Abstract
By in vitro evolution experiment, we have first succeeded in acquiring higher active mutants of a synthase that is a key enzyme essential for bacterial synthesis of biodegradable polyester, polyhydroxyalkanoate (PHA). Aeromonas caviae FA440 synthase, termed PhaCAc, was chosen as a good target for evolution, since it can synthesize a PHA random copolyester of 3-hydroxybutyrate and 3-hydroxyhexanoate [P(3HB-co-3HHx)] that is a tough and flexible material compared to polyhydroxybutyrate (PHB) homopolyester. The in vitro enzyme evolution system consists of PCR-mediated random mutagenesis targeted to a limited region of the phaCAc gene and screening mutant enzymes with higher activities based on two types of polyester accumulation system by using Escherichia coli for the synthesis of PHB (by JM109 strain) (S. Taguchi, A. Maehara, K. Takase, M. Nakahara, H. Nakamura, and Y. Doi, FEMS Microbiol. Lett. 198:65-71, 2001) and of P(3HB-co-3HHx) {by LS5218 [fadR601 atoC(Con)] strain}. The expression vector for the phaCAc gene, together with monomer-supplying enzyme genes, was designed to synthesize PHB homopolyester from glucose and P(3HB-co-3HHx) copolyester from dodecanoate. Two evolved mutant enzymes, termed E2-50 and T3-11, screened through the evolution system exhibited 56 and 21% increases in activity toward 3HB-coenzyme A, respectively, and consequently led to enhanced accumulation (up to 6.5-fold content) of P(3HB-co-3HHx) in the recombinant LS5218 strains. Two single mutations in the mutants, N149S for E2-50 and D171G for T3-11, occurred at positions that are not highly conserved among the PHA synthase family. It should be noted that increases in the 3HHx fraction (up to 16 to 18 mol%) were observed for both mutants compared to the wild type (10 mol%).
Polyhydroxyalkanoates (PHAs) have been drawing much attention as biodegradable polyesters that can be used in place of conventional petrochemical-based plastics. At present, ca. 130 different PHAs are known to be constituents of many bacterial storage polyesters, and their biosynthesis genes have been identified and isolated (24). To realize sustainable industrial processes based on the use of PHA biopolyester, the high manufacturing cost needs to be reduced by establishing an efficient recombinant production system of PHA with the desired properties. Until now, microbial PHA production has been achieved by the coordinated fermentation of natural PHA-producing bacteria or by the gene dosage effect of PHA biosynthesis genes, such as the PHA synthase (PhaC) gene, in recombinants. In several studies, however, the level of accumulated intracellular PHA was not always enhanced, or was only slightly enhanced, by amplification of the wild-type phaC genes (24).
In vitro evolution of the enzymes related to PHA biosynthesis would be a very powerful approach for realizing productivity improvement and quality alteration of PHA, which is not possible with naturally occurring enzymes. The PhaC is a key enzyme essential for PHA biosynthesis catalyzing the polymerization of (R)-3-hydroxyacyl-coenzyme A (3HA-CoA) monomers into water-insoluble granule, and the reaction mechanism of this intriguing enzyme has been investigated biochemically (24). Its performance is closely related to the properties of the polyesters generated, such as molecular weight, polydispersity, and compositional variation upon copolymerization. Previously, we established an in vivo assay system for the PhaCRe of Ralstonia eutropha as a model based on PCR-mediated random mutagenesis and two analytical procedures for screening mutant enzymes (28, 29, 29a). This in vivo system enables us to readily estimate the synthase activity by monitoring the accumulation level of poly(3-hydroxybutyrate) [P(3HB) or PHB] within the cells of recombinant Escherichia coli. As demonstrated for other bioactive polypeptides (1, 26, 27, 30-33, 36), efficient use of bacterial cells is often effective for exploring the sequence space of an enzyme, which evolves in an aimed direction. Thus, it is of interest to elucidate the versatility of such an in vivo system.
Aeromonas caviae FA440 PHA synthase (PhaCAc) has been considered an attractive target for this sort of application because it can synthesize not only PHB homopolyester but also random copolyesters of 3HB and 3-hydroxyhexanoate [P(3HB-co-3HHx)] from renewable carbon sources such as alkanoates or plant oils (4, 21). Bacterial random copolyesters are more ductile, easier to mold, and tougher than PHB homopolyester (25). Thus, random copolyesters of 3HB and longer-chain (R)-3-hydroxyalkanoate (3HA) seem to confer preferable mechanical properties for practical use. In this sense, in vitro molecular breeding of PhaCAc would be very useful for improving the production and controlling the compositional variation of copolyesters. We have cloned and analyzed the PHA biosynthetic locus of A. caviae, including three defined structural genes: phaCAc, phaJAc [encoding (R)-specific enoyl-CoA hydratase], and phaPAc (encoding a granule-associated protein) (5-7). The PHA biosynthetic pathway in A. caviae proceeds, through the function of PhaJAc, from enoyl-CoA derivatives of the fatty acid β-oxidation pathway (7).
