Abstract
1. Different techniques were used to generate sudden ramp extension movements of the wrist while the subjects were either relaxed or maintaining a weak voluntary contraction in the wrist flexors. Afferent responses to the displacements were recorded with a tungsten micro-electrode inserted into a fascicle of the median nerve supplying one of the wrist flexor muscles, and e.m.g. responses were recorded with needle electrodes inserted into the same muscle.
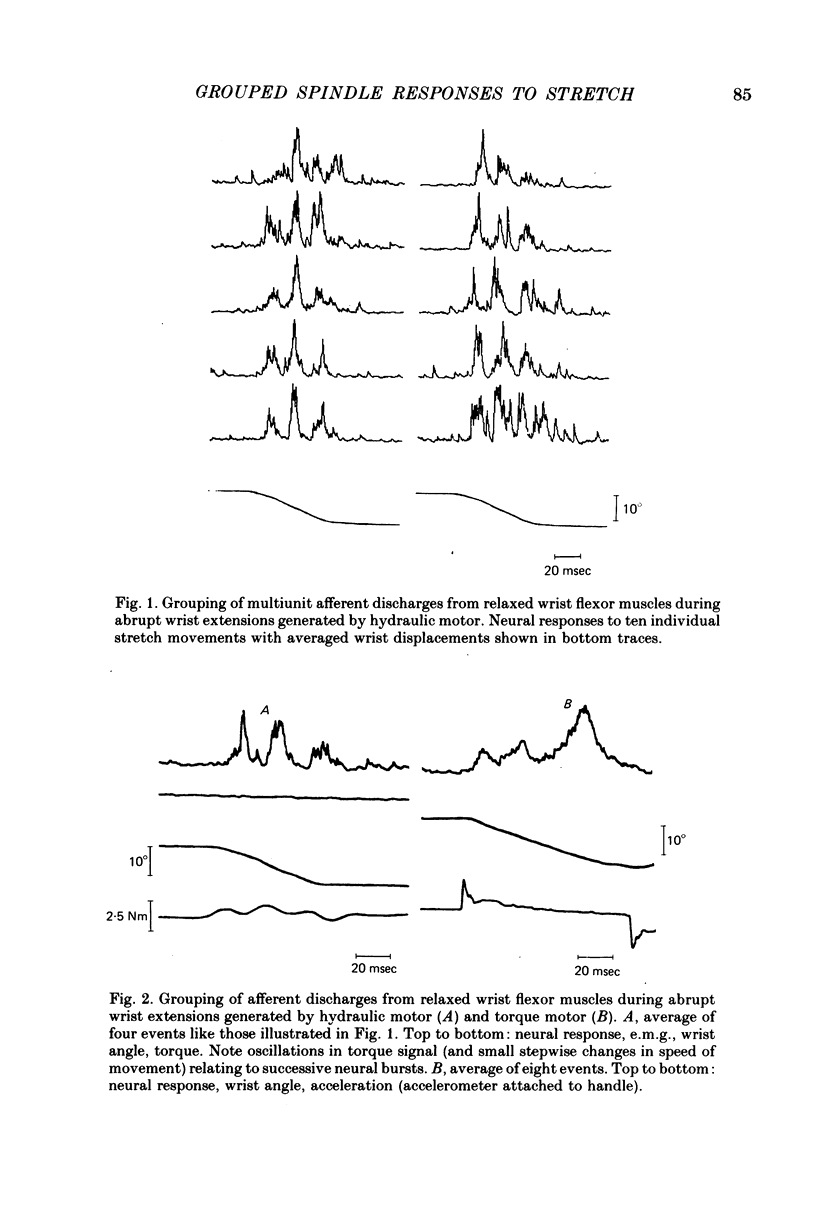
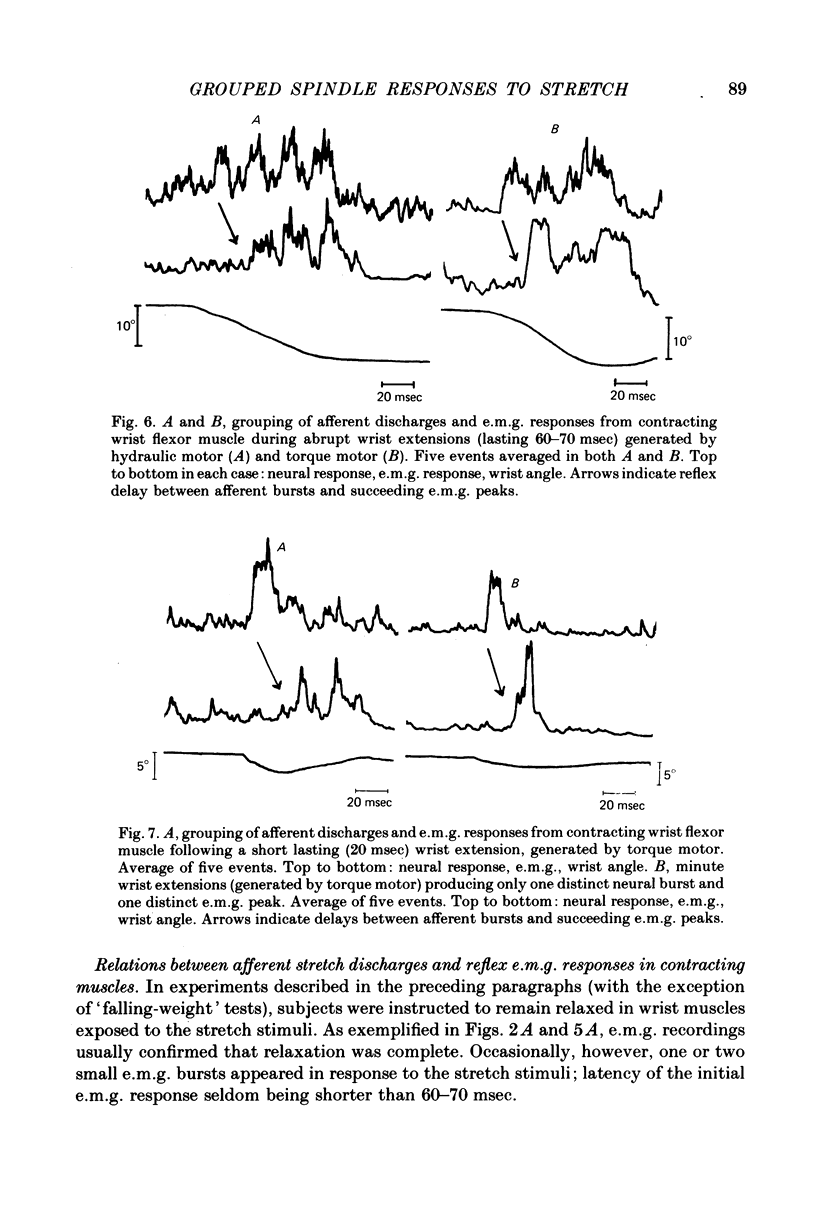
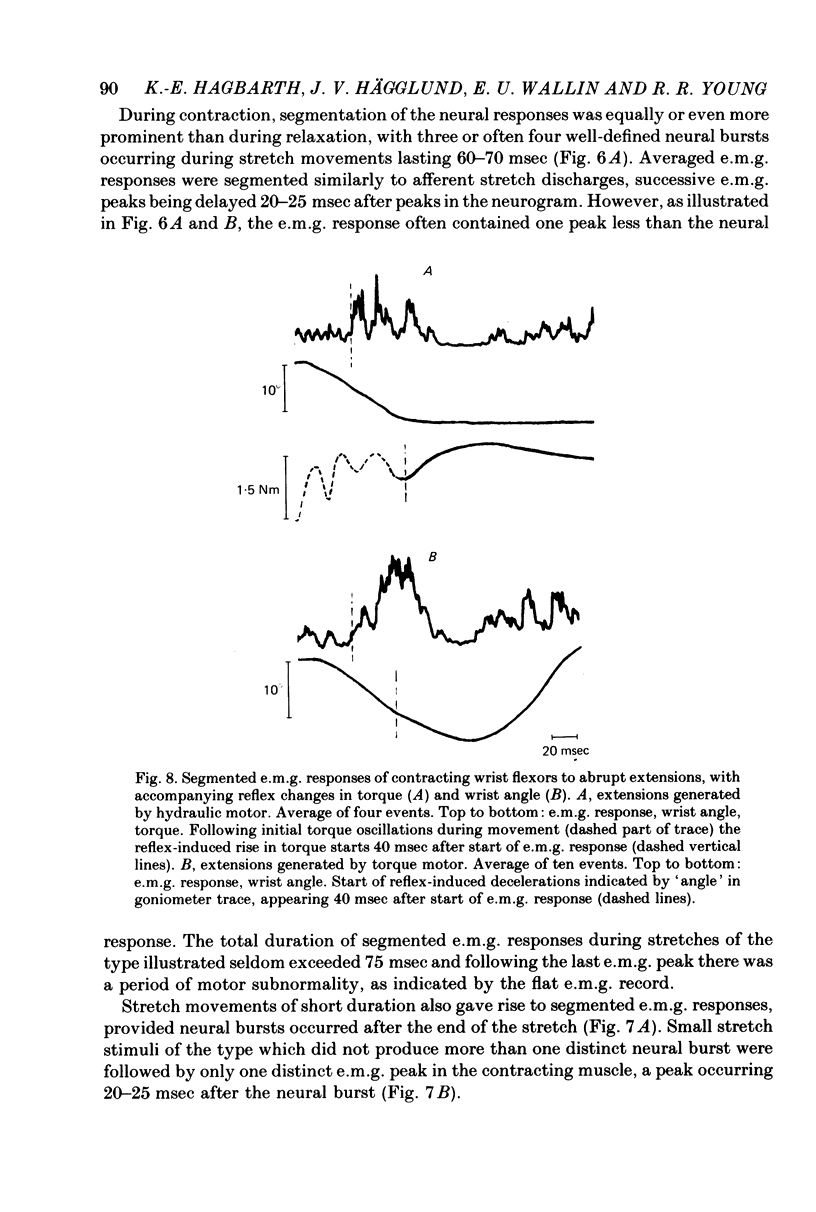
2. With the wrist flexors either relaxed or contracting, extensions at 100-200°/sec for 60-70 msec (generated by either an hydraulic motor or a torque motor) produced segmented afferent responses with two to four afferent bursts, separated by intervals of 20-30 msec. The successive neural peaks, occuring during the stretch phase, were correlated to mechanical vibrations sensed by a strain gauge and sometimes also by a wrist goniometer. With the flexor muscles contracting, the successive peaks in the neurogram were followed by similar peaks in the e.m.g, the delay between neural and e.m.g. peaks being 20-25 msec.
