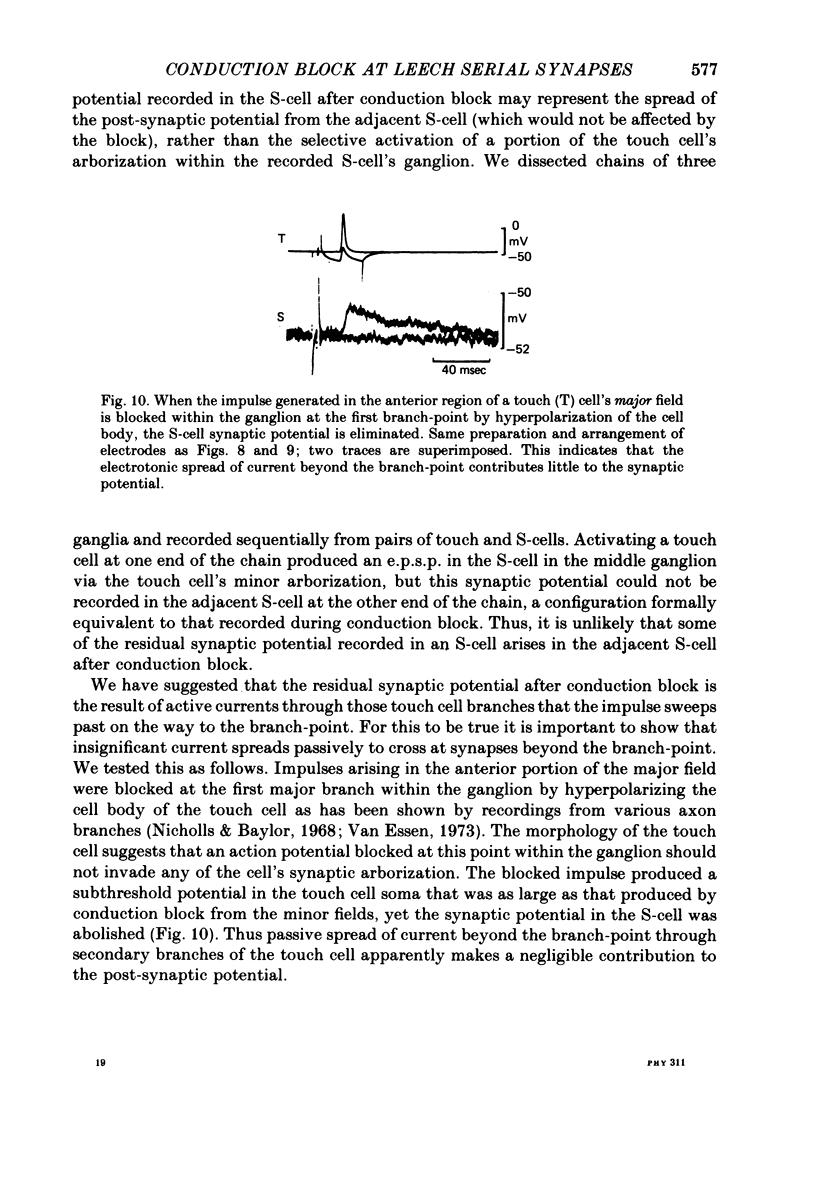
1. Touch sensory neurones in the leech excite a rapidly conducting interneurone called the S-cell. Although the electrical synaptic connexion between the two cells is monosynaptic by physiological criteria, intracellular staining reveals that the touch cells and the S-cell do not make contact, but instead are linked by a pair of small interneurones. 2. The electrical coupling between touch cells and S-cells rectifies, in that depolarizing current but not hyperpolarizing current passes from the touch cell into the S-cell. The rectifying junction is between the touch cells and coupling interneurones, while the connexion between coupling interneurones and the S-cell passes current in both directions. 3. Selective destruction of the coupling interneurones by intracellular injection of a protease interrupts the disynaptic electrical connexion between touch and S-cells. 4. The touch cell's geometry and membrane properties account for the failure of impulses that are generated in certain portions of the receptive field in the skin to propagate beyond the first branch-points of the touch cell axon within the ganglion. Conduction block at branch-points is used to examine physiologically the spatial distribution of contacts between the touch cell and the coupling interneurones. In addition, it is shown that under natural conditions branch-point failure presynaptically reduces the effectiveness of the electrical synaptic connexions.
