Abstract
dl-2-amino-Δ2-thiazolin-4-carbonic acid (dl-ATC) is a substrate for cysteine synthesis in some bacteria, and this bioconversion has been utilized for cysteine production in industry. We cloned a DNA fragment containing the genes involved in the conversion of l-ATC to l-cysteine from Pseudomonas sp. strain BS. The introduction of this DNA fragment into Escherichia coli cells enabled them to convert l-ATC to cysteine via N-carbamyl-l-cysteine (l-NCC) as an intermediate. The smallest recombinant plasmid, designated pTK10, contained a 2.6-kb insert DNA fragment that has l-cysteine synthetic activity. The nucleotide sequence of the insert DNA revealed that two open reading frames (ORFs) encoding proteins with molecular masses of 19.5 and 44.7 kDa were involved in the l-cysteine synthesis from dl-ATC. These ORFs were designated atcB and atcC, respectively, and their gene products were identified by overproduction of proteins encoded in each ORF and by the maxicell method. The functions of these gene products were examined using extracts of E. coli cells carrying deletion derivatives of pTK10. The results indicate that atcB and atcC are involved in the conversion of l-ATC to l-NCC and the conversion of l-NCC to cysteine, respectively. atcB was first identified as a gene encoding an enzyme that catalyzes thiazolin ring opening. AtcC is highly homologous with l-N-carbamoylases. Since both enzymes can only catalyze the l-specific conversion from l-ATC to l-NCC or l-NCC to l-cysteine, it is thought that atcB and atcC encode l-ATC hydrolase and N-carbamyl-l-cysteine amidohydrolase, respectively.
l-cysteine has been widely used in medicines, cosmetics, and food additives. It has mainly been produced via acid or alkaline hydrolysis of human and animal hairs. However, these methods result in low yields and give rise to unpleasant odors and problems of waste treatment (11). In addition, products extracted from hair do not qualify for medical use because of sanitary problems.
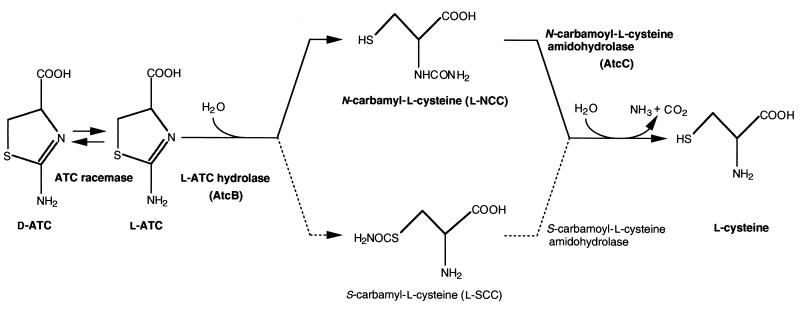
A bioconversion, instead of hydrolysis of hairs, for producing l-cysteine has also been developed (14, 15). Some bacteria, especially in the genus Pseudomonas (14, 15, 21), have potent activities of asymmetrical hydrolysis of dl-2-amino-Δ2-thiazolin-4-carbonic acid (dl-ATC) to form l-cysteine. dl-ATC is a desirable substrate for the bioconversion since the method for chemical synthesis of dl-ATC is not complicated (14, 15). The total cost for l-cysteine synthesis using dl-ATC is favorable for the large-scale production in industry because the price of dl-ATC is less than that of l-cysteine. Cultured bacteria have been used for commercial production of l-cysteine. As summarized in Fig. 1, bacterial conversion of dl-ATC to l-cysteine consists of the following three successive steps (21): (i) enzymatic racemization of d-ATC to l-ATC; (ii) a ring-opening hydrolysis of l-ATC with an enzyme called ATC hydrolase, to produce either N-carbamyl-l-cysteine (l-NCC) or S-carbamyl-l-cysteine (l-SCC) as intermediates; and (iii) hydrolysis of these intermediates to l-cysteine with an enzyme. As a result, the whole racemic ATC is converted to l-cysteine.
FIG. 1.
The process of bioconversion from dl-ATC to l-cysteine. Solid lines indicate a metabolic pathway in Pseudomonas species, including the BS strain used in this study (21). Dotted lines indicate an alternative pathway (14, 15).
In order to investigate the molecular mechanisms of the reactions catalyzed by Pseudomonas sp. strain BS, we cloned the genes involved in the conversion of l-ATC to l-cysteine. l-ATC was hydrolyzed to l-NCC by a gene product of atcB, and subsequent hydrolysis from l-NCC to l-cysteine was catalyzed by l-NCC amidohydrolase encoded by atcC.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strain BS (FERM P-16965, Fermentation Research Institute, Agency of Industrial Science and Technology, Japanese Ministry of International Trade and Industry), which is able to convert dl-ATC to l-cysteine, was isolated from soil and identified as a Pseudomonas sp. by the methods described by Sano et al. (15). Escherichia coli DH5α (12) was used as the host strain for cloning and subcloning of DNA fragments isolated from strain BS. E. coli BL21(DE3) (19) and pT7-7 plasmids were used for overexpression of proteins under the control of the T7 promoter (19). pBluescript SK+ (pBS-SK; Stratagene) was used as a cloning and an expression vector.
