Abstract
Aerobic biotransformation of the diaryl ethers 2,7-dichlorodibenzo-p-dioxin and 1,2,3,4-tetrachlorodibenzo-p-dioxin by the dibenzo-p-dioxin-utilizing strain Sphingomonas wittichii RW1, producing corresponding metabolites, was demonstrated for the first time. Our strain transformed 2,7-dichlorodibenzo-p-dioxin, yielding 4-chlorocatechol, and 1,2,3,4-tetrachlorodibenzo-p-dioxin, producing 3,4,5,6-tetrachlorocatechol and 2-methoxy-3,4,5,6-tetrachlorophenol; all of these compounds were unequivocally identified by mass spectrometry both before and after N,O-bis(trimethylsilyl)-trifluoroacetamide derivatization by comparison with authentic standards. Additional experiments showed that strain RW1 formed a second metabolite, 2-methoxy-3,4,5,6-tetrachlorophenol, from the original degradation product, 3,4,5,6-tetrachlorocatechol, by methylation of one of the two hydroxy substituents.
Polychlorinated dibenzo-p-dioxins and dibenzofurans are compounds of considerable environmental concern due to their persistence and toxicity (2). These halogenated compounds have become ubiquitous pollutants because they can be accidentally released (10) after they are formed as unwanted by-products during synthesis of haloaromatic compounds, such as agricultural pesticides (3), or during incineration of industrial or domestic wastes (12, 16). In addition to diaryl ether-transforming isolates belonging to genera such as Terrabacter (7, 11) and Pseudomonas (7), workers have isolated and characterized several strains belonging to the genus Sphingomonas which are able to use diaryl ethers, such as dibenzofurans and dibenzo-p-dioxins, as sole sources of carbon and energy (for reviews, see references 2 and 21). Some of these isolates are known to be effective biocatalysts, even catabolizing some di- and trichlorinated dibenzo-p-dioxins and dibenzofurans (7, 9, 19). However, aerobic catabolism of 2,7-dichloro- and 1,2,3,4-tetrachlorodibenzo-p-dioxins, yielding the corresponding metabolites, has not been demonstrated previously.
Previous studies showed that in Sphingomonas wittichii RW1 the catabolism of dibenzofurans and dibenzo-p-dioxins is initiated by a ring-hydroxylating dioxygenase (1, 18, 20), which introduces two hydroxy functions in the angular positions of the corresponding diaryl ethers, thereby producing unstable hemiacetals. The intermediate decays in the case of dibenzo-p-dioxins, spontaneously yielding the corresponding 2,2′,3-trihydroxydiphenyl ether, which is then subjected to meta cleavage and enzymatic hydrolysis, yielding catechol and the corresponding C6 body 2-hydroxy-cis,cis-muconic acid (for reviews, see references 2 and 21 ). However, although recently reported transformation experiments monitoring the disappearance of selected mono- to tetrachlorinated congeners of dibenzofurans and dibenzo-p-dioxins indicated that even some of the tetrachlorodibenzo-p-dioxins are depleted by the metabolic activity of the bacteria employed (15), there is still a lack of information concerning aerobic bacterial catabolism of tetrachlorinated dibenzo-p-dioxins, such as 1,2,3,4-tetrachlorodibenzo-p-dioxin.
In this paper, we describe aerobic biotransformation of 2,7-dichlorochlorodibenzo-p-dioxin and 1,2,3,4-tetrachlorodibenzo-p-dioxin by S. wittichii RW1 and characterize the resulting metabolic intermediates. In addition, we show that strain RW1 transformed 3,4,5,6-tetrachlorocatechol to the corresponding methoxyphenol, an activity not previously described for this organism.