In the present study, through an in vitro evolution system, we succeeded in acquiring the PhaCAc mutants with higher CoA release activities that led to enhanced accumulation and increased 3HHx fractions of P(3HB-co-3HHx) in E. coli LS5218 [fadR601 atoC(Con)] via the fatty acid β-oxidation pathway. We also carried out functional mapping of the amino acid residues responsible for the synthase activity of PhaCAc based on the correlation between synthase activity and level of PHB accumulation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture condition.
E. coli JM109 (35) was used in all standard cloning procedures and was used as the host strain for screening mutants of A. caviae PHA synthase (PhaCAc) and for PHB accumulation from glucose. For accumulation of P(3HB-co-3HHx) copolyester from dodecanoate, E. coli LS5218 [fadR601 atoC(Con)] (23) was used as the host strain. Plasmid pBSEE32phbAB, derivative of pBluescript II SK(+), was constructed for biosynthesis of PHB and PHA copolyester, as illustrated in Fig. 1. The plasmid pBSEE32phbAB carries PHA polycistronic genes for PhaPAc (granule-associated protein), PhaCAc, and PhaJAc [(R)-specific enoyl-CoA hydratase] with promoter derived from A. caviae FA440 (5) and the genes for the 3HB-CoA monomer substrate supplying enzymes PhbARe (β-ketothiolase) and PhbBRe (NADPH-dependent acetoacetyl-CoA reductase) from R. eutropha H16 (14). The phaPCJAc gene-containing fragment was subcloned once into the EcoRI site of pBluescript II SK(+), and the resultant plasmid pBSEE32 was combined at the BamHI site with the phbABRe gene-containing fragment to generate pBSEE32phbAB. For PHB accumulation, recombinant JM109 strains were grown on Luria-Bertani (LB) medium containing 2% glucose. M9 medium supplemented with 10 mM sodium dodecanoate was used for measuring P(3HB-co-3HHx) accumulation. All cells were cultivated for 14 h (PHB accumulation) or 72 h [P(3HB-co-3HHx) accumulation] at 37°C. For P(3HB-co-3HHx) accumulation in the recombinant LS5218 strain, the sodium dodecanoate was solubilized with 0.4% (vol/vol) Brij-35 (18). Brij-35 is a detergent (formally termed polyoxyethylene dodecyl ether) for solubilizing dodecanoate. When needed, ampicillin (50 μg/ml) was added to the medium.
FIG. 1.
Plasmid vector pBSEE32phbAB, used for synthesizing PHB homopolyester and P(3HB-co-3HHx) copolyester. Three genes derived from A. caviae—phaPAc, phaCAc, and phaJAc—encode a granule-associated protein, PHA synthase, and (R)-specific enoyl-CoA hydratase, respectively. These genes are presumably transcribed under the control of native promoter (5), PphaAc. The 3HB-CoA monomer substrate-supplying enzymes, β-ketothiolase and NADPH-dependent acetoacetyl-CoA reductase, are encoded by phbARe and phbBRe, respectively.
DNA manipulation and sequencing.
Standard recombinant DNA manipulations (19) were used for isolation of plasmid DNA, restriction digestion, ligation, and transformation of E. coli. All restriction enzymes and related reagents for DNA manipulation were commercially available and used according to the suppliers' recommendations. All other chemicals were of biochemical analytical grade and were used without further purification. DNA sequencing for analysis of the mutation points was carried out by the dideoxy chain termination method with the Prism 310 DNA sequencer or the Prism 377 DNA sequencer (Applied Biosystems, Inc.) by using the BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems). Nucleotide sequence data, deduced amino acid sequences, and the predicted hydropathy profile of the PhaCAc were analyzed with GENETYX-MAC software (Software Development Co., Tokyo, Japan).
Random mutagenesis by error-prone PCR.
Random mutagenesis of the PstI-XhoI region in the phaCAc gene was carried out by error-prone PCR. The forward primer used was 5′-GCTGCTGCAGACCAATC-3′ (the underlined sequence indicates a PstI site), and the reverse primer was 5′-GCCTCATTTTGCGCCTCG-3′ (the underlined sequence indicates a XhoI site). PCR conditions used were a 100.5-μl reaction solution containing 2.5 nM pBSEE32phbAB, 0.025 U of Taq DNA polymerase, 0.1 μM concentrations (each) of two primers, 0.2 mM concentrations of each deoxynucleotide triphosphate, 10 mM Tris-HCl (pH 8.8), and 50 mM KCl, with the addition of 5 mM MgCl2 and 10% dimethyl sulfoxide (27). PCR was carried out by using a program of 25 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 2 min with a GeneAmp PCR System 9700 (Perkin-Elmer/Applied Biosystems).
Screening mutants leading to enhanced PHA accumulation and changed monomer compositions in PHA copolyester.
Figure 2 shows a schematic diagram for construction of an in vivo screening system with the pBSEE32phbAB plasmid. The target region for PCR mutagenesis within the phaCAc gene was a 1,012-bp PstI-XhoI fragment containing 57% of the phaCAc gene, as shown in Fig. 2. After amplification, a mixture of PstI-XhoI fragments including the randomly mutagenized genes was religated into the same restriction sites of pBSEE32phbAB to generate a mutant library. The recombinants harboring phaCAc mutants were grown on the LB plates supplemented with 2% glucose, 0.5 μg of Nile red/ml, and 50 μg of ampicillin/ml. The change in PHB accumulation resulting from the introduction of mutations into the phaCAc gene was judged on the basis of intensity of the pinkish pigmentation of the cells caused by Nile red staining (22). For precise quantification of the cellular PHB accumulation, mutants were cultivated in LB medium with 2% glucose at 37°C for 14 h. The cellular PHB content was determined by analytical high-performance liquid chromatography (HPLC) after the cellular PHB was converted to crotonic acid by treatment with hot concentrated sulfuric acid (H2SO4) (10, 28, 29, 29a). HPLC data allowed the distribution profile of the PHB accumulation level of the mutant population (300 clones).