Media, culture conditions, and chemicals.
Strain BS was grown in Luria-Bertani (LB) medium (12) at 30°C. E. coli DH5α cells carrying the genomic DNA library of strain BS were grown in M9 agar plates containing 0.1% dl-ATC as the sole nitrogen source instead of NH4Cl and supplemented with thiamine (1 μg/ml) as a means of selection for gene cloning. For general purpose, E. coli DH5α cells carrying recombinant plasmids were grown in LB medium containing ampicillin (100 μg/ml). dl-ATC, l-ATC, and d-ATC were kindly donated by Nihon-Rikagakuyakuhin Co. and Ajinomoto Co. (Tokyo, Japan). l-NCC and N-carbamyl-d-cysteine (d-NCC) were purchased from Wako Chemicals Co., Osaka, Japan.
Cloning of genes encoding enzymes involved in l-cysteine production from dl-ATC.
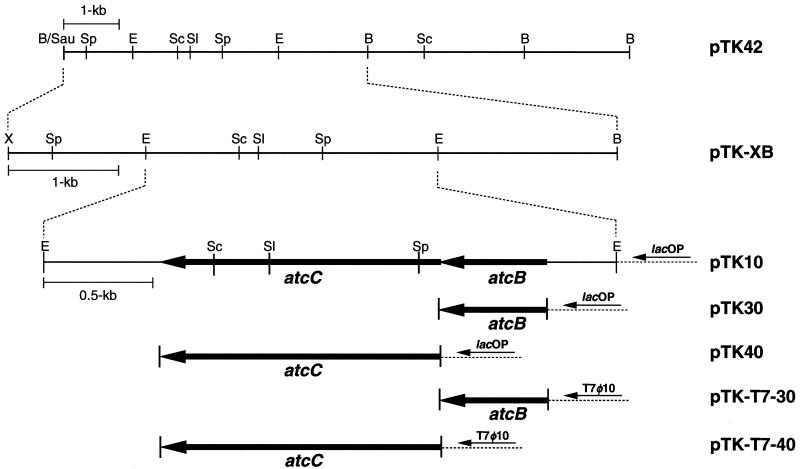
The experimental approach used for cloning of the genes involved in the conversion of dl-ATC to l-cysteine was to select the clones of the transformed E. coli cells that had the ability to utilize dl-ATC as a nitrogen source. Since E. coli does not grow on a medium containing dl-ATC as the sole nitrogen source, it was a feasible host for this experimental approach. The genomic DNA of strain BS was partially digested with Sau3AI, and 6- to 10-kb DNA fragments were separated by sucrose gradient centrifugation (12). The DNA fragments were ligated into the BamHI site of pBS-SK. E. coli DH5α was transformed by using ligated DNA, and the cells were spread onto an LB agar plate containing ampicillin (100 μg/ml) and 5-bromo-4-chloro-3-indolyl-β-d(−)-galactopyranoside (X-Gal) (20 μg/ml). White colonies were replicated to M9 selective plates, described above, and incubated at 37°C for a few days. Recombinant clones having the ability to utilize dl-ATC as a nitrogen source were expected to form colonies. This process yielded two clones, one of which was designated pTK42 and was used in further studies (Fig. 2).
FIG. 2.
Restriction map of a 10-kb insert DNA fragment of pTK42 and structures of its deletion derivatives. ORF1 and ORF2 are located downstream of the lac promoter in the pBS-SK vector (lacOP) in the same directions in pTK10, pTK30, and pTK40. In pTK-T7-30 and pTK-T7-40, ORF1 and ORF2 are located downstream of the T7 promoter in the pT7-7 expression vector (T7φ10) (19) in the same directions, respectively. Insert DNAs were prepared by PCR amplification with upstream primers p-30-U (5′-AATCTAGAGGAGCAGCATGAGTGATAAGCCAT-3′) for pTK30 and p-30T7-U (5′-GGAATTCCATATGAGTGATAAGCCATTGCACCC-3′) for pTK-T7-30 and with a common downstream primer, p-30-D (5′-AAGGATCCTTGCGGCTGGATGAGCGGCG-3′), for pTK30 and pTK-T7-30. For pTK40 and pTK-T7-40, insert DNAs were amplified with upstream primers p-40-U (5′-AATCTAGAGGTCAGCAATGAGCGTAATTGAA-3′) and p-40T7-U (5′-GGAATTCCATATGAGCGTAATTGAAAACACGACCC-3′), respectively, and with a common downstream primer, p-40-D (5′-AAGGATCCATCGGCGTCGGCTGCAAAGT-3′). The amplified DNA fragments were inserted into XbaI- and BamHI-digested pBS-SK and NdeI- and BamHI-digested pT7-7 (19). Arrows indicate the region and direction of each ORF and promoters. Abbreviations for restriction enzymes: B, BamHI; E, EcoRI; Sau, Sau3AI; Sc, SacI; Sl, SalI; Sp, SphI; X, XbaI.