Full text
PDF









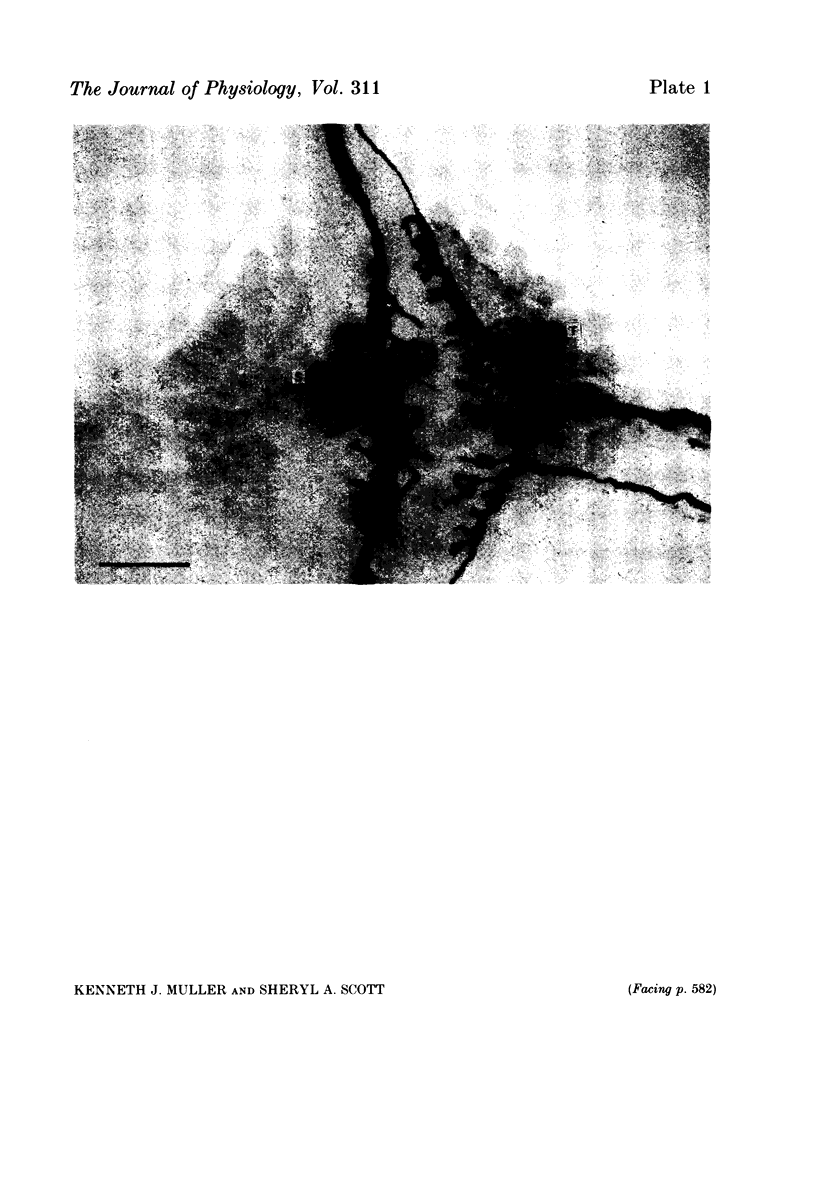
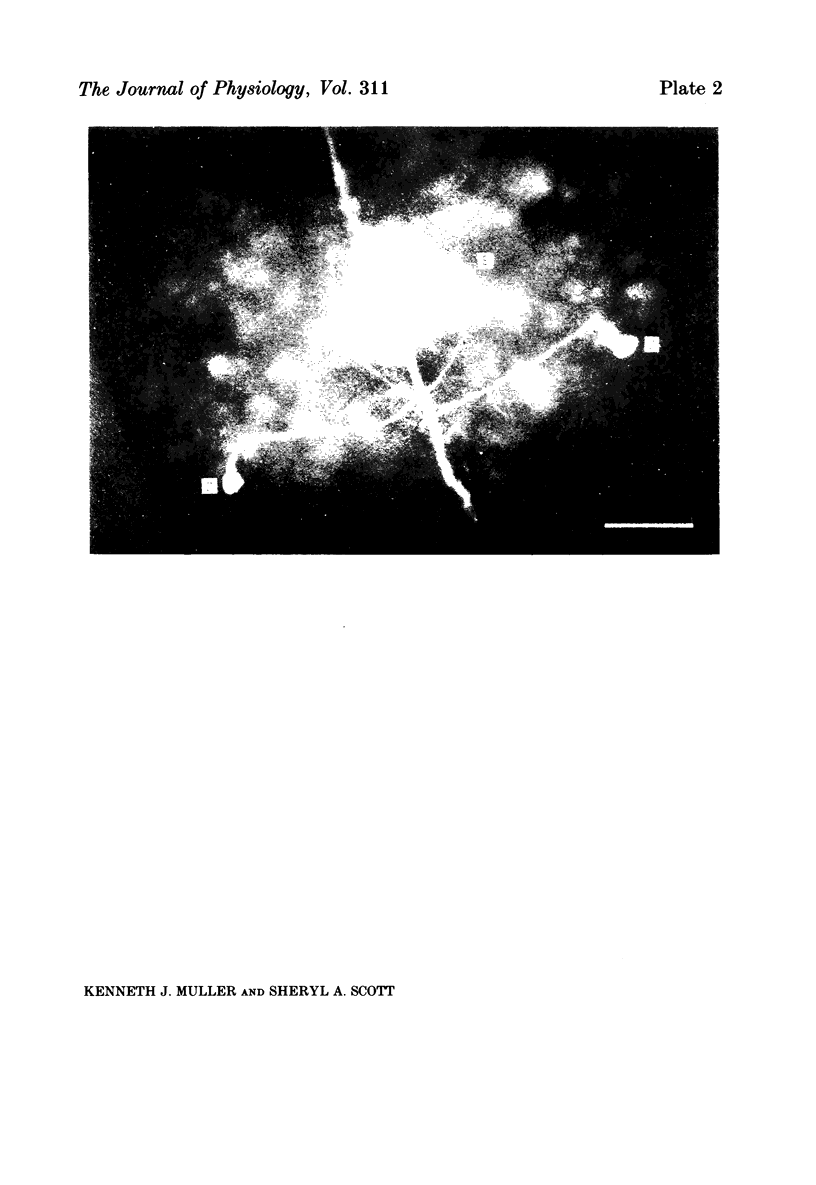
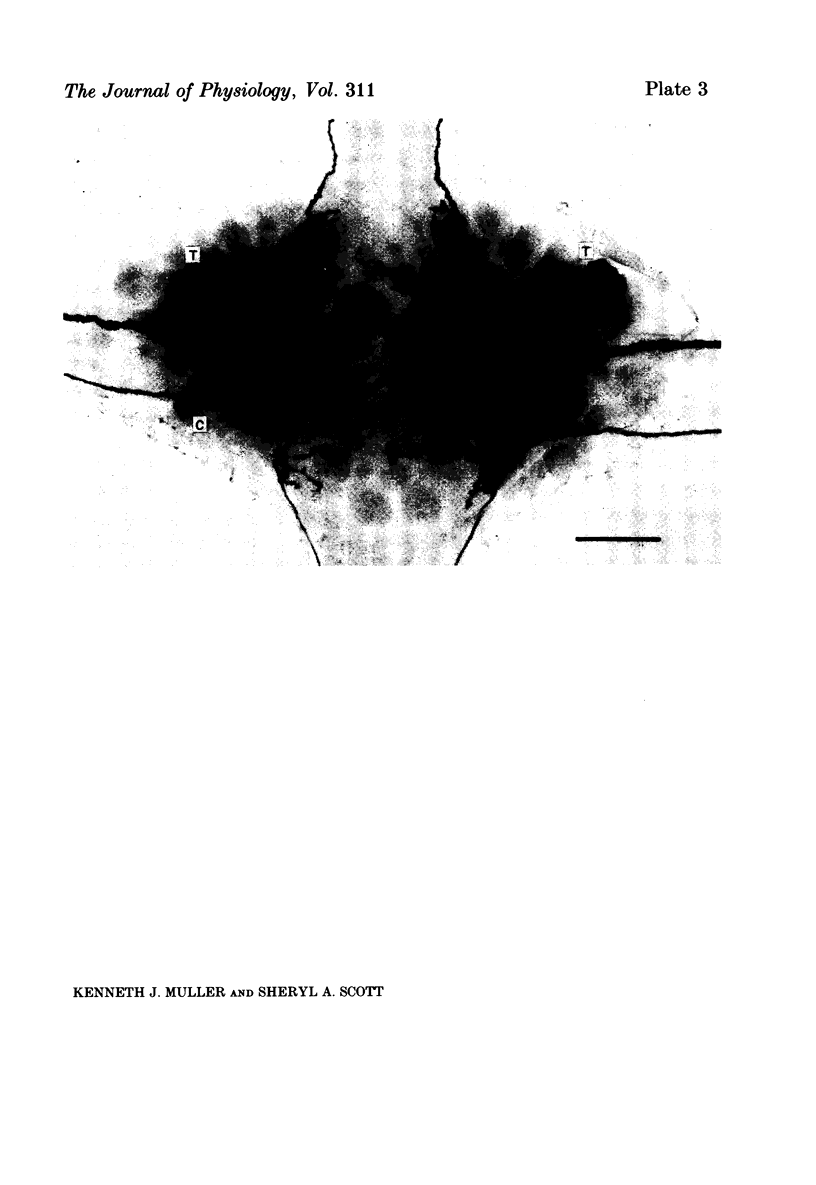
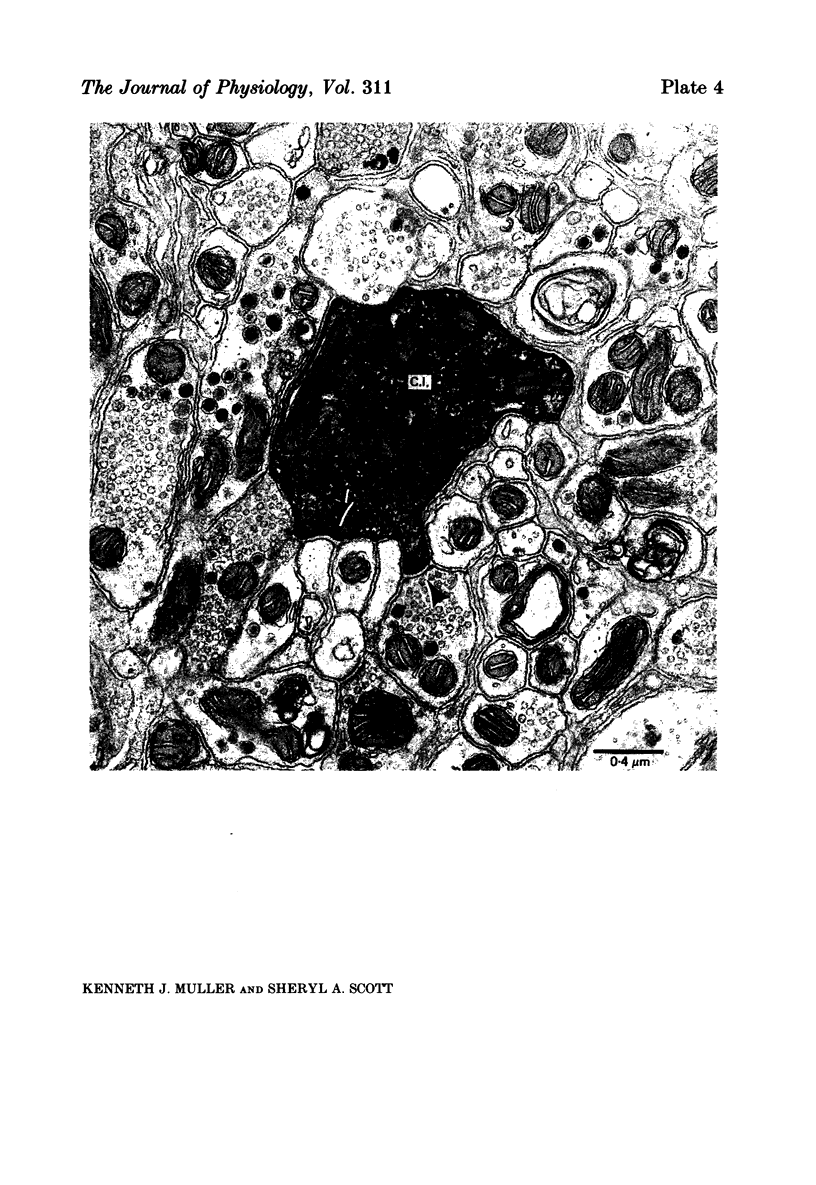

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwood H. L., Bittner G. D. Matching of excitatory and inhibitory inputs to crustacean muscle fibers. J Neurophysiol. 1971 Jan;34(1):157–170. doi: 10.1152/jn.1971.34.1.157. [DOI] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. Intermittent conduction in the spinal cord. J Physiol. 1935 Aug 22;85(1):73–103. doi: 10.1113/jphysiol.1935.sp003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nicholls J. G. Chemical and electrical synaptic connexions between cutaneous mechanoreceptor neurones in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):591–609. doi: 10.1113/jphysiol.1969.sp008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Hille B., Obara S. Voltage threshold in excitable cells depends on stimulus form. J Neurophysiol. 1970 Sep;33(5):585–594. doi: 10.1152/jn.1970.33.5.585. [DOI] [PubMed] [Google Scholar]
- Bowling D., Nicholls J., Parnas I. Destruction of a single cell in the central nervous system of the leech as a means of analysing its connexions and functional role. J Physiol. 1978 Sep;282:169–180. doi: 10.1113/jphysiol.1978.sp012455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. H., Raymond S. A., Lettvin J. Y. Multiple meaning in single visual units. Brain Behav Evol. 1970;3(1):72–101. doi: 10.1159/000125464. [DOI] [PubMed] [Google Scholar]