FIG. 2.
In vitro enzyme evolution and screening system for evolved A. caviae PHA synthase (PhaCAc), leading to enhanced PHA accumulation and changed monomer composition in PHA copolyester. A schematic flow diagram of the system is illustrated. PCR-mediated random mutagenesis toward the target region (between restriction enzyme sites PstI and XhoI) of the phaCAc gene, preparation of a mutant library, primary plate assay of PHB accumulation in E. coli JM109 cells with Nile red dye, secondary HPLC assay based on conversion of PHB to crotonic acid, and nucleotide sequence determination and activity assay of the PhaCAc samples were carried out. The plasmid carrying genes for PhaCAc mutants which exhibited >50% PHB accumulation were introduced into E. coli LS5218, and the recombinants cultivated on M9 medium supplemented with 10 mM sodium dodecanoate as a sole carbon source were subjected to GC analysis.
Plasmids harboring mutants that exhibited >50% PHB accumulation were individually introduced into E. coli LS5218 to examine the accumulation level and monomer composition of P(3HB-co-3HHx). E. coli LS5218 recombinants were cultivated on M9 medium with 10 mM dodecanoate and 50 μg of ampicillin/ml at 37°C for 72 h (8). To determine the cellular P(3HB-co-3HHx) content and composition, ca. 30 mg of dry cells was subjected to methanolysis with a solution consisting of 1.7 ml of methanol, 0.3 ml of 98% sulfuric acid, and 2.0 ml of chloroform at 100°C for 140 min to convert the constituents to their methyl esters. The addition of 1 ml of water to the reaction mixture induced phase separation. The lower chloroform layer was used for gas chromatography (GC) analysis on a Shimadzu GC-17A system equipped with a Neutra Bond-1 capillary column (30 m by 0.25 mm) and a flame ionization detector (11).
Enzyme assay and electrophoresis.
Whole-cell extracts of the recombinant E. coli were prepared by sonication. In addition, each soluble fraction was obtained by centrifugation (18,000 × g, 4°C, 10 min). The synthase activity in each soluble fraction was determined spectrophotometrically by a discontinuous assay according to a previous method (9) with chemically synthesized (R)-3HB-CoA and 5,5′-dithiobis(2-nitrobenzoic acid). One unit of enzyme activity was defined as the amount required to catalyze the transformation of 1 mol of substrate/min/mg of total cellular proteins. The concentration of total cellular proteins was determined by the method of Bradford (3) by using a Bio-Rad Protein Assay kit (Hercules, Calif.) and bovine serum albumin as the standard.
The cell extracts containing 10 μg of soluble protein were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by the standard procedure. Western blot analysis of PhaCAc was carried out with a specific antiserum to the C-terminal oligopeptide of PhaCAc prepared previously (8).
Analytical prodecures of the P(3HB-co-3HHx) copolyester.
The copolyesters accumulated in the cells were extracted with chloroform for 48 h and purified by reprecipitation with hexane. Molecular mass data of copolyesters were obtained by gel permeation chromatography by using the Shimadzu 10A system with RID-10A refractive-index detector with serial columns of Shodex K802 and K806 M. Polystyrene standards with low polydispersity were used to construct a calibration curve (13). The 500-MHz 1H nuclear magnetic resonance spectra of a CDCl3 solution of copolyesters were obtained as described previously (11).
RESULTS
In vitro evolution of the synthase PhaCAc.
The in vitro evolution system for PhaCAc was constructed according to the scheme illustrated in Fig. 2. Basically, the system was designed so that the level of accumulated PHB or P(3HB-co-3HHx) copolyester was attributable only to the change in PhaCAc activity by maintaining the physiological and genetic background equivalent among mutants of PhaCAc. The plasmid, pBSEE32phbAB, is a bifunctional expression vector capable of synthesizing PHB homopolyester from excess glucose in strain JM109 and synthesizing P(3HB-co-3HHx) copolyester from a sole carbon source, dodecanoate, in strain LS5218 [fadR601 atoC(Con)]. For convenience of DNA manipulation (available restriction enzyme sites were restricted to PstI and XhoI), PCR random mutagenesis was targeted to the region (57% of total amino acids) encoding the N-terminal half, including the putative active center site (Cys319) of PhaCAc (6). Gene libraries were created by mutagenic PCR under conditions that averaged one to two amino acid substitutions per gene. Mutants of the phaCAc gene were conducted by colony (here termed “clone”) formation of transformant E. coli JM109 cells. The assay for detection of PHB accumulated within the cells was carried out semiquantitatively by a sensitive staining method employing Nile red (22). For more precise estimation of PHB content, E. coli cells harboring mutants screened by the plate assay described above were subjected to a crotonic acid conversion reaction system, followed by HPLC analysis.