Identification of gene products.
Proteins encoded in the insert DNA fragment carried on pTK10, a deletion derivative of pTK42 (Fig. 2), were identified by overproduction of gene products and by the maxicell method (13).
For overproduction of proteins, DNA fragments containing open reading frame 1 (ORF1) and ORF2 were amplified by PCR and inserted into NdeI- and BamHI-digested pT7-7 (19), which has the T7 promoter as described in the legend of Fig. 2. The resultant plasmid pTK-T7-30 or pTK-T7-40 has ORF1 or ORF2 downstream of the T7 promoter, and the gene products of these ORFs could be overproduced in E. coli strain BL-21(DE3), which expresses T7 RNA polymerase. Five-milliliter cultures of BL-21(DE3) harboring pTK-T7-30, pTK-T7-40, or pT7-7 (control vector) were grown to the mid-logarithmic phase (optical density at 600 nm = 0.6), and isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration, 0.4 mM) was added to induce the expression of T7 RNA polymerase. After 3 h of incubation at 37°C, cells were harvested by centrifugation and disrupted by sonication (Sonifier 450D; Branson) for 3 min at output 3. The disrupted cells were centrifuged at 10,000 × g for 30 min, and the resultant supernatants were collected. Proteins in the supernatant were separated by sodium dodecyl sulfate-14% polyacrylamide gel electrophoresis (SDS-14% PAGE) and visualized by Coomassie brilliant blue staining.
For the maxicell method, E. coli DH5α harboring either pTK10 or pBS-SK was cultured in M9 medium and exposed to UV radiation at a lethal dose. After 12 h of incubation with d-cycloserine, Pro-mix l-[35S] in vitro cell labeling mix (Amersham Pharmacia Biotech) was added to the culture, and plasmid-encoding proteins were specifically labeled. The labeled proteins were analyzed by SDS-12.5% PAGE and visualized using a radio image analyzer (BAS2000; Fujix).
Assay for l-cysteine synthetic activity, including activities of l-ATC hydrolase and l-NCC amidohydrolase.
To prepare a crude extract containing l-ATC hydrolase and/or l-NCC hydrolase activities, E. coli cells harboring pTK10, pTK30, or pTK40 were collected by centrifugation and disrupted by sonication (Sonifier 450D; Branson). The disrupted cells were centrifuged at 10,000 × g for 30 min, and the supernatants were collected. The resultant supernatant was added to the standard reaction mixture containing buffer A (50 mM Tris-HCl [pH 8.0], 10 mM dithiothreitol, 2.5 mM hydroxylamine) and 0.5% dl-ATC as a substrate. Reactions were performed at 30°C and stopped by the addition of trichloroacetic acid (final concentration, 5%). Hydrolyzed products of dl-ATC in the reaction mixtures were separated by a cellulose thin-layer chromatography (TLC) plate (Merck) developed with a solvent of 1-butanol-acetic acid-water at the ratio of 2:1:1 (21) and visualized by iodine or ninhydrin staining. The Gaitonde ninhydrin method was used for quantitative analysis of cysteine synthetic activity (3). Cysteine concentration in the reaction mixture was colorimetrically measured by the absorbance at 560 nm.
Other procedures.
The nucleotide sequence was determined by the dideoxy chain termination method with some specific primers using an ABI PRISM Big-Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems) and an ABI PRISM 310 Genetic Analyzer (Applied Biosystems).
Nucleotide sequence accession number.
The nucleotide sequence data reported in this work will appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession number AB070707.
RESULTS
Cloning of genes encoding enzymes involved in l-cysteine production from dl-ATC.
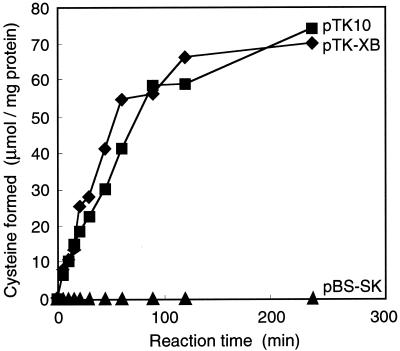
The experimental approach used for cloning of the genes involved in the conversion of dl-ATC to l-cysteine was to select the clones of the transformed E. coli cells that had the ability to utilize dl-ATC as a nitrogen source. E. coli transformants can grow on a medium containing dl-ATC if they have an activity to hydrolyze l-NCC or l-SCC, resulting in NH3. A Sau3AI partially digested genomic DNA library of strain BS was constructed by insertions within the BamHI site of pBS-SK. Approximately 4,000 recombinant clones were screened for their ability to form a colony on the selective plate containing dl-ATC. Two clones formed colonies, and they were also ampicillin resistant. Plasmid DNAs were prepared from these colonies and analyzed by restriction enzyme digestion. The fact that the patterns of the two plasmids were exactly the same indicated that both isolated insert DNAs represent the same region. Figure 2 shows the restriction map of one of the cloned DNAs (pTK42) and its deletion derivatives. Cysteine synthetic activity was detected by the Gaitonde ninhydrin method in the crude extract of E. coli harboring pTK-XB and pTK10 (Fig. 3). No cysteine synthetic activity was observed when d-ATC was used as a substrate (data not shown; see also Fig. 8).