- Frank E., Jansen J. K., Rinvik E. A multisomatic axon in the central nervous system of the leech. J Comp Neurol. 1975 Jan 1;159(1):1–13. doi: 10.1002/cne.901590102. [DOI] [PubMed] [Google Scholar]
- Grossman Y., Parnas I., Spira M. E. Mechanisms involved in differential conduction of potentials at high frequency in a branching axon. J Physiol. 1979 Oct;295:307–322. doi: 10.1113/jphysiol.1979.sp012970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y., Spira M. E., Parnas I. Differential flow of information into branches of a single axon. Brain Res. 1973 Dec 21;64:379–386. doi: 10.1016/0006-8993(73)90191-1. [DOI] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976 Jul;259(2):367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Nicholls J. G. Conductance changes, an electrogenic pump and the hyperpolarization of leech neurones following impulses. J Physiol. 1973 Mar;229(3):635–655. doi: 10.1113/jphysiol.1973.sp010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY D., MELLON D., Jr SYNAPTIC ACTIVATION AND RECEPTIVE FIELDS IN CRAYFISH INTERNEURONS. Comp Biochem Physiol. 1964 Dec;13:275–300. doi: 10.1016/0010-406x(64)90025-8. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Failure of neuromuscular propagation in rats. J Physiol. 