Screening 8337 clones of the total libraries for PHB accumulation exhibited ca. 15% inactivation, corresponding to an optimal mutation rate of two to three nucleotides per sequence (32). All of the active clones thus obtained were roughly classified into three groups: similar (S), medium (M), and low (L), in which the levels of PHB accumulation were, respectively, similar to, slightly lower than, and clearly lower than the level of the wild-type clone. The numbers of clones belonging to the S, M, and L groups were 145, 4,350, and 2,755, respectively. All of the 145 group S clones and the arbitrarily selected clones belonging to group M (129) and group L (23), as well as the inactive clones (3), were subjected to HPLC analysis, allowing further precise estimation of the PHB accumulation level.
Figure 3 shows the distribution profile (the so-called fitness landscape [12]) of the PHB accumulation level for the whole mutant population (300 clones) selected arbitrarily. The fitness landscape obtained here shows that the wild-type enzyme is located near the top of the hill, apparently indicating that the present enzyme is well optimized for PHB accumulation.
FIG. 3.
Distribution pattern of the level of PHB accumulation of the random mutant population. The broken line indicates the average PHB accumulation by the wild-type clone. Ten mutants, which were subjected to synthase activity assay (see Fig. 4), are marked by arrows.
Acquirement of PhaCAc mutants with higher activities.
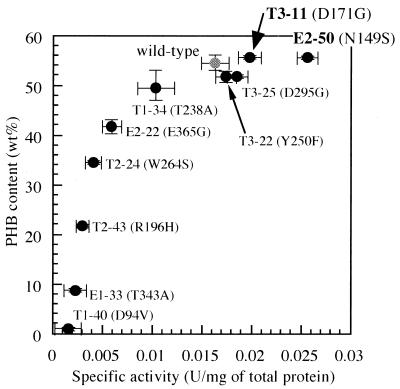
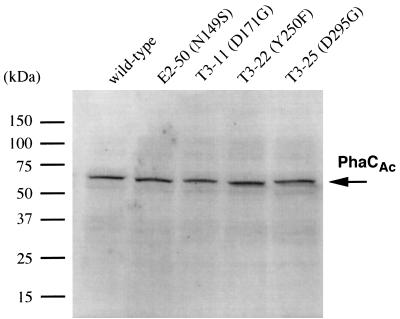
We assayed the synthase activities of 10 mutants (marked by arrows in Fig. 3), together with the wild-type enzyme. Figure 4 represents a biphasic pattern of the relationship between enzyme activity toward 3HB-CoA and PHB content in dry cells, keeping linearity up to a PHB content of ca. 45 wt% and then gradually reaching a plateau corresponding to a content of ca. 55 wt%. This suggests that insufficient monomer substrate is supplied for the wild-type and for mutants that exhibit activities exceeding 0.01 U/mg. PhbCRe activity was correlative with the level of PHB accumulation up to 50 wt% in dry cells. Almost the same expression level of the phaCAc gene was obtained among the wild type and mutants (E2-50, T3-11, T3-22, and T3-25) by Western blot analysis (Fig. 5), suggesting that a comparative study can be performed among these enzymes with regard to the specific activity. Thus, the improvement in the PhaCAc activity of mutants E2-50 and T3-11 prompted us to further examine their potential for enhanced accumulation of the P(3HB-co-3HHx) copolyester.
FIG. 4.
Correlation between PhaCAc activity and the level of PHB accumulation revealed by mutation experiment. CoA release activity toward (R)-3HB-CoA and PHB content was assayed for 10 mutant enzymes [T1-40 (D94V), E1-33 (T343A), T2-43 (R196H), T2-24 (W264S), E2-22 (E365G), T1-34 (T238A), T3-22 (Y250F), T3-25 (D295G), T3-11 (D171G), and E2-50 (N149S)] and the wild-type enzyme. The plots are indicated by a gray circle (for the wild type) and black circles (for the mutants). The data shown are means from three independent experiments.
FIG. 5.
Western blot analysis of recombinant production of the wild-type and mutants of PhaCAc. A 10-μg protein sample was subjected to SDS-PAGE. Western blot analysis was performed with the PhaCAc C-terminal specific antibody raised from rabbits. Indication of each sample is given in the corresponding lane.
Enhancement of PHA content and 3HHx fraction of the P(3HB-co-3HHx) copolyester.
Mutants, in which the level of PHB accumulation was higher than or identical to that in the wild type, were next applied to the P(3HB-co-3HHx) copolyester production system in strain LS5218. Table 1 shows the accumulation level and monomer composition of the PHA copolyester. The recombinant E. coli LS5218 harboring the wild-type phaCAc gene resulted in 10 mol% P(3HB-co-3HHX) accumulation with a 2 wt% PHA content in dry cells. As expected, two recombinants harboring the mutants, E2-50 and T3-11, exhibited enhancement of the PHA content in dry cells (6.5- and 3-fold higher than the content of the wild type, respectively). In addition, higher fractions of 3HHx in the PHA copolyester were observed in both recombinants: 18 mol% 3HHx for E2-50 and 16 mol% 3HHx for T3-11. No longer-chain 3HA unit such as C8 or C10 (carbon atoms) was detected by GC.