FIG. 3.
Cysteine synthetic activity of E. coli carrying the deletion derivatives of pTK42. E. coli cells carrying pTK-XB, pTK10, or pBS-SK were used for preparing crude extracts. The cysteine synthetic reactions were performed at 30°C in 1-ml reaction mixtures each containing 0.5% dl-ATC. Synthesized cysteine was measured by the Gaitonde ninhydrin method (3).
FIG. 8.
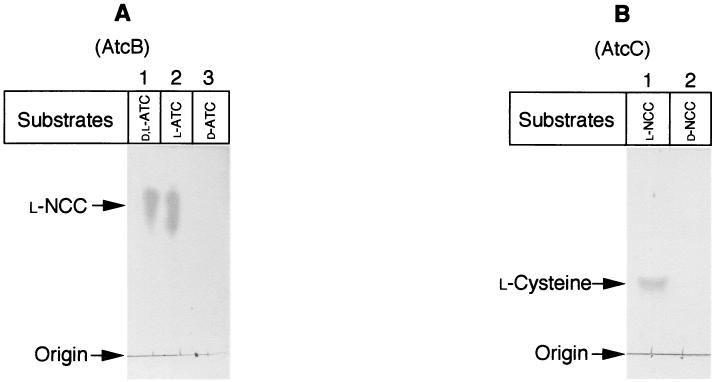
The l-specific conversion from l-ATC to l-NCC by AtcB (A) and from l-NCC to l-cysteine by AtcC (B). Crude extracts (20 μg) of E. coli cells carrying pTK30 (A) or pTK40 (B) were mixed with dl-ATC (lane 1), l-ATC (lane 2), and d-ATC (lane 3) (A) or l-NCC (lane 1) and d-NCC (lane 2) (B). Reactions were performed in 100-μl reaction mixtures containing buffer A with 0.5% substrates and incubated at 30°C for 1 h. Two microliters of the reaction mixture was spotted onto a TLC plate, and hydrolyzed products were analyzed and visualized by iodine staining (A) or ninhydrin staining (B).
DNA and amino acid sequence analyses.
The nucleotide sequence of the 3.8-kb region of pTK10 (Fig. 2) was determined as shown in Fig. 4. Analysis of the nucleotide sequence revealed two putative ORFs, designated ORF1 and ORF2. Since these ORFs were in the same orientation and 4 bp, including the stop codon of ORF1 and the initiation codon of ORF2, were overlapped, they may constitute an operon. ORF1 and ORF2 are composed of 519 and 1,260 bp, respectively. ORF1 and ORF2 encode 173 and 420 amino acids, respectively, and each ORF was preceded by possible ribosome-binding sites (underlined in Fig. 4). Upstream of ORF1, sequences similar to the −10 and −35 consensus sequences for E. coli σ70-dependent promoters were found (underlined in Fig. 4).
FIG. 4.
Nucleotide sequence of the 3.8-kb region of pTK10. Nucleotides and amino acids are numbered at the right of these sequence lines. Sequences similar to the −10 and −35 consensus sequences for E. coli promoters and ribosome-binding sites (RBS) are underlined.
