Abstract
Mechanisms for Fe(III) oxide reduction were investigated in Geothrix fermentans, a dissimilatory Fe(III)-reducing microorganism found within the Fe(III) reduction zone of subsurface environments. Culture filtrates of G. fermentans stimulated the reduction of poorly crystalline Fe(III) oxide by washed cell suspensions, suggesting that G. fermentans released one or more extracellular compounds that promoted Fe(III) oxide reduction. In order to determine if G. fermentans released electron-shuttling compounds, poorly crystalline Fe(III) oxide was incorporated into microporous alginate beads, which prevented contact between G. fermentans and the Fe(III) oxide. G. fermentans reduced the Fe(III) within the beads, suggesting that one of the compounds that G. fermentans releases is an electron-shuttling compound that can transfer electrons from the cell to Fe(III) oxide that is not in contact with the organism. Analysis of culture filtrates by thin-layer chromatography suggested that the electron shuttle has characteristics similar to those of a water-soluble quinone. Analysis of filtrates by ion chromatography demonstrated that there was as much as 250 μM dissolved Fe(III) in cultures of G. fermentans growing with Fe(III) oxide as the electron acceptor, suggesting that G. fermentans released one or more compounds capable of chelating and solubilizing Fe(III). Solubilizing Fe(III) is another strategy for alleviating the need for contact between cells and Fe(III) oxide for Fe(III) reduction. This is the first demonstration of a microorganism that, in defined medium without added electron shuttles or chelators, can reduce Fe(III) derived from Fe(III) oxide without directly contacting the Fe(III) oxide. These results are in marked contrast to those with Geobacter metallireducens, which does not produce electron shuttles or Fe(III) chelators. These results demonstrate that phylogenetically distinct Fe(III)-reducing microorganisms may use significantly different strategies for Fe(III) reduction. Thus, it is important to know which Fe(III)-reducing microorganisms predominate in a given environment in order to understand the mechanisms for Fe(III) reduction in the environment of interest.
The mechanisms for microbial reduction of insoluble Fe(III) oxides in subsurface environments are of interest because models of microbial Fe(III) reduction may aid in understanding important aspects of aquifer biogeochemistry. For example, the production of Fe(II) as the result of microbial Fe(III) reduction can significantly influence the water quality of pristine aquifers (4, 19). Microbial Fe(III) reduction can also be an important process for the oxidation of organic contaminants in polluted aquifers (17, 18). Furthermore, Fe(III) oxides are likely to be the major electron acceptor supporting the growth of organisms in the subsurface that are capable of immobilizing radioactive metals such as uranium, technetium, and cobalt (13, 14).
Microbial reduction of insoluble Fe(III) oxides may be kinetically constrained by the need for Fe(III)-reducing microorganisms to directly contact insoluble Fe(III) oxides (16, 26). In some environments the need for Fe(III)-reducing microorganisms to contact Fe(III) oxides may be alleviated by humic substances and other extracellular quinones that can serve as soluble electron shuttles between Fe(III)-reducing microorganisms and Fe(III) oxides (20, 21, 29, 32). However, many subsurface environments, especially those that are low in natural organic matter, may not have significant quantities of electron-shuttling compounds. In such instances the addition of humic acids or other quinones can stimulate Fe(III) reduction and the oxidation of organic contaminants coupled to Fe(III) reduction (3, 9, 20, 21, 25, 29). Another mechanism for stimulating Fe(III) reduction in subsurface environments is to add Fe(III)-chelating compounds which solubilize Fe(III) from Fe(III) oxide, making the Fe(III) more accessible for microbial reduction (24-26).
Molecular studies have indicated that microorganisms in the Geobacteraceae family are the predominant Fe(III)-reducing microorganisms in a variety of sedimentary environments in which Fe(III) reduction is an important terminal electron-accepting process (31, 34, 35). Studies with Geobacter metallireducens, a well-studied member of the Geobacteraceae (12, 22), have suggested that this organism requires contact with Fe(III) oxide in order to reduce it (28). G. metallireducens could not reduce Fe(III) oxide that it could not contact without the addition of an exogenous electron shuttle, and G. metallireducens did not appear to solubilize Fe(III) from Fe(III) oxide prior to Fe(III) reduction (28).