TABLE 1.
Accumulation of P(3HB-co-3HHx) in E. coli LS5218 recombinantsa and molecular weights of P(3HB-co-3HHx) copolyester samples
| Mutant (amino acid substitution) | Mean ± SDb
|
Molecular wtc
|
||
|---|---|---|---|---|
| PHA content (wt%) | 3HHx fraction (mol%) | Mn (104) | Mw/Mn | |
| Wild type | 2 ± 1 | 10 ± 1 | 98.4 | 2.6 |
| E2-13 (Q45L) | 3 ± 1 | 11 ± 1 | ||
| T1-34 (D67E) | 2 ± 1 | 12 ± 1 | ||
| E1-19 (V148A) | 1 ± 1 | 9 ± 1 | ||
| E2-50 (N149S) | 13 ± 3 | 18 ± 1 | 48.3 | 4.1 |
| E1-34 (M151T) | 1 ± 0 | 10 ± 0 | ||
| T3-27 (K165R) | 2 ± 0 | 9 ± 1 | ||
| T3-11 (D171G) | 6 ± 1 | 16 ± 0 | 75.0 | 3.5 |
| E1-27 (F203L) | 0.5 ± 0 | 11 ± 1 | ||
| T1-44 (T234S) | 2 ± 0 | 11 ± 0 | ||
| T3-22 (Y250F) | 2 ± 0 | 11 ± 0 | ||
| T3-25 (D295G) | 1 ± 0 | 11 ± 1 | ||
| T1-41 (G303S) | 3 ± 1 | 11 ± 1 | ||
| T1-12 (R335Q) | 2 ± 0 | 12 ± 0 | ||
Cells were cultivated in 100 ml of M9 medium (containing 10 mM sodium dodecanoate and 0.4% [vol/vol] Brij-35) for 72 h at 37°C.
The data shown are the means of three or four independent experiments.
Mn, number-average molecular weight; Mw, weight-average molecular weight; Mw/Mn, polydispersity.
In fact, for three copolyesters (wild type, E2-50, and T3-11) extracted from recombinant E. coli strains, their number-average molecular weights were determined to be 48.3 × 104 to 98.4 × 104 by gel permeation chromatography analysis. It was also found that the PHA compositions of 3HB and 3HHx obtained by GC analysis were almost identical to those obtained by 1H nuclear magnetic resonance analysis.
Functional mapping of amino acid residues of PhaCAc correlated with PHA accumulation level.
Nucleotide sequence analysis revealed that various mutants carrying one to three point mutations were evenly distributed over the targeted region. The ratio of transitions and transversions was four to six. As for nonsynonymous mutations, there were 55 single mutants, 17 were double mutants, and 5 were triple mutants, and 15 clones had in multiples the same mutations found in single mutants. Figure 6 shows the positions (marked by bars) where amino acid residues were substituted for 40 clones carrying single mutations selected arbitrarily from the mutant libraries. All of the clones were divided into three groups in order of PHB accumulation level, as follows: group A, <10 wt%; group B, 10 to 50 wt%; and group C, >50 wt% (see corresponding panels Fig. 6A to C). The α/β hydrolase fold region (16), which is commonly found among various synthases (across classes I to III), was also assigned to positions 270 to 516 of PhaCAc from analysis with Pfam as a domain database (2). The detailed functional mapping is presented below.
FIG. 6.
Functional mapping of amino acid residues responsible for PhaCAc activity based on PHB accumulation level. (A to C) Functional mapping profiles are arranged by dividing into the three respective groups, A to C, based on the PHB accumulation level. (D) Predicted hydropathy profile of PhaCAc. Amino acid-substituted positions, which were identified in this study, are marked with vertical bars on the box corresponding to the primary sequence of PhaCAc. Vertical broken lines in the box are the target region (positions 36 to 372) for error-prone PCR. A region consisting of ca. 250 amino acids carrying an “α/β hydrolase fold” (16) is commonly shared with all synthases belonging to classes I to III and is assigned to positions 270 to 516 of PhaCAc. The corresponding region is shaded. A putative active site residue, cysteine (C319, marked by a large solid triangle), is located within the α/β hydrolase fold region. The N-terminal nonconserved region (positions 1 to 93) is dotted. On each bar, the amino acid substitution is attached, with the average level of the PHB accumulation given in parentheses. Based on the multiple alignment of amino acid sequences of various PHA synthases, mutations falling into highly conserved positions are denoted by a small closed triangle on the top of each bar. Mutations leading to a markedly lowered level of PHB accumulation are denoted with single (1 to 9%) or double (0%) closed circles at the top of the bar (group A), whereas mutations leading to the barely influenced level of PHB accumulation by mutation are denoted by an open circle at the top of the bar (group C). In panel D, the mutation position of R177H (T1-38) is indicated at the corresponding position by an arrowhead.