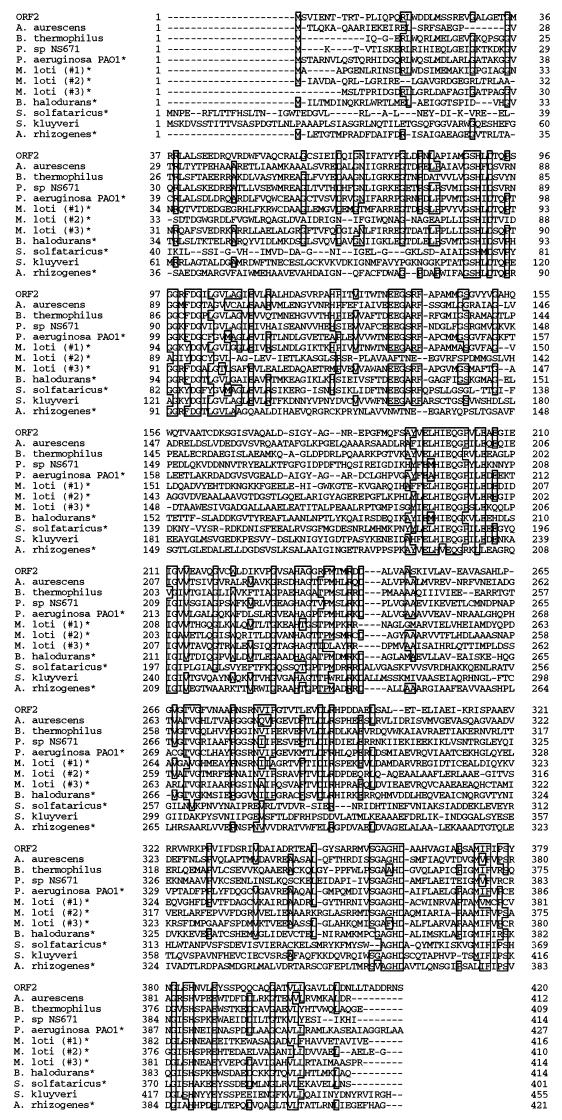
A computer-aided homology search using the FASTA program (10) revealed that ORF2 is homologous with N-carbamoyl-l-amino acid amidohydrolases (l-N-carbamoylase), including N-carbamoyl-β-alanine amidohydrolase, from Arthrobacter aurescens (23), Bacillus stearothermophilus (2, 9), Pseudomonas sp. NS671 (22), and Saccharomyces kluyveri (Z. Gojkovic, M. Sandrini, and J. Piskur, unpublished results [GenBank accession number AF333185]). ORF2 is also homologous with putative l-N-carbamoylase obtained in the genome sequence projects. Figure 5 shows the multiple alignment of five amino acid sequences of l-N-carbamoylase that have been identified by their enzymatic activity and seven amino acid sequences of putative l-N-carbamoylase ordered by the best homology scores (10). The sequence identities among these 10 sequences range from 31.7 to 47.2%.
FIG. 5.
Multiple alignment of the amino acid sequences of ORF2 and l-N-carbamoylase. The amino acid sequence of ORF2 was aligned with those of other known and putative l-N-carbamoylases from A. aurescens (41.4% identical) (23), B. stearothermophilus (37.1% identical) (2, 9), Pseudomonas sp. strain NS671 (31.7% identical) (22), Pseudomonas aeruginosa PAO1 (47.2% identical) (18), Mesorhizobium loti (41.6% [#1], 44.4% [#2], and 39.5% [#3] identical) (5), Bacillus halodurans (35.0% identical) (20), Sulfolobus solfataricus (37.6% identical) (16), S. kluyveri (41.5% identical) (Z. Gojkovic, M. Sandrini, and J. Piskur, unpublished results [GenBank accession number AF333185]), and Agrobacterium rhizogenes (36.8% identical) (8). Since M. loti has three homologous enzymes, they were identified as #1 to #3. Multiple alignment was done by GENETYX-MAC (Hitachi Software Co. Ltd.). Putative enzymes are indicated by asterisks.
Identification of gene products of the ORFs.
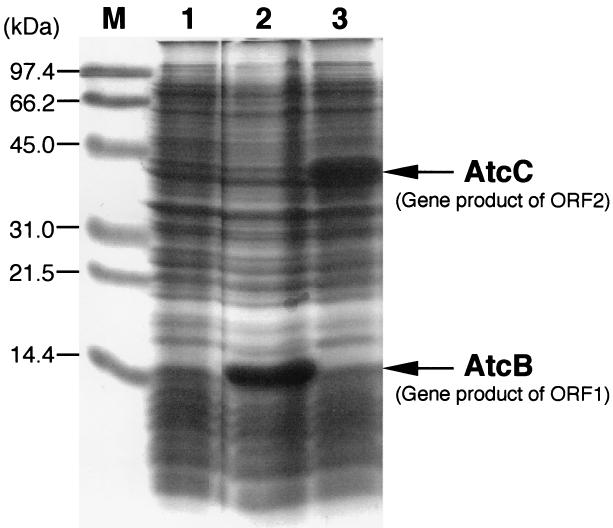
As shown in Fig. 6, in the E. coli harboring pTK-T7-30 or pTK-T7-40, proteins with molecular masses of about 20 and 45 kDa, corresponding to the calculated masses of proteins encoded by ORF1 and ORF2, respectively, were overproduced. The maxicell method was also used to identify proteins encoded in pTK10 (13). Approximately 20- and 45-kDa proteins were produced only by the E. coli harboring pTK10, whereas these proteins were not detected in the E. coli harboring pBS-SK as a vector plasmid (data not shown). These results indicate that the 20- and the 45-kDa proteins are products of ORF1 and ORF2, respectively, which is in agreement with the expected sizes deduced from the DNA sequences.