Geothrix fermentans is a dissimilatory Fe(III)-reducing microorganism that is phylogenetically distinct from the Geobacteraceae yet has many physiological similarities with members of the Geobacteraceae (7). Like many of the Geobacteraceae, G. fermentans is a strict anaerobe that conserves energy to support growth from the complete oxidation of organic acids to carbon dioxide, with Fe(III) serving as the sole electron acceptor. As is commonly observed in the Geobacteraceae, G. fermentans has the ability to use alternative electron acceptors such as Mn(IV), nitrate, fumarate, and the humic acid analog 2,6-anthraquinone disulfonate, or it can grow fermentatively with some intermediates of the citric acid cycle as the substrate (7).
G. fermentans and closely related strains have been recovered in culture from the Fe(III) reduction zone of petroleum-contaminated aquifers (1, 7). Molecular analyses have indicated that although their numbers are always much lower than those of the Geobacteraceae, Geothrix species are often enriched in aquifer sediments when Fe(III) reduction becomes an important terminal electron-accepting process (31, 34). Therefore, in order to learn more about the potential mechanisms for Fe(III) oxide reduction in subsurface environments, it was of interest to evaluate the mechanisms for Fe(III) oxide reduction in G. fermentans. The results suggest that, unlike G. metallireducens, G. fermentans releases compounds which can serve as electron shuttles and can solubilize Fe(III) from Fe(III) oxide. This is the first demonstration of a dissimilatory Fe(III)-reducing microorganism that, in the absence of added exogenous electron shuttles or chelators, does not need to directly contact insoluble Fe(III) oxide in order to reduce the Fe(III).
MATERIALS AND METHODS
Source of organism and culturing techniques.
G. fermentans ATCC 700665 was obtained from our laboratory culture collection. Strict anaerobic culturing techniques (2, 27) were used throughout. The organism was routinely grown in the previously described (23) defined medium with 100 mmol of synthetic poorly crystalline Fe(III) oxide (23) per liter as the electron acceptor and 20 mM lactate as the electron donor. All incubations were at 30°C in the dark.
Effect of culture filtrates on reduction of Fe(III) oxide by cell suspensions.
In order to determine whether G. fermentans produced any soluble factors to promote Fe(III) oxide reduction, the effect of cell filtrates on Fe(III) oxide reduction was examined. Cultures of G. fermentans grown on lactate-Fe(III) oxide medium were passed through a filter (0.2-μm pore diameter) to remove cells. The filtrate was oxidized by stirring in air for 1 h in order to remove dissolved Fe(II) and then filtered to remove the precipitated Fe(III). The filtrate was diluted in various proportions with fresh medium. In some instances, the filtrate was treated with heat (100°C, 1 h) or protease (protease K; 5 mg/ml, 1 h). The filtrate-medium mixtures (10 ml) were added to pressure tubes, bubbled with N2-CO2 (80:20) to remove dissolved oxygen, and sealed with butyl rubber stoppers. Poorly crystalline Fe(III) oxide (10 mmol/liter) was added from a concentrated anaerobic stock. When noted, 10 μM AQDS (anthraquinone-2,6-disulfonate) was added from a concentrated stock. A washed cell suspension of G. fermentans that had been grown in lactate-Fe(III)-citrate medium was prepared as previously described (28) and added to the filtrate-medium mixtures to provide ca. 0.4 mg of cell protein per ml. Over time, subsamples were taken anaerobically, and the concentration of HCl-extractable Fe(II) was determined with ferrozine as previously described (1).
Release of electron-shuttling compounds.
In order to determine whether G. fermentans produced a molecule with the ability to shuttle electrons from the cell to Fe(III) oxide that the cell could not contact, synthetic poorly crystalline Fe(III) oxide was incorporated into porous alginate beads as previously described (28). The amount of Fe(III) exposed on the surface of the beads was calculated to be 10% of the total Fe(III) in the bead (28). Previous studies have demonstrated that molecules with a molecular mass of up to 12 kDa are able to diffuse into such beads (5).
Beads were added to G. fermentans cultures in lactate-Fe(III) oxide medium after reduction of the free Fe(III) oxide had begun in order to provide a final Fe(III) oxide concentration of 50 mmol of entrapped Fe(III) oxide per liter. Then 50 μM AQDS was added to some of the cultures at the same time as the entrapped Fe(III) oxide. HCl-extractable Fe(II) in the bulk culture was determined with ferrozine as described above. The Fe(II) within the beads was determined with the same procedure with the exception that the beads were extracted in 0.5 N HCl for 12 h, as previously described (28).