DISCUSSION
Our final goal is to develop a methodology for economically feasible production of PHA that would be practical and use environment-friendly materials. To achieve this, there have been many attempts by genetic engineering to use PHA biosynthesis genes and synthase and monomer-supplying enzymes (24). To further optimize the PHA production system, we initiated the in vitro evolution of enzymes related to PHA biosynthesis. An initial trial was carried out to establish an in vivo assay system for the key enzyme, PHA synthase (derived from R. eutropha), based on the linear relationship between the synthase activity and the PHB production level (28, 29, 29a). This allowed the synthase activity to be readily estimated in vivo by monitoring the level of PHB accumulation in E. coli JM109. In contrast, in the case of PhaCAc, the relationship between synthase activity and PHB accumulation showed a biphasic profile in the range measured (Fig. 3). This difference points to the fact that sufficient supplying of 3HB-CoA monomer substrate should be required for reliable acquirement of higher active mutant, as can be seen in the case of R. eutropha synthase (28, 29, 29a). Therefore, enhancement in the supplying ability of the 3HB-CoA generated by PhbABRe enzymes would enable us to readily acquire the higher active mutant. This point should be taken into consideration for the construction of this type evolution strategy, in which PHA biosynthesis is governed by the association of the synthase reaction with the monomer-supplying pathway.
When strain LS5218 was used as the host, positive selection of the target mutants, such as E2-50 and T3-11, could be achieved, possibly due to the sufficient supplying of monomers for P(3HB-co-3HHx) synthesis, although for strain JM109 their PHB contents were estimated to be almost identical to that of the wild type. Plasmid pBSEE32phbAB is a bifunctional expression vector capable of synthesizing both PHB and PHA copolyester. For PHB biosynthesis, 3HB-CoA monomer substrate may mainly be supplied via the PhbABRe-mediated acetyl-CoA dimerization pathway in strain JM109. On the other hand, in strain LS5218, 3HHx-CoA may be channeled mostly by the PhaJAc-mediated β-oxidation pathway into the biosynthesis of P(3HB-co-3HHx) copolyester. It might be argued that both of the above pathways function to generate flux of the 3HB-CoA constituent of the PHA copolyester. In our previous study (8) a vector construct harboring only the phaPCJAc gene cluster (5) synthesized 7 mol% P(3HB-co- 3HHx) under the same culture conditions as were employed here. Almost the same 3HHx fraction in the PHA copolyester was obtained regardless of the contribution from the phbABRe pathway. These findings strongly suggest that the PhaJAc-mediated β-oxidation pathway is a major supplier of 3HB-CoA monomer for the PHA copolyester.
Increased 3HHx fractions in P(3HB-co-3HHx) were observed for both of the evolved mutants, E2-50 and T3-11, which led to enhanced PHA contents (Table 1). In our previous study, the expression level of the wild-type phaCAc gene was enhanced by the presence of the phaPAc gene, consequently giving rise to a higher PHA content and 3HHx fraction in the accumulated P(3HB-co-3HHx) in two other recombinants of A. caviae (6) and R. eutropha (5, 13). It has been unclear whether such a concomitant relationship between PHA content and the 3HHx fraction can be accounted for by either PhaCAc function alone or PhaPAc-assisted PhaCAc activation (6). In the present study, PhaPAc function did not vary among the mutants employed. Accordingly, PhaCAc function is presumed to be a primary contributor to the enhancement of PHA content and the 3HHx fraction, whereas PhaPAc is not a direct positive effector in itself. However, this concomitant relationship is not always evident. In fact, a mutant producing PHA copolyester with a much lower 3HHx fraction (or a much higher 3HB fraction) could be isolated with no decrease in PHA content. This suggests that compositional variation upon copolymerization could also be controlled to a certain extent by artificial alteration of PhaCAc.
As to functional mapping based on mutational variation, the following discussion can be done. In group A (<10 wt% of PHB accumulation in dry cells), it is feasible that many of the mutations yielded deleterious effects on PHB accumulation ability, since amino acids at their substituted positions are highly conserved among all synthases. Cysteine at position 319 (C319) has been considered essential for covalent catalysis with respect to multiple sequence alignment (15, 17, 24, 34), and there is experimental evidence that replacement by alanine leads to inactivation of the PhaCAc (6). This finding is supported by the mutation (C319A) obtained here. Similarly, other positions important for proper structure formation and/or activity exhibition of the PhaCAc could be defined by mutations such as D94V, N160Y, L182S, Y251C, and T349A. Among mutants categorized in group C (>50 wt% of PHB accumulation), one can know neutral or beneficial mutations for PHB accumulation. Evolved mutants (E2-50 and T3-11) exhibiting higher synthase activities than that of the wild type were found to possess the single mutations N149S and D171G, respectively. Neither of these positions is strictly conserved for amino acid substitution. Interestingly, a mutation at position 203 (F203L), which is highly conserved in class I and II synthases, had no negative effect on PHB accumulation. The mutations listed in group B (10 to 50 wt% of PHB accumulation) are concentrated with a 60% population in the region ranging from 160 to 220 amino acid positions, a finding which corresponds to a predicted hydrophobic portion (shaded region in the hydropathy profile of Fig. 6D). Mutant T1-38, which belongs to group B, exhibited extremely unstable PHB accumulation, ranging from 5 to 48 wt%, as a result of the R177H mutation. Thus, it is likely that this mutation causes structural fluctuation of the phaCAc gene product within the bacterial cell. Throughout the three groups, the nonconserved region (positions 1 to 93) seems relatively tolerant to amino acid substitutions in terms of PHB accumulation ability. This is coincident with the results revealed by analysis of a truncated R. eutropha PHA synthase lacking 36 or even 100 amino acids (20). These findings suggest the reliability of in vivo functional mapping of PhaCAc based on the level of PHB accumulation.