The resting cells used for biotransformation experiments were produced as previously described (6, 9) by routinely employing dibenzofuran as the substrate (dibenzo-p-dioxin-grown cells exhibited similar activities [data not shown]). However, the strain employed in the previously described experiments, Sphingomonas sp. strain RW1, has only recently been proposed to be a member of a new species, S. wittichii (22), the name which we used in this study. The substrates tested were dissolved in acetone (concentration, 10 mg/ml, unless indicated otherwise). Then an appropriate aliquot of a stock solution was added to a sterilized Erlenmeyer flask, and the acetone was evaporated by flushing the flask with N2. For production of metabolites, 200-ml portions of a cell suspension (optical density at 578 nm, 8.0) were added to 2-liter Erlenmeyer flasks containing 10 mg of the appropriate dibenzo-p-dioxin and incubated at 160 rpm and 28°C for 5 days. The biotransformation was monitored and quantified by employing 100-ml Erlenmeyer flasks; each flask contained 10 ml of cell suspension (optical density at 578 nm, 8.0) and 0.5 mg of a dibenzo-p-dioxin. Every 24 h, a set of triplicate flasks was removed, 2 ml of ortho-phosphoric acid was added to each flask to stop the reaction, and the flasks were immediately frozen and stored at −70°C. After incubation for 120 h, all flasks were thawed, and the contents were extracted as described below. However, for routine determination of recovery rates, 500 μg of 2-chlorodibenzo-p-dioxin and 5 ng of 2,3,4,5-tetrachlorophenol were added to each flask prior to extraction.
The flasks and the corresponding controls were extracted four times with 0.2 volume of chilled ethyl acetate. This procedure was repeated after the pH of the remaining water phase was adjusted to 3.5 with ortho-phosphoric acid. Extracts were dried over anhydrous sodium sulfate, and the ethyl acetate was evaporated under reduced pressure. Aliquots were analyzed directly with a liquid chromatography-mass spectrometry system as described below. However, for gas chromatography-mass spectrometry analysis, samples were derivatized prior to measurement by using BSTFA [N,O-bis(trimethylsilyl)-trifluoroacetamide] to form trimethylsilyl (TMS) derivatives by incubation at 60°C for 1 h (8), or they were methylated by using iodomethane (4).
Metabolites present in extracts obtained from biotransformation experiments were detected and characterized initially (data not shown) with a liquid chromatography-mass spectrometry-mass spectrometry (Qstar Pulsar; Applied Biosystems, Foster City, Calif.) system consisting of a reverse-phase high-performance liquid chromatograph (Agilent 1100; Agilent, Waldbronn, Germany) equipped with a Lichrocart RP-18 column (125 by 30 mm; 5 μm; Merck, Darmstadt, Germany) by using mass and UV-visible light detection after filtration of the samples. The aqueous solvent system (flow rate, 1.0 ml/min) contained 0.1% (wt/vol) acetic acid and 60% methanol. The derivatives obtained were analyzed by high-resolution gas chromatography ion trap mass spectrometry without further purification. For gas chromatography-mass spectrometry analyses, we used a Trace GC 2000 system (Thermoquest, Austin, Tex.) linked to a Finnigan Polaris Q mass spectrometer (Thermoquest) with a 60-m DB-5 mass spectrometry column. The initial temperature, 60°C, was maintained for 2 min, and then the temperature was increased to 290°C by 10 min and was held at 290°C for 10 min. In all experiments, which were generally repeated three times, additional controls were employed; these controls consisted of cells that were heat inactivated (75°C for 20 min) and cultures that were poisoned (10 mM NaN3). Metabolites were identified and quantified by comparison of their mass spectra, retention times, and peak areas with those of authentic standards.
Dibenzofuran, 2,3,4,5-tetrachlorophenol, BSTFA, and iodomethane were obtained from Sigma-Aldrich (St. Louis, Mo.). 3,4,5,6-Tetrachlorocatechol and substituted dibenzo-p-dioxins were purchased from Accustandard (New Haven, Conn.) or Sigma-Aldrich. The solvents employed and ortho-phosphoric acid were obtained from Merck. 2-Methoxy-3,4,5,6-tetrachlorophenol was synthesized by methylating 3,4,5,6-tetrachlorocatechol using iodomethane and subsequently purifying the desired reaction product by column chromatography. All chemicals employed were of the highest quality commercially available.
Biotransformation of 2,7-dichlorodibenzo-p-dioxin by S. wittichii RW1 gave rise to a polar metabolite, which we identified as 4-chlorocatechol. Diagnostic peaks were present at m/z 288 (M+), m/z 273 (M+ − CH3), m/z 200 [M+ − CH3 − Si(CH3)3], m/z 185 [M+ − CH3 − Si(CH3)3 − CH3], and m/z 170 [M+ − CH3 − Si(CH3)3 − CH3 − CH3], and the mass spectrum was undistinguishable from that of the TMS derivative of authentic 4-chlorocatechol. In addition, we detected a compound exhibiting an m/z of 328 (35Cl), indicating the presence of an O-methylated dichlorotrihydroxydiphenyl ether. This was not unexpected on biochemical grounds, as catabolism of dioxins by S. wittichii RW1 proceeds via the corresponding trihydroxdiphenyl ethers (19, 20). As shown in Fig. 1, after 120 h of incubation about 47% of the depleted 2,7-dichlorodibenzo-p-dioxin had been converted into 4-chlorocatechol.