In order to determine if the culture filtrates of G. fermentans contained water-soluble quinones that might function as an electron shuttle, filtrate samples (2 μl) were repeatedly spotted on a silica gel 60 plate until a spot was visible under UV light at 254 nm (8). Samples were resolved using an isopropanol-ethanol-water-acetic acid (30:35:30:5) system designed to mobilize polar natural pigments (10). Plates were air dried, and spots visualized under UV light.
Potential for Fe(III) solubilization.
In order to determine whether G. fermentans solubilized Fe(III) from Fe(III) oxide during growth on Fe(III) oxide, subsamples were taken from a culture grown on Fe(III) oxide over time and analyzed for HCl-extractable Fe(II) (as described above) and dissolved Fe(II) and Fe(III). Dissolved Fe(II) and Fe(III) in culture filtrates was determined by ion chromatography as previously described (28). In some instances, the concentrations of dissolved Fe(II) and Fe(III) were also analyzed by first determining the concentration of Fe(II) with ferrozine as described above and then reducing Fe(III) to Fe(II) with hydroxylamine in order to measure total dissolved iron with ferrozine, as previously described (1). The concentration of dissolved Fe(III) was then calculated as the difference between total dissolved iron and dissolved Fe(II).
RESULTS
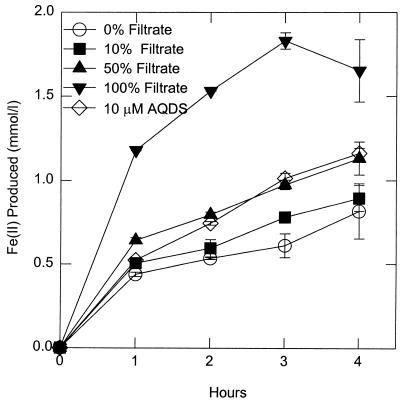
The filtrate from a culture of G. fermentans stimulated the reduction of Fe(III) oxide by washed cell suspensions of G. fermentans (Fig. 1). When the cells were suspended in undiluted filtrate, the rate of Fe(III) oxide reduction was higher than when cells were suspended in fresh medium with 10 μM of the electron-shuttling compound AQDS. Treating the filtrate with protease or heat did not diminish the stimulatory effect of the filtrate (data not shown). This stimulation of Fe(III) oxide reduction with the culture filtrate of G. fermentans contrasts with previous results with G. metallireducens in which the culture filtrate did not stimulate Fe(III) oxide reduction (28), and the filtrate of G. fermentans did not stimulate the reduction of Fe(III) oxide by cell suspensions of G. metallireducens.
FIG. 1.
Reduction of poorly crystalline Fe(III) oxide by a cell suspension of G. fermentans in freshwater medium with various percentages of filtrate from a G. fermentans culture. Error bars indicate range of duplicates; in some cases the symbol may obscure the error bar.
Release of an electron shuttle.
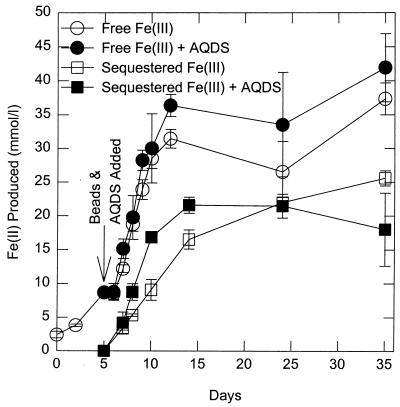
In order to determine whether G. fermentans might be releasing an electron-shuttling compound, the ability of G. fermentans to reduce Fe(III) oxide sequestered within microporous alginate beads was evaluated. Previous studies have demonstrated that the nominal pore size (ca. 12 kDa) of the beads prevents dissimilatory Fe(III)-reducing microorganisms from contacting the Fe(III) oxide but permits the entry of the electron-shuttling compound AQDS (28). G. fermentans readily reduced the Fe(III) oxide within the beads when beads were added to a culture growing on Fe(III) oxide (Fig. 2). The Fe(II) that was produced remained within the beads. This demonstrated that the Fe(III) was reduced within the beads and that there was a compound in the medium capable of shuttling electrons to Fe(III) oxide that G. fermentans could not directly contact. The addition of AQDS to the cultures did not significantly stimulate the rate of reduction of either the free Fe(III) in the medium or the Fe(III) sequestered within the beads. This suggested that the reduction of the Fe(III) oxide in the AQDS-free medium was not limited by a lack of electron-shuttling compounds in the medium.