FIG. 6.
Identification of the proteins encoded by ORF1 and ORF2. The cell extracts of E. coli carrying pT7-7 (lane 1), pTK-T7-30 (lane 2), and pTK-T7-40 (lane 3) were analyzed by SDS-14% PAGE.
Functions of the gene products of ORF1 and ORF2.
To identify the functions of each ORF, we constructed deletion derivatives of pTK10 as shown in Fig. 2. pTK30 and pTK40 contain a 575-bp DNA fragment including ORF1 and a 1,340-bp DNA fragment including ORF2, respectively, under the control of the lac promoter provided by the plasmid vector. These DNA fragments were amplified by PCR with specific primers containing the XbaI site (upstream primers) and BamHI site (downstream primers). The primer sequences are presented in the legend of Fig. 2. Amplified DNA fragments were inserted into XbaI- and BamHI-digested pBS-SK.
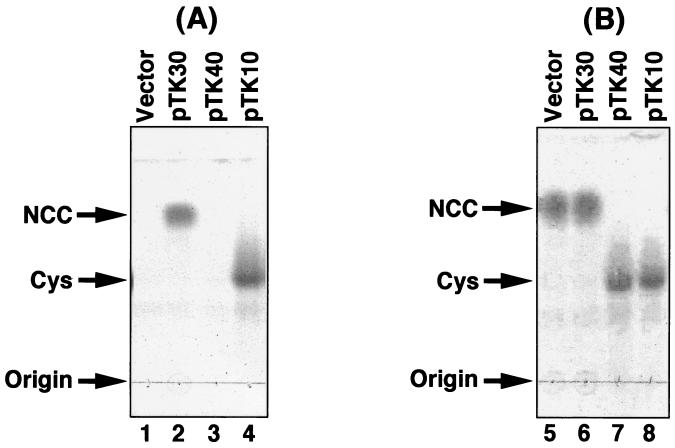
To determine the functions of the gene products of the ORFs, we examined ATC hydrolase activity and the hydrolyzed intermediate. A crude extract of E. coli cells carrying pTK30 or pTK40 was prepared as described in Materials and Methods. The activities to convert dl-ATC to a hydrolyzed intermediate and then to convert the intermediate to l-cysteine were analyzed by TLC. As shown in Fig. 7A, dl-ATC was converted to a hydrolyzed intermediate by the E. coli extract carrying pTK30 (lane 2), whereas no hydrolyzed product was observed in the reaction mixture containing E. coli extract carrying pTK40 (lane 3). The Rf value of the intermediate is 0.85, which is almost the same as that of authentic l-NCC (Rf = 0.84) and very different form that of l-SCC (Rf = 0.43) (21). As demonstrated in Fig. 3, cysteine was produced from dl-ATC with E. coli extract carrying pTK10 (lane 4). To further identify the intermediate, a reaction mixture (10 ml) containing 70 mM dl-ATC and the E. coli extract carrying pTK30 was prepared, and the reaction was performed at 30°C for 20 h. The intermediate was purified by the method described by Tamura et al. (21). Analyses of the purified intermediate by two-dimensional 1H nuclear magnetic resonance indicated that the intermediate was NCC, not SCC (data not shown). In addition, the fact that no hydrolyzed intermediate was observed when d-ATC was used as a substrate in the reaction mixture containing the E. coli extract carrying pTK30 indicates that the product of ORF1 can only catalyze the l-specific conversion from l-ATC to l-NCC (Fig. 8A). The gene product of ORF1 encodes l-ATC hydrolase.
FIG. 7.
Functions of gene products of ORF1 and ORF2. (A) Hydrolysis of dl-ATC by the gene product of atcB. Crude extracts of E. coli cells carrying pBS-SK (control vector) (lane 1), pTK30 (lane 2), pTK40 (lane 3), or pTK10 (lane 4) (20 μg each) were mixed with 0.5% dl-ATC in 100-μl reaction mixtures and incubated at 30°C for 3 h. Two microliters of the reaction mixture was spotted onto a TLC plate, and hydrolyzed products were analyzed and visualized by iodine staining. (B) Hydrolysis of l-NCC by the gene product of atcC. l-NCC was produced from dl-ATC in a 100-μl reaction mixture by incubating 0.5% dl-ATC with a crude extract of E. coli cells carrying pTK30 (20 μg) at 30°C for 12 h. The crude extract of E. coli cells carrying pBS-SK (control vector) (lane 1), pTK30 (lane 2), pTK40 (lane 3), or pTK10 (lane 4) (20 μg each) was further added to the aliquoted reaction mixtures (20 μl each), and the reactions were continued for a further 3 h. The hydrolyzed products were analyzed by TLC and visualized by iodine staining. l-NCC and l-cysteine are represented as NCC and Cys, respectively. Origin, origin of development of TLC.