FIG. 1.
Biotransformation of 2,7-dichlorodibenzo-p-dioxin (2,7-DCDD) by dibenzofuran-grown resting cells of S. wittichii RW1. Symbols: ▪, 2,7-dichlorodibenzo-p-dioxin; □, chlorocatechol.
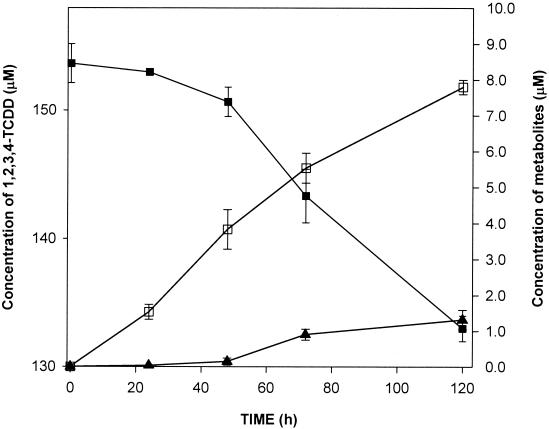
However, in the case of 1,2,3,4-tetrachlorodibenzo-p-dioxin, two metabolites accumulated. One was characterized as 3,4,5,6-tetrachlorocatechol (Fig. 2A), with diagnostic peaks at m/z 392 (M+), m/z 377 (M+ − CH3), m/z 304 [M+ − CH3 − Si(CH3)3], m/z 289 [M+ − CH3 − Si(CH3)3 − CH3], and m/z 274 [M+ − CH3 − Si(CH3)3 − CH3 − CH3)]; the other was characterized as 2-methoxy-3,4,5,6-tetrachlorophenol (Fig. 2B), with diagnostic peaks at m/z 334 (M+), m/z 319 (M+ − CH3), m/z 304 (M+ − CH3 − CH3), and m/z 289 [M+ − (CH3)3]. The mass spectra of these two metabolites were undistinguishable from those of the authentic compounds. Again, detection of minute amounts of a metabolite with an m/z of 396 (35Cl) implied that the corresponding O-methylated tetrachlorinated trihydroxydiphenyl ether was present, thus confirming the expected catabolic sequence for 1,2,3,4-tetrachlorodibenzo-p-dioxin in our strain. When the biotransformation was monitored over time (Fig. 3), it became evident that after 120 h of incubation about 37% of the 1,2,3,4-tetrachlorodibenzo-p-dioxin consumed had been converted into 3,4,5,6-tetrachlorocatechol and about 6% had been converted into 2-methoxy-3,4,5,6-tetrachlorophenol. Poisoned controls and controls in which heat-inactivated cells were used did not show formation of metabolites from the two dioxins. Although formation of the chlorocatechols detected and the presence of the chlorinated trihydroxydiphenyl ethers were in agreement with the previously described catabolism of dibenzo-p-dioxin or 2,3-dichlorodibenzo-p-dioxin by this strain (19, 20), the data did not explain formation of the 2-methoxy-3,4,5,6-tetrachlorophenol. By either incubating 3,4,5,6-tetrachlorocatechol with resting cells or adding it to growing cultures, we could demonstrate that 2-methoxy-3,4,5,6-tetrachlorophenol was indeed formed upon methylation of one of the hydroxy groups by our strain, probably as a means of detoxification. Again, poisoned or heat-inactivated controls failed to produce this metabolite of 3,4,5,6-tetrachlorocatechol.
FIG. 2.
Identification of 3,4,5,6-tetrachlorocatechol and 2-methoxy-3,4,5,6-tetrachlorophenol as metabolites produced from 1,2,3,4-tetrachlorodibenzo-p-dioxin by S. wittichii RW1 by mass spectrometry. (A) 3,4,5,6-Tetrachlorocatechol (TMS derivative); (B) 2-methoxy-3,4,5,6-tetrachlorophenol (TMS derivative).
FIG. 3.