FIG. 2.
Concentration of HCl-extractable Fe(II) when G. fermentans was grown with synthetic poorly crystalline Fe(III) oxide both free in solution and entrapped in alginate beads in the presence and absence of 50 μM AQDS. The results are means of triplicate incubations for each treatment.
The reason that the added AQDS did not stimulate Fe(III) reduction may have been that there already was a significant quantity of electron-shuttling capacity in the cultures. When the electron-shuttling capacity of the culture filtrate was evaluated with an assay previously devised to assay the electron-shuttling capacity of solutions of humic substances (20), the filtrate was found to contain electron shuttles capable of accepting 52 μM electron equivalents from G. fermentans that could subsequently be transferred to Fe(III). This is equivalent to the electron-shuttling capacity of 26 μM AQDS. As expected, uninoculated medium did not have any electron-shuttling capacity.
The culture filtrates of G. fermentans were examined by thin-layer chromatography to determine if they contained a water-soluble quinone that might serve as an electron shuttle (Table 1). The isopropanol-ethanol-water-acetic acid solvent system mobilized water-soluble quinones such as alizarin, AQDS, emodin, lawsone, purpurin, and vitamin K, but not hydrophobic quinones such as coenzyme Q. Culture filtrates of Fe(III) oxide-grown G. fermentans contained a fluorescent compound that migrated similarly to the hydrophilic quinone standards (Table 1). Filtrates from cultures of G. fermentans grown with Fe(III) citrate or fumarate as the electron acceptor did not contain a similar fluorescent compound. Culture filtrates of G. metallireducens, which does not produce electron-shuttling compounds (28), also did not contain any fluorescent compounds which migrated in this solvent system.
TABLE 1.
Rf values for quinone standards and cell supernatants used for TLC
| Sample or standard | Rf valuea |
|---|---|
| G. fermentans | |
| Fe(III) oxide-grown culture filtrate | 0.75 |
| Fe(III) citrate-grown culture filtrate | — |
| Fumarate-grown culture filtrate | — |
| G. metallireducens Fe(III) oxide-grown culture filtrate | — |
| Alizarin | 0.54 |
| AQDS | 0.98 |
| Coenzyme Q | 0. |
| Emodin | 0.87 |
| Lawsone | 0.83 |
| Purpurin | 0.99 |
| Vitamin K | 0.49 |
—, no spot detected.
Solubilization of Fe(III).
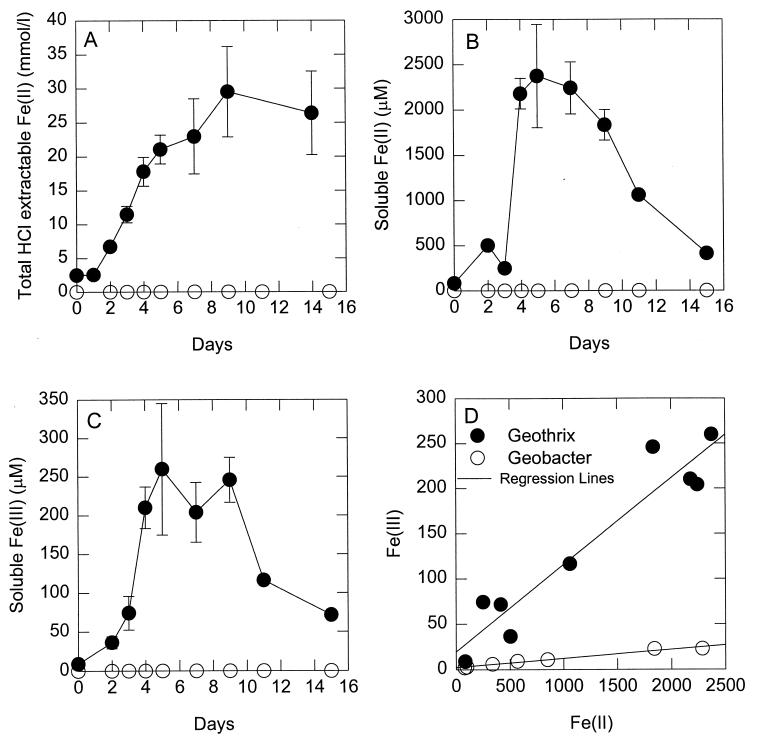
The possibility that G. fermentans might also promote Fe(III) oxide reduction by solubilizing Fe(III) was evaluated by monitoring concentrations of dissolved Fe(III) over time during growth on Fe(III) oxide. As previously observed in cultures of G. metallireducens (28), the production of total HCl-extractable Fe(II) was accompanied by a release of dissolved Fe(II) (Fig. 3). Soluble Fe(II) increased in parallel with the increase in HCl-extractable Fe(II) during the first 5 days and then declined, presumably due to the consumption of dissolved Fe(II) in the formation of Fe(II)-containing solid forms, such as siderite and magnetite (28). The increase in dissolved Fe(II) was accompanied by an increase in soluble Fe(III), which then decreased in parallel with the decrease in soluble Fe(II) (Fig. 3C).