It can be demonstrated in part that in vivo functional mapping provides some insights for examining amino acid residues that are related to the activity and protein structuring of PhaCAc. A comparison of the X-ray crystal structures of the wild-type and mutant enzymes obtained here elucidates some of the mechanisms by which the evolved enzymes are activated, but at present these structures are not yet available because of the difficulty of overproduction of the active form of PhaCAc. In this initial study, random mutations were introduced into a limited region (the N-terminal half). The C-terminal half of PhaCAc should also be targeted to explore beneficial mutations for improvements of the enzyme properties. The next project is directed to further accumulation of beneficial mutations, saturation mutagenesis at hot spots for enzyme improvement (26), and recombination of sets of improved genes.
In summary, the in vitro evolution of PHA synthase (PhaCAc) has extended the capacity for enhanced accumulation and changed the monomer composition of PHA copolyester by using a catalytic function not available from a natural source. This approach allowed us to engineer the catalytic properties of the synthase from which we have no tertiary structure and very little knowledge of the catalytic mechanism. Furthermore, the in vitro evolution strategy is generally applicable to explore any other properties, such as thermostability and substrate specificity, of any enzymes related to PHA biosynthesis.
Acknowledgments
We thank Y. Ichikawa and R. Nakazawa for DNA sequencing (Bioarchitect Research Group, RIKEN Institute).
This research was financially supported by a grant for Ecomolecular Science Research to RIKEN Institute and a President's Special Research Grant (to S.Taguchi) funded by the RIKEN Institute.
REFERENCES
- 1.Arnold, F. H. 1998. Design by directed evolution. Acc. Chem. Res. 31:125-131. [Google Scholar]
- 2.Bateman, A., E. Birney, R. Durbin, S. R. Eddy, K. L. Howe, and E. L. L. Sonnhammer. 2000. The Pfam protein families database. Nucleic Acids Res. 28:263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Doi, Y., S. Kitamura, and H. Abe. 1995. Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 28:4822-4828. [Google Scholar]
- 5.Fukui, T., and Y. Doi. 1997. Cloning and analysis of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) biosynthesis genes of Aeromonas caviae. J. Bacteriol. 179:4821-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukui, T., T. Kichise, T. Iwata, and Y. Doi. 2001. Characterization of 13 kDa granule-associated protein in Aeromonas caviae and biosynthesis of polyhydroxyalkanoates with altered molar composition by recombinant bacteria. Biomacromolecules 2:148-153. [DOI] [PubMed] [Google Scholar]
- 7.Fukui, T., N. Shiomi, and Y. Doi. 1998. Expression and characterization of (R)-specific enoyl-coenzyme A hydratase involved in polyhydroxyalkanoate biosynthesis by Aeromonas caviae. J. Bacteriol. 180:667-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukui, T., S. Yokomizo, G. Kobayashi, and Y. Doi. 1999. Co-expression of polyhydroxyalkanoate synthase and (R)-enoyl-CoA hydratase genes of Aeromonas caviae establishes copolyester biosynthesis pathway in Escherichia coli. FEMS Microbiol. Lett. 170:69-75. [DOI] [PubMed] [Google Scholar]
- 9.Gerngross, T. U., K. D. Snell, O. P. Peoples, A. J. Sinskey, E. Csuhai, S. Masamune, and J. Stubbe. 1994. Overexpression and purification of the soluble polyhydroxyalkanoate synthase from Alcaligenes eutrophus: evidence for a required posttranslational modification for catalytic activity. Biochemistry 33:9311-9320. [DOI] [PubMed] [Google Scholar]
- 10.Karr, D. B., J. K. Waters, and D. W. Emerich. 1983. Analysis of poly-β-hydroxybutyrate in Rhizobium japonicum bacteroids by ion-exclusion high-pressure liquid chromatography and UV detection. Appl. Environ. Microbiol. 46:1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato, M., H. J. Bao, C. K. Kang, T. Fukui, and Y. Doi. 1996. Production of a novel copolyester of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids by Pseudomonas sp. 61-3 from sugars. Appl. Microbiol. Biotechnol. 45:363-370. [Google Scholar]
- 12.Kauffman, S., and S. Levin. 1987. Towards a general theory of adaptive walks on rugged landscapes. J. Theor. Biol. 128:11-45. [DOI] [PubMed] [Google Scholar]
- 13.Kichise, T., T. Fukui, Y. Yoshida, and Y. Doi. 1999. Biosynthesis of polyhydroxyalkanoates (PHA) by recombinant Ralstonia eutropha and effects of PHA synthase activity on in vivo PHA biosynthesis. Int. J. Biol. Macromol. 25:69-77. [DOI] [PubMed] [Google Scholar]
- 14.Matsusaki, H., H. Abe, K. Taguchi, T. Fukui, and Y. Doi. 2000. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyalkanoates) by recombinant bacteria expressing the PHA synthase gene phaC1 from Pseudomonas sp. 61-3. Appl. Microbiol. Biotechnol. 53:401-409. [DOI] [PubMed] [Google Scholar]