When the E. coli extract that produces a gene product of ORF2 was incubated with l-NCC, which was produced by incubating dl-ATC with a crude extract containing the product of ORF1, l-NCC was completely converted to l-cysteine (Fig. 7B, lanes 7 and 8). Since no conversion was observed when l-NCC was incubated with E. coli extract of a vector and pTK30 transformants (Fig. 7B, lanes 5 and 6, respectively), it seems reasonable to conclude that l-cysteine was produced by an ORF2 gene product, not by spontaneous hydrolysis of l-NCC. These results showed that ORF2 encodes l-NCC amidohydrolase. As well as the product of ORF1, l-NCC, but not d-NCC, can be hydrolyzed to l-cysteine by the product of ORF2 (Fig. 8B).
ORF1 and ORF2 were named atcB and atcC, respectively, since the genes were involved in ATC hydrolysis.
DISCUSSION
In this study, we isolated the gene encoding l-ATC hydrolase and l-NCC amidohydrolase from Pseudomonas sp. strain BS, which is able to convert dl-ATC to l-cysteine. Many bacteria, especially in the genus Pseudomonas, that have an activity to convert dl-ATC to l-cysteine have been isolated (14, 15, 21), and two biosynthetic pathways have been described (21). As an intermediate, one pathway produces l-NCC and another pathway produces l-SCC (Fig. 1). We showed the existence of the former pathway by identifying the gene involved in l-cysteine synthesis from dl-ATC in strain BS.
The 2.6-kb insert DNA fragment in pTK10 was sequenced, and two ORFs (atcB and atcC) were found. l-ATC hydrolase encoded by atcB did not share extensive homology with other enzymes whose functions have been reported in detail. However, recently, an atcB homologue has also been cloned from Pseudomonas sp., although the only report on this homologue is a Japanese patent (1). The homologue also has l-ATC hydrolase activity, and its amino acid sequence is 53.5% identical with AtcB (1). l-NCC amidohydrolase encoded by atcC shares strong homology with l-N-carbamoylase (23). l-N-Carbamoylase catalyzes the conversion of N-carbamyl-l-amino acids to l-amino acids (23). The gene for l-N-carbamoylase has been cloned in Pseudomonas sp. (22), A. aurescens, (23), and B. stearothermophilus (2, 9). In Pseudomonas sp. strain NS671, hyuC constitutes an operon with hyuA and hyuB, which are involved in the conversion of d- and l-5-substituted hydantoins to corresponding N-carbamyl-d- and N-carbamyl-l-amino acids, respectively (22). Although it is not clear whether HyuC can hydrolyze l-NCC and whether AtcC can hydrolyze N-carbamyl-l-amino acids other than l-NCC, the similarity of the two enzymes could be due to the similarity of the substrates. Furthermore, the similarity of ATC-utilizing enzymes and hydantoin-utilizing enzymes suggests that these enzymes are evolutionarily oriented from the same origin and may have the same physiological functions in Pseudomonas. d-hydantoinase, the product of hyuA, may be involved in the catabolism of uracil and thymine (6), and it has gained considerable attention for its ability to synthesize the less commonly occurring d-amino acids by coupling with l-n-carbamoylase (6). ATC-utilizing enzymes may also be involved in amino acid synthesis in bacteria. Since a branched cyclic dodecylpeptide antibiotic bacteriocin that contains thiazolin is produced in some strains of Bacillus (7) and since some siderophores (bacterially produced iron-chelating compounds), such as yersiniabactin and anguibactin, also contain thiazolin (4), atcB and atcC may be involved in the synthesis or degradation of these thiazolin-containing metabolites. Further analysis of the metabolism of thiazolin-containing compounds is needed to elucidate the detailed biological functions of atcB and atcC.
Recently, we have also cloned an ATC racemase gene (17) and a regulator gene for ATC utilization in strain BS. Further research on these genes is now in progress.
REFERENCES
- 1.Asada, Y., T. Omachi, and Y. Tamura. March 2001. Pseudomonas D,L-2-amino-Δ2-thiazolin-4-carboxylic acid hydrolase and N-carbamoyl-l-cysteine amidohydrolase genes for l-cysteine biosynthesis. Japan Patent, Kokai Tokkyo Koho, JP2001069985.