FIG. 3.
Concentrations of HCl-extractable Fe(II) and soluble Fe(II) and Fe(III) during the growth of G. fermentans on poorly crystalline Fe(III) oxide. (A to C) Symbols: •, inoculated media; ○, uninoculated media. (A) Total HCl-extractable Fe(II); (B) soluble Fe(II); (C) soluble Fe(III); (D) covariance of soluble Fe(II) and Fe(III) during growth of G. fermentans and G. metallireducens.
As was seen in a previous analysis of dissolved Fe(III) in cultures of G. metallireducens (28), there was a strong correlation between the concentrations of dissolved Fe(II) and Fe(III) (Fig. 3D). In the previous study with G. metallireducens, it was determined that the apparent Fe(III) was an artifact due to the presence of Fe(II) (28). However, at any given concentration of Fe(III) in the G. fermentans cultures, the concentrations of Fe(III) were much higher than could be attributed to analytical artifact (Fig. 3D). Furthermore, independent analysis of dissolved Fe(III) with the ferrozine method gave the same estimates of dissolved Fe(III) as were measured with ion chromatography.
DISCUSSION
These results provide the first demonstration of a dissimilatory Fe(III)-reducing microorganism that can use insoluble Fe(III) oxide as a terminal electron acceptor without directly contacting the Fe(III) oxide. To our knowledge, the release of Fe(III) chelators by dissimilatory Fe(III)-reducing microorganisms has not been proposed previously as a strategy for reducing Fe(III) derived from Fe(III) oxide. As discussed in detail below, the possibility that Fe(III)-reducing microorganisms release electron-shuttling compounds as a mechanism for Fe(III) oxide reduction has been proposed (30, 33) but never directly demonstrated. The release of electron-shuttling and Fe(III)-chelating compounds by G. fermentans shown here contrasts with the results for G. metallireducens, which does not produce electron-shuttling compounds or Fe(III) chelators (28). Thus, these two dissimilatory Fe(III)-reducing microorganisms, which molecular studies have demonstrated are enriched within the Fe(III) reduction zone of subsurface environments (31, 34), may compete for Fe(III) via significantly different mechanisms.
Electron shuttling and Fe(III) solubilization.
The fact that culture filtrates of G. fermentans stimulated the reduction of Fe(III) oxide by washed cell suspensions suggests that G. fermentans releases one or more compounds which aid it in reducing insoluble Fe(III). The finding that G. fermentans could reduce Fe(III) oxide sequestered within microporous beads indicates that at least one of these compounds is an electron shuttle that can transport electrons from G. fermentans to Fe(III) oxide that is not in contact with the cell. Further evidence for the presence of one or more electron-shuttling compounds in G. fermentans cultures was the finding that culture filtrates had an electron-shuttling capacity equivalent to 26 μM AQDS.