1958 Mar 11;140(3):440–461. [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverack M. S. Mechanoreceptors, photoreceptors and rapid conduction pathways in the leech, Hirudo medicinalis. J Exp Biol. 1969 Feb;50(1):129–140. doi: 10.1242/jeb.50.1.129. [DOI] [PubMed] [Google Scholar]
- Magni F., Pellegrino M. Patterns of activity and the effects of activation of the fast conducting system on the behaviour of unrestrained leeches. J Exp Biol. 1978 Oct;76:123–135. doi: 10.1242/jeb.76.1.123. [DOI] [PubMed] [Google Scholar]
- Muller K. J., Carbonetto S. The morphological and physiological properties of a regenerating synapse in the C.N.S. of the leech. J Comp Neurol. 1979 Jun 1;185(3):485–516. doi: 10.1002/cne.901850305. [DOI] [PubMed] [Google Scholar]
- Muller K. J., McMahan U. J. The shapes of sensory and motor neurones and the distribution of their synapses in ganglia of the leech: a study using intracellular injection of horseradish peroxidase. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):481–499. doi: 10.1098/rspb.1976.0090. [DOI] [PubMed] [Google Scholar]
- Muller K. J., Nicholls J. G. Different properties of synapses between a single sensory neurone and two different motor cells in the leech C.N.S. J Physiol. 1974 Apr;238(2):357–369. doi: 10.1113/jphysiol.1974.sp010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K. J., Scott S. A. Correct axonal regeneration after target cell removal in the central nervous system of the leech. Science. 1979 Oct 5;206(4414):87–89. doi: 10.1126/science.482931. [DOI] [PubMed] [Google Scholar]