- 2.Batisse, N., P. Weigel, M. Lecocq, and V. Sakanyan. 1997. Two amino acid amidohydrolase genes encoding l-stereospecific carbamoylase and aminoacylase are organized in a common operon in Bacillus stearothermophilus. Appl. Environ. Microbiol. 63:763-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaitonde, M. K. 1967. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 104:627-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gehring, A. M., E. DeMoll, J. D. Fetherston, I. Mori, G. F. Mayhew, F. R. Blattner, C. T. Walsh, and R. D. Perry. 1998. Iron acquisition in plague: modular logic in enzymatic biogenesis of yersiniabactin by Yersinia pestis. Chem. Biol. 5:573-586. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 6.Kim, G. L., D. E. Lee, and H.-S. Kim. 2000. Functional expression and characterization of the two cyclic amidohydrolase enzymes, allantoinase and a novel phenylhydantoinase, from Escherichia coli. J. Bacteriol. 182:7021-7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konz, D., A. Klens, K. Schörgendorfer, and M. A. Marahiel. 1997. The bacitracin biosynthesis operon of Bacillus licheniformis ATCC 10716: molecular characterization of three multi-modular peptide synthetase. Chem. Biol. 4:927-937. [DOI] [PubMed] [Google Scholar]
- 8.Moriguchi, K., Y. Maeda, M. Satou, M. Kataoka, N. Tanaka, and K. Yoshida. 2000. Analysis of unique variable region of a plant root inducing plasmid, pRil724, by the construction of its physical map and library. DNA Res. 7:157-163. [DOI] [PubMed] [Google Scholar]
- 9.Mukohara, Y., T. Ishikawa, K. Watabe, and H. Nakamura. 1993. Molecular cloning and sequencing of the gene for a thermostable N-carbamyl-l-amino acid amidohydrolase from Bacillus stearothermophilus strain NS1122A. Biosci. Biotechnol. Biochem. 57:1935-1937. [DOI] [PubMed] [Google Scholar]
- 10.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryu, O. H., J. Y. Ju, and C. S. Shin. 1997. Continuous l-cysteine production using immobilized cell reactors and product extractors. Proc. Biochem. 32:201-209. [Google Scholar]
- 12.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1990. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 13.Sancar, A., R. P. Wharton, S. Seltzer, B. M. Kacinski, N. D. Clarke, and W. D. Rupp. 1981. Identification of the uvrA gene product. J. Mol. Biol. 148:45-62. [DOI] [PubMed] [Google Scholar]
- 14.Sano, K., and K. Mitsugi. 1978. Enzymatic production of l-cysteine from d,l-2-amino-Δ2-thiazoline-4-carboxylic acid by Pseudomonas thiazolinophilum: optimal conditions for the enzyme formation and enzymatic reaction. Agric. Biol. Chem. 42:2315-2321. [Google Scholar]
- 15.Sano, K., K. Yokozeki, F. Tamura, N. Yasuda, I. Noda, and K. Mitsugi. 1977. Microbial conversion of dl-2-amino-Δ2-thiazoline-4-carboxylic acid to l-cysteine and l-cysteine: screening of microorganisms and identification of products. Appl. Environ. Microbiol. 34:806-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.She, Q., R. K. Singh, F. Confalonieri, Y. Zivanovic, G. Allard, M. J. Awayez, C. C. Chan-Weiher, I. G. Clausen, B. A. Curtis, A. DeMoors, G. Erauso, C. Fletcher, P. M. Gordon, I. Heikamp-De Jong, A. C. Jeffries, C. J. Kozera, N. Medina, X. Peng, H. P. Thi-Ngoc, P. Redder, M. E. Schenk, C. Theriault, N. Tolstrup, R. L. Charlebois, W. F. Doolittle, M. Duguet, T. Gaasterland, R. A. Garrett, M. A. Ragan, C. W. Sensen, and J. Van Der Oost. 2001. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc. Natl. Acad. Sci. USA 98:7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiba, T. April 2001. 2-Aminothiazolin-4-carboxylate racemase and gene encoding therefor. U.S. patent 6,214,590 B1.
- 18.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. F. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 19.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 20.Takami, H., K. Nakasone, Y. Takaki, G. Maeno, R. Sasaki, N. Masui, F. Fuji, C. Hirama, Y. Nakamura, N. Ogasawara, S. Kuhara, and K. Horikoshi. 2000. Complete genome sequence of the alkaliphilic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis. Nucleic Acids Res. 28:4317-4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura, Y., M. Nishino, T. Ohmachi, and Y. Asada. 1998. N-carbamoyl-l-cysteine as an intermediate in the bioconversion from d,l-2-amino-Δ2-thiazoline-4-carboxylic acid to l-cysteine by Pseudomonas sp. ON-4a. Biosci. Biotechnol. Biochem. 62:2226-2229. [DOI] [PubMed] [Google Scholar]
- 22.Watabe, K., T. Ishikawa, Y. Mukohara, and H. Nakamura. 1992. Cloning and sequencing of the genes involved in the conversion of 5-substituted hydantoins to the corresponding l-amino acids from the native plasmid of Pseudomonas sp. strain NS671. J. Bacteriol. 174:962-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilms, B., A. Wiese, C. Syldatk, R. Mattes, J. Altenbuchner, and M. Pietzsch. 1999. Cloning, nucleotide sequence and expression of a new l-N-carbamoylase gene from Arthrobacter aurescens DSM 3747 in E. coli. J. Biotechnol. 68:101-113. [DOI] [PubMed] [Google Scholar]