The electron-shuttling compound produced by G. fermentans has not yet been identified. The results with the heat and protease treatments suggest that it is not a protein. The fact that the shuttle promoted the reduction of Fe(III) oxide within the beads suggests that it is water soluble and is smaller than 12 kDa. The analysis of the culture filtrates via thin-layer chromatography suggested that G. fermentans releases a compound with characteristics similar to a water-soluble quinone during growth on Fe(III) oxide. Extracellular quinones, such as AQDS and humic acids, are known to serve as electron shuttles between dissimilatory Fe(III)-reducing microorganisms and Fe(III) oxides (20, 21). However, unlike AQDS or humic acids, which stimulate Fe(III) oxide reduction by a variety of dissimilatory Fe(III)-reducing microorganisms, the electron-shuttling compound(s) in G. fermentans cultures did not affect Fe(III) oxide reduction by washed cell suspensions of G. metallireducens. Whatever the nature of the electron shuttle, it is clearly very effective in promoting Fe(III) oxide reduction by G. fermentans, because the addition of AQDS to provide additional electron-shuttling capacity did not further stimulate the rate of Fe(III) oxide reduction in G. fermentans cultures.
G. fermentans also solubilized significant quantities of Fe(III) during the most active phase of Fe(III) reduction. Solubilization of Fe(III) from insoluble Fe(III) oxide represents an alternative strategy for reducing Fe(III) without the need for direct contact with the Fe(III) oxide in order to reduce it. Previous studies have demonstrated that synthetic Fe(III) chelators can greatly stimulate the activity Fe(III)-reducing microorganisms in subsurface sediments (25, 26). To our knowledge, the studies with G. fermentans represent the first indication that dissimilatory Fe(III)-reducing microorganisms might produce chelating compounds which can solubilize Fe(III). The nature of the Fe(III) chelator in these cultures and whether it is distinct from the electron-shuttling compound(s) have yet to be determined.
Physiological, ecological, and evolutionary implications.
It has generally been considered that microorganisms which use insoluble Fe(III) oxide as an electron acceptor must establish direct contact with the Fe(III) oxide in order to reduce it (15). Although it has been demonstrated that this might not necessarily be the case in environments which contain humic acids or other extracellular quinones which can serve as electron-shuttling compounds (20, 21), until recently the need for cell-Fe(III) oxide contact was assumed to still apply to cultures growing in defined media as well as organic material-poor environments.
Several previous studies have suggested that Fe(III)-reducing microorganisms might produce extracellular electron shuttles to promote Fe(III) oxide reduction. The release of a c-type cytochrome thought to serve as an electron shuttle between Geobacter sulfurreducens and Fe(III) oxides was proposed previously (33), but subsequent studies demonstrated that this was not a likely mechanism for Fe(III) reduction in G. sulfurreducens (11) or the closely related G. metallireducens (28). The finding that a diffusible compound thought to be a quinone was present in cultures of Shewanella oneidensis led to the suggestion that it might release quinones as an electron shuttle to transfer electrons to Fe(III) oxide, but the role of this putative shuttle in electron transfer to Fe(III) oxide was not examined (30). The finding that G. fermentans releases both electron-shuttling and Fe(III)-solublizing compounds clearly demonstrates that direct contact between cells and Fe(III) oxide may not be necessary for insoluble Fe(III) oxides to serve as an electron acceptor.
The conclusion that different Fe(III)-reducing microorganisms have evolved fundamentally different strategies for Fe(III) oxide reduction has evolutionary and ecological implications. For example, it will be interesting to determine whether the direct-contact model, represented by G. metallireducens, or the electron-shuttling/Fe(III) solubilization model, represented by G. fermentans, is more prevalent among the wide phylogenetic diversity of dissimilatory Fe(III)-reducing microorganisms, which extends throughout the Archaea and the Bacteria (14). Furthermore, the selective advantage of the two strategies for accessing Fe(III) oxides in different sedimentary environments warrants further investigation.
The fact that G. fermentans can reduce Fe(III) that it does not directly contact might be expected to give it a competitive advantage over other Fe(III)-reducing microorganisms, such as G. metallireducens, which must directly contact the Fe(III) oxide. However, molecular analysis of the Fe(III)-reducing microorganisms in various sedimentary environments in which Fe(III) reduction is the predominant terminal electron-accepting process have indicated that Geobacter species are present in much higher numbers than Geothrix species (31, 34, 35). Clearly, other physiological considerations must also play a role in the outcome of competition between these organisms. Further investigations to better elucidate these factors are in progress.