- Muller K. J., Scott S. A. Removal of the synaptic target permits terminal sprouting of a mature intact axon. Nature. 1980 Jan 3;283(5742):89–90. doi: 10.1038/283089a0. [DOI] [PubMed] [Google Scholar]
- Muller K. J. Synapses between neurones in the central nervous system of the leech. Biol Rev Camb Philos Soc. 1979 May;54(2):99–134. doi: 10.1111/j.1469-185x.1979.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Baylor D. A. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol. 1968 Sep;31(5):740–756. doi: 10.1152/jn.1968.31.5.740. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Purves D. A comparison of chemical and electrical synaptic transmission between single sensory cells and a motoneurone in the central nervous system of the leech. J Physiol. 1972 Sep;225(3):637–656. doi: 10.1113/jphysiol.1972.sp009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J. G., Purves D. Monosynaptic chemical and electrical connexions between sensory and motor cells in the central nervous system of the leech. J Physiol. 1970 Aug;209(3):647–667. doi: 10.1113/jphysiol.1970.sp009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I., Bowling D. Killing of single neurons by intracellular injection of proteolytic enzymes. Nature. 1977 Dec 15;270(5638):626–628. doi: 10.1038/270626a0. [DOI] [PubMed] [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. J Neurophysiol. 1972 Nov;35(6):903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Parnas I., Segev I. A mathematical model for conduction of action potentials along bifurcating axons. J Physiol. 1979 Oct;295:323–343. doi: 10.1113/jphysiol.1979.sp012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- TAUC L., HUGHES G. M. Modes of initiation and propagation of spikes in the branching axons of molluscan central neurons. J Gen Physiol. 1963 Jan;46:533–549. doi: 10.1085/jgp.46.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. C. The contribution of membrane hyperpolarization to adaptation and conduction block in sensory neurones of the leech. J Physiol. 1973 May;230(3):509–534. doi: 10.1113/jphysiol.1973.sp010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. G., Adal M. N., Nicholls J. G. Regeneration of synaptic connections by sensory neurons in leech ganglia maintained in culture. Proc R Soc Lond B Biol Sci. 1977 Dec 30;199(1137):567–585. doi: 10.1098/rspb.1977.0164. [DOI] [PubMed] [Google Scholar]
- Yau K. W. Receptive fields, geometry and conduction block of sensory neurones in the central nervous system of the leech. J Physiol. 1976 Dec;263(3):513–538. doi: 10.1113/jphysiol.1976.sp011643. [DOI] [PMC free article] [PubMed] [Google Scholar]