- 15.Müh, U., A. J. Sinskey, D. P. Kirby, W. S. Lane, and J. Stubbe. 1999. PHA synthase from Chromatium vinosum: cysteine 149 is involved in covalent catalysis. Biochemistry 38:826-837. [DOI] [PubMed] [Google Scholar]
- 16.Ollis, D. L., E. Cheah, M. Cygler, B. Dijkstra, F. Frolow, S. M. Franken, M. Harel, S. J. Remington, I. Silman, J. Schrag, J. L. Sussman, K. H. G. Verschueren, and A. Goldman. 1992. The α/β hydrolase fold. Protein Eng. 5:197-211. [DOI] [PubMed] [Google Scholar]
- 17.Rehm, B. H. A., and A. Steinbüchel. 1999. Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int. J. Biol. Macromol. 25:3-19. [DOI] [PubMed] [Google Scholar]
- 18.Salanitro, J. P., and W. S. Wegener. 1971. Growth of Escherichia coli on short-chain fatty acids: growth characteristics of mutants. J. Bacteriol. 108:885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Schubert, P., N. Krüger, and A. Steinbüchel. 1991. Molecular analysis of the Alcaligenes eutrophus poly(3-hydroxybutyrate) biosynthesis operon: identification of the N terminus of poly(3-hydroxybutyrate) synthase and identification of the promoter. J. Bacteriol. 173:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimamura, E., K. Kasuya, G. Kobayashi, T. Shiotani, Y. Shima, and Y. Doi. 1994. Physical properties and biodegradability of microbial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 27:878-880. [Google Scholar]
- 22.Spiekermann, P., B. H. A. Rehm, R. Kalscheuer, D. Baumeister, and A. Steinbüchel. 1999. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 171:73-80. [DOI] [PubMed] [Google Scholar]
- 23.Spratt, S. K., C. L. Ginsburgh, and W. D. Nunn. 1981. Isolation and genetic characterization of Escherichia coli mutants defective in propionate metabolism. J. Bacteriol. 146:1166-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinbüchel, A., and S. Hein. 2001. Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv. Biochem. Eng. Biotechnol. 71:81-123. [DOI] [PubMed] [Google Scholar]
- 25.Sudesh, K., H. Abe, and Y. Doi. 2000. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci. 25:1503-1555. [Google Scholar]
- 26.Taguchi, S., S. Komada, and H. Momose. 2000. The complete amino acid substitutions at positional 131 that are positively involved in cold adaptation of subtilisin BPN′. Appl. Environ. Microbiol. 66:1410-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taguchi, S., K. Kuwasako, A. Suenaga, M. Okada, and H. Momose. 2000. Functional mapping against Escherichia coli for the broad-spectrum antimicrobial peptide, thanatin, based on an in vivo monitoring assay system. J. Biochem. 128:745-754. [DOI] [PubMed] [Google Scholar]
- 28.Taguchi, S., A. Maehara, K. Takase, M. Nakahara, H. Nakamura, and Y. Doi. 2001. Analysis of mutational effects of polyhydroxybutyrate (PHB) polymerase on bacterial PHB accumulation using an in vivo assay system. FEMS Microbiol. Lett. 198:65-71. [DOI] [PubMed] [Google Scholar]
- 29.Taguchi, S., A. Maehara, K. Takase, M. Nakahara, H. Nakamura, and Y. Doi. 2001. In vivo assay system of the polymerase as a key enzyme for PHA biosynthesis. RIKEN Rev. 42:3-6. [Google Scholar]
- 29a.Taguchi, S., H. Matsusaki, K. Matsumoto, K. Takase, K. Taguchi, and Y. Doi. Biosynthesis of biodegradable polyesters from renewable carbon sources by recombinant bacteria. Polym. Int., in press.
- 30.Taguchi, S., K. Nakagawa, M. Maeno, and H. Momose. 1994. In vivo monitoring system for structure-function relationship analysis of the antibacterial peptide apidaecin. Appl. Environ. Microbiol. 60:3566-3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taguchi, S., A. Ozaki, and H. Momose. 1998. Engineering of a cold-adapted protease by sequential random mutagenesis and a screening system. Appl. Environ. Microbiol. 64:492-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taguchi, S., A. Ozaki, K. Nakagawa, and H. Momose. 1996. Functional mapping of amino acid residues responsible for antibacterial action of apidaecin. Appl. Environ. Microbiol. 62:4652-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taguchi, S., A. Ozaki, T. Nonaka, Y. Mitsui, and H. Momose. 1999. A cold-adapted protease engineered by experimental evolution system. J. Biochem. 126:689-693. [DOI] [PubMed] [Google Scholar]
- 34.Wodzinska, J., K. D. Snell, A. Rhomberg, A. J. Sinskey, K. Biemann, and J. Stubbe. 1996. Polyhydroxybutyrate synthase: evidence for covalent catalysis. J. Am. Chem. Soc. 118:6319-6320. [Google Scholar]
- 35.Yanisch-Perron, C., C. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 36.Zhao, H., and F. H. Arnold. 1999. Directed evolution converts subtilisin E into a functional equivalent of thermitase. Protein Eng. 12:47-53. [DOI] [PubMed] [Google Scholar]