In summary, the findings presented here, as well as recent biochemical studies (6), suggest that phylogenetically distinct dissimilatory Fe(III)-reducing microorganisms transfer electrons to Fe(III) oxide via different mechanisms. Therefore, it is important to develop models of Fe(III) oxide reduction in sedimentary environments based on the physiology of the Fe(III)-reducing microorganisms that actually predominate in the environment of interest.
Acknowledgments
We thank Richard Vachet and Jihyeon Lim of the Department of Chemistry for their assistance.
This research was supported by grant N00014-96-1-0382 from the Office of Naval Research and by the Natural and Accelerated Bioremediation Research (NABIR) program, Biological and Environmental Research (BER), U.S. Department of Energy grant DE-FG02-00ER62985. This material is based upon work supported by the Cooperative State Research Extension, Education Service, U.S. Department of Agriculture, Massachusetts Agricultural Experiment Station, under Project No. MASS00787.
REFERENCES
- 1.Anderson, R. T., J. Rooney-Varga, C. V. Gaw, and D. R. Lovley. 1998. Anaerobic benzene oxidation in the Fe(III) reduction zone of petroleum-contaminated aquifers. Environ. Sci. Technol. 32:1222-1229. [Google Scholar]
- 2.Balch, W. E., G. E. Fox, L. J. Magrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43:260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley, P. M., F. H. Chapelle, and D. R. Lovley. 1998. Humic acids as electron acceptors for anaerobic microbial oxidation of vinyl chloride and dichloromethane. Appl. Environ. Microbiol. 64:3102-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapelle, F. H., and D. R. Lovley. 1992. Competitive exclusion of sulfate reduction by Fe(III)-reducing bacteria: a mechanism for producing discrete zones of high-iron ground water. Ground Water 30:29-36. [Google Scholar]
- 5.Cheetham, P., K. Blunt, and C. Bucke. 1979. Physical studies on cell immobilization using calcium alginate gels. Biotechnol. Bioeng. 21:2155-2168. [Google Scholar]
- 6.Childers, S., and D. Lovley. 2001. Differences in Fe(III) reduction in the hyperthermophilic archaeon, Pyrobaculum islandicum, versus mesophilic Fe(III)-reducing bacteria. FEMS Microbiol. Lett. 195:253-258. [DOI] [PubMed] [Google Scholar]
- 7.Coates, J. D., D. J. Ellis, C. V. Gaw, and D. R. Lovley. 1999. Geothrix fermentens gen. nov. sp. nov., an acetate-oxidizing Fe(III) reducer capable of growth via fermentation. Int. J. Syst. Bacteriol. 49:1615-1622. [DOI] [PubMed] [Google Scholar]
- 8.Collins, M. 1985. Analysis of isoprenoid quinones, p. 329-366. In G. Gottschalk (ed.), Methods in microbiology, vol. 18. Academic Press, London, England. [Google Scholar]
- 9.Finneran, K., and D. Lovley. 2001. Anaerobic degradation of methyl tert-butyl ether (MTBE) and tert-butyl alcohol (TBA). Environ. Sci. Technol. 35:1785-1790. [DOI] [PubMed] [Google Scholar]
- 10.Isaksen, M. 1991. Natural pigments, p. 625-662. In J. Sherma and B. Fried (ed.), Handbook of thin-layer chromatography, vol. 55. Marcel Dekker, Inc., New York, N.Y. [Google Scholar]
- 11.Lloyd, J. R., E. Blunt-Harris, and D. R. Lovley. 1999. The periplasmic 9.6-kilodalton c-type cytochrome of Geobacter sulfurreducens is not an electron shuttle to Fe(III). J. Bacteriol. 181:7647-7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonergan, D. J., H. Jenter, J. D. Coates, E. J. P. Phillips, T. Schmidt, and D. R. Lovley. 1996. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J. Bacteriol. 178:2402-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovley, D. R. 2000. Fe(III) and Mn(IV) reduction, p. 3-30. In D. Lovley (ed.), Environmental microbe-metal interactions. ASM Press, Washington, D.C.