Biotransformation of 1,2,3,4-tetrachlorodibenzo-p-dioxin (1,2,3,4-TCDD) by dibenzofuran-grown resting cells of S. wittichii RW1. Symbols: ▪, 1,2,3,4-tetrachlorodibenzo-p-dioxin; □, 3,4,5,6-tetrachlorocatechol; ▴, 2-methoxy-3,4,5,6-tetrachlorophenol.
2,7-Dichlorodibenzo-p-dioxin was not catabolized by two strains recently reported to attack a range of chlorinated dibenzofurans and dibenzo-p-dioxins (7). However, as demonstrated previously for the structural analogue 2,7-dibenzofuran (9), our strain is able to catabolize this compound, which we demonstrated by characterizing the metabolite detected as 4-chlorocatechol (Fig. 4A). In 1,2,3,4-tetrachlorodibenzo-p-dioxin, all of the chlorine substituents are present in just one of the two available benzene rings. Therefore, positions 1 and 4 are not available for an initial angular attack by the ring-hydroxylating dioxygenase of S. wittichii RW1. Nevertheless, as our identification of the degradation product 3,4,5,6-tetrachlorocatechol illustrated, an attack can take place at the nonsubstituted ring, thus giving rise to the metabolite detected. However, the additional product which we identified as 2-methoxy-3,4,5,6-tetrachlorophenol was produced by our organism by O-methylation of the 3,4,5,6-tetrachlorocatechol (Fig. 4B). This is not unusual as such activity leading to biotransformation of hydroxy functions attached to aromatic nuclei into methoxy groups has been reported previously for other bacterial strains (5, 17). However, this is the first report describing biotransformation of 2,7-dichloro- and 1,2,3,4-tetrachlorodibenzo-p-dioxins by a bacterial strain together with identification of the corresponding metabolites. Although our strain does not utilize these two chlorinated dibenzo-p-dioxins as sole sources of carbon and energy, the chlorocatechols identified are indeed degradable by appropriate bacterial strains (13). In view of the rather low turnover rates which we determined for these two substrates (the rates for 2,7-dichlorodibenzo-p-dioxin and 1,2,3,4-tetrachlorodibenzo-p-dioxin were 2 and 1%, respectively, of the rate previously reported for dibenzo-p-dioxin turnover by S. wittichii RW1 [19]), it remains to be seen if production of genetically altered hybrid strains, as reported previously for chlorobiphenyl-catabolizing bacteria (14), will enable more efficient degradation of these noxious compounds.
FIG. 4.
Proposed pathways for the biotransformation of 2,7-dichlorodibenzo-p-dioxin (A) and 1,2,3,4-tetrachlorodibenzo-p-dioxin (B) by S. wittichii RW1.
Acknowledgments
This work was performed under the German-Korean cooperation scheme run jointly by DFG and KOSEF (grant no. 466 KOR 113/160/0-1 and 2001-6-202-02-2).
REFERENCES
- 1.Bünz, P. V., and A. M. Cook. 1993. Dibenzofuran 4,4a-dioxygenase from Sphingomonas sp. strain RW1: angular dioxygenation by a three-component enzyme system. J. Bacteriol. 175:6467-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bünz, P. V., and S. Schmidt. 1997. The microbial degradation of halogenated diaryl ethers. Biotechnol. Adv. 15:621-632. [DOI] [PubMed] [Google Scholar]
- 3.Buser, H. R., and H. P. Bosshardt. 1976. Determination of polychlorinated dibenzo-p-dioxins and dibenzofurans in commercial pentachlorophenols by combined gas chromatography-mass spectrometry. J. Assoc. Off. Anal. Chem. 59:562-569. [PubMed] [Google Scholar]
- 4.Cox, A. D., J. R. Brisson, V. Varma, and M. B. Perry. 1996. Structural analysis of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr. Res. 290:43-58. [DOI] [PubMed] [Google Scholar]