- 14.Lovley, D. R. 2000. Dissimilatory Fe(III) and Mn(IV)-reducing prokaryotes. In M. Dworkin, S. Falkow, E. Rosenberg, K. Schleifer, and E. Stackebrandt (ed.), The prokaryotes. Springer-Verlag, New York, N.Y. http://www.prokaryotes.com.
- 15.Lovley, D. R. 1991. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 55:259-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovley, D. R. 1997. Microbial Fe(III) reduction in subsurface environments. FEMS Microbiol. Rev. 20:305-315. [Google Scholar]
- 17.Lovley, D. R. 1997. Potential for anaerobic bioremediation of BTEX in petroleum-contaminated aquifers. J. Ind. Microbiol. 18:75-81. [Google Scholar]
- 18.Lovley, D. R., M. J. Baedecker, D. J. Lonergan, I. M. Cozzarelli, E. J. P. Phillips, and D. I. Siegel. 1989. Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature 339:297-299. [Google Scholar]
- 19.Lovley, D. R., F. H. Chapelle, and E. J. P. Phillips. 1990. Fe(III)-reducing bacteria in deeply buried sediments of the Atlantic Coastal Plain. Geology 18:954-957. [Google Scholar]
- 20.Lovley, D. R., J. D. Coates, E. L. Blunt-Harris, E. J. P. Phillips, and J. C. Woodward. 1996. Humic substances as electron acceptors for microbial respiration. Nature 382:445-448. [Google Scholar]
- 21.Lovley, D. R., J. L. Fraga, E. L. Blunt-Harris, L. A. Hayes, E. J. P. Phillips, and J. D. Coates. 1998. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim. Hydrobiol. 26:152-157. [Google Scholar]
- 22.Lovley, D. R., S. J. Giovannoni, D. C. White, J. E. Champine, E. J. P. Phillips, Y. A. Gorby, and S. Goodwin. 1993. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 159:336-344. [DOI] [PubMed] [Google Scholar]
- 23.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovley, D. R., and J. C. Woodward. 1996. Mechanisms for chelator stimulation of microbial Fe(III)-oxide reduction. Chem. Geol. 132:19-24. [Google Scholar]
- 25.Lovley, D. R., J. C. Woodward, and F. H. Chapelle. 1996. Rapid anaerobic benzene oxidation with a variety of chelated Fe(III) forms. Appl. Environ. Microbiol. 62:288-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovley, D. R., J. C. Woodward, and F. H. Chapelle. 1994. Stimulated anoxic biodegradation of aromatic hydrocarbons using Fe(III) ligands. Nature 370:128-131. [DOI] [PubMed] [Google Scholar]
- 27.Miller, T. L., and M. J. Wolin. 1974. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl. Microbiol. 27:985-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nevin, K. P., and D. R. Lovley. 2000. Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter metallireducens. Appl. Environ. Microbiol. 66:2248-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nevin, K. P., and D. R. Lovley. 2000. Potential for nonenzymatic reduction of Fe(III) via shuttling in subsurface sediments. Environ. Sci. Technol. 34:2472-2478. [Google Scholar]
- 30.Newman, D. K., and R. Kolter. 2000. A role for excreted quinones in extracellular electron transfer. Nature 405:93-97. [DOI] [PubMed] [Google Scholar]
- 31.Rooney-Varga, J. N., R. T. Anderson, J. L. Fraga, D. Ringelberg, and D. R. Lovley. 1999. Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. Appl. Environ. Microbiol. 65:3056-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott, D. T., D. M. McKnight, E. L. Blunt-Harris, S. E. Kolesar, and D. R. Lovley. 1998. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 32:2984-2989. [Google Scholar]
- 33.Seeliger, S., R. Cord-Ruwisch, and B. Schink. 1998. A periplasmic and extracellular c-type cytochrome of Geobacter sulfurreducens acts as a ferric iron reductase and as an electron carrier to other acceptors or to partner bacteria. J. Bacteriol. 180:3686-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snoeyenbos-West, O. L., K. P. Nevin, R. T. Anderson, and D. R. Lovley. 2000. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microb. Ecol. 39:153-167. [DOI] [PubMed] [Google Scholar]
- 35.Stein, L., M. La Duc, T. Grundl, and K. Nealson. 2001. Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments. Environ. Microbiol. 3:10-18. [DOI] [PubMed] [Google Scholar]