- 5.Dhar, K., and J. P. N. Rosazza. 2000. Purification and characterization of Streptomyces griseus catechol O-methyltransferase. Appl. Environ. Microbiol. 66:4877-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortnagel, P., H. Harms, R. M. Wittich, S. Krohn, H. Meyer, V. Sinnwell, H. Wilkes, and W. Francke. 1990. Metabolism of dibenzofuran by Pseudomonas sp. strain HH69 and the mixed culture HH27. Appl. Environ. Microbiol. 56:1148-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habe, H., J. S. Chung, J. H. Lee, K. Kasuga, T. Yoshida, H. Nojiri, and T. Omori. 2001. Degradation of chlorinated dibenzofurans and dibenzo-p-dioxins by two types of bacteria having angular dioxygenases with different features. Appl. Environ. Microbiol. 67:3610-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jianzhen, Y., R. C. Flagan, and J. H. Seinfeld. 1998. Identification of products containing -COOH, —OH, and -C O in atmospheric oxidation of hydrocarbons. Environ. Sci. Technol. 32:2357-2370. [Google Scholar]
- 9.Keim, T., W. Francke, S. Schmidt, and P. Fortnagel. 1999. Catabolism of 2,7-dichloro- and 2,4,8-trichlorodibenzofuran by Sphingomonas sp. strain RW1. J. Ind. Microbiol. Biotechnol. 23:359-363. [DOI] [PubMed] [Google Scholar]
- 10.Meharg, A. A., and D. Osborn. 1995. Dioxins released from chemical accidents. Nature 375:353-354. [DOI] [PubMed] [Google Scholar]
- 11.Monna, L., T. Omori, and T. Kodama. 1993. Microbial degradation of dibenzofuran, fluorene, and dibenzo-p-dioxin by Staphylococcus auriculans DBF63. Appl. Environ. Microbiol. 59:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh, J. E., K. T. Lee, J. W. Lee, and Y. S. Chang. 1999. The evaluation of PCDD/Fs from various Korean incinerators. Chemosphere 38:2097-2108. [Google Scholar]
- 13.Potrawfke, T., K. N. Timmis, and R. M. Wittich. 1998. Degradation of 1,2,3,4-tetrachlorobenzene by Pseudomonas chlororaphis RW71. Appl. Environ. Microbiol. 64:3798-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reineke, W. 1998. Development of hybrid strains for the mineralization of chloroaromatics by patchwork assembly. Annu. Rev. Microbiol. 52:287-331. [DOI] [PubMed] [Google Scholar]
- 15.Schreiner, G., T. Wiedmann, H. Schimmel, and K. Ballschmiter. 1997. Influence of the substitution pattern on the microbial degradation of mono- to tetrachlorinated dibenzo-p-dioxins and dibenzofurans. Chemosphere 34:1315-1331. [Google Scholar]
- 16.Shin, D. H., S. M. Choi, J. E. Oh, and Y. S. Chang. 1999. Evaluation of polychlorinated dibenzo-p-dioxin/dibenzofuran emission in ten municipal solid waste incinerators. Environ. Sci. Technol. 33:2657-2666. [Google Scholar]
- 17.Valo, R., and M. Salkinoja-Salonen. 1986. Microbial transformation of polychlorinated phenoxyphenols. J. Gen. Appl. Microbiol. 32:505-517. [Google Scholar]
- 18.Wilkes, H., W. Francke, R. M. Wittich, H. Harms, S. Schmidt, and P. Fortnagel. 1992. Mechanistic investigation on microbial degradation of diaryl ethers—analysis of isotope-labelled reaction products. Naturwissenschaften 79:269-271. [Google Scholar]
- 19.Wilkes, H., R. M. Wittich, K. N. Timmis, P. Fortnagel, and W. Francke. 1996. Degradation of chlorinated dibenzofurans and dibenzo-p-dioxins by Sphingomonas sp. strain RW1. Appl. Environ. Microbiol. 62:367-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wittich, R. M., H. Wilkes, V. Sinnwell, W. Francke, and P. Fortnagel. 1992. Metabolism of dibenzo-p-dioxin by Sphingomonas sp. strain RW1. Appl. Environ. Microbiol. 58:1005-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittich, R. M. 1998. Degradation of dioxin-like compounds by microorganisms. Appl. Microbiol. Biotechnol. 49:489-499. [DOI] [PubMed] [Google Scholar]
- 22.Yabuuchi, E., H. Yamamoto, S. Terakubo, N. Okamura, T. Naka, N. Fujiwara, K. Kobayashi, Y. Kosako, and A. Hiraishi. 2001. Proposal of Sphingomonas wittichii sp. nov. for strain RW1(T), known as a dibenzo-p-dioxin metabolizer. Int. J. Syst. E vol. Microbiol. 51:281-292. [DOI] [PubMed] [Google Scholar]