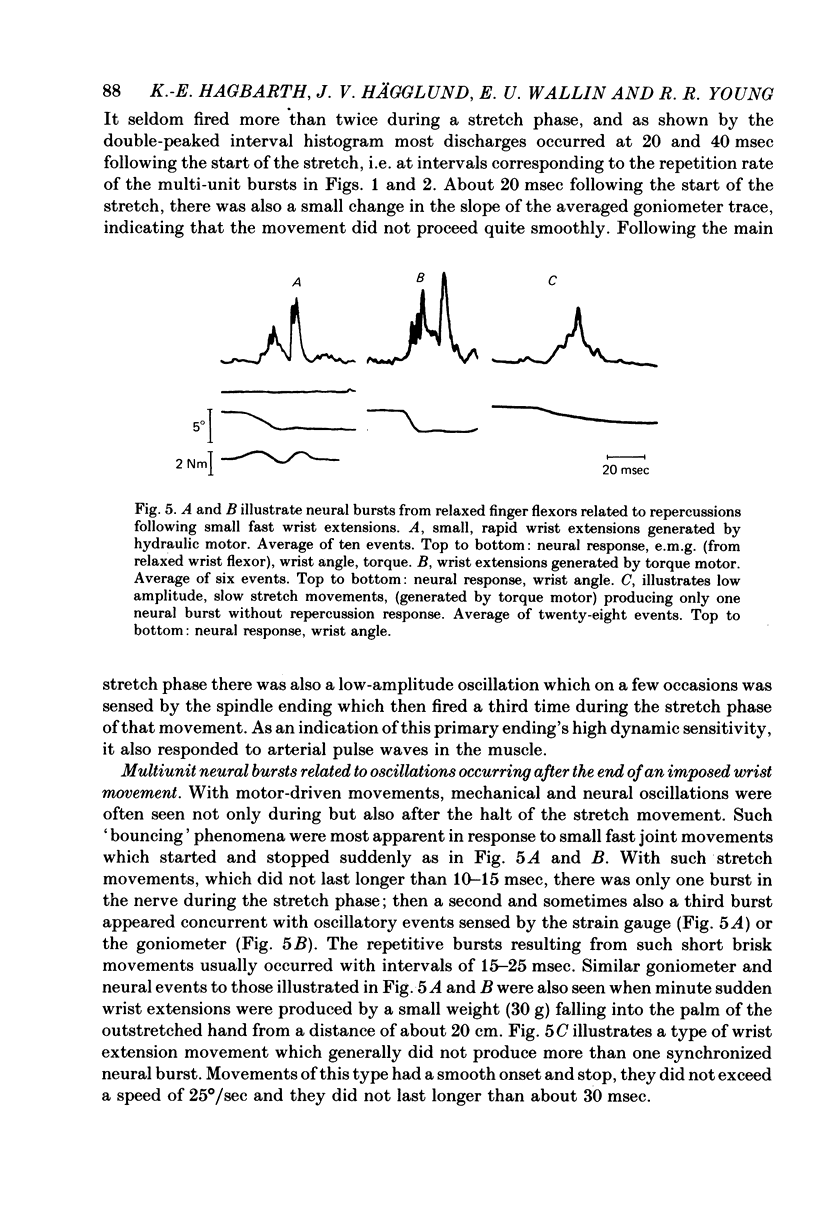
3. Small abrupt extension movements of 1-2° lasting only 10-15 msec often produced segmented afferent responses with one neural burst occuring during the stretch phase and another 15-20 msec later, corresponding to a mechanical oscillatory event succeeding the stretch. The oscillation and the second neural burst were not present with small extension movements of smooth onset and halt. With the flexor muscles contracting, stimuli producing one afferent burst produced only one e.m.g. peak, whereas double-peaked afferent discharges produced double-peaked e.m.g. responses, the delay between individual neural e.m.g. peaks being 20-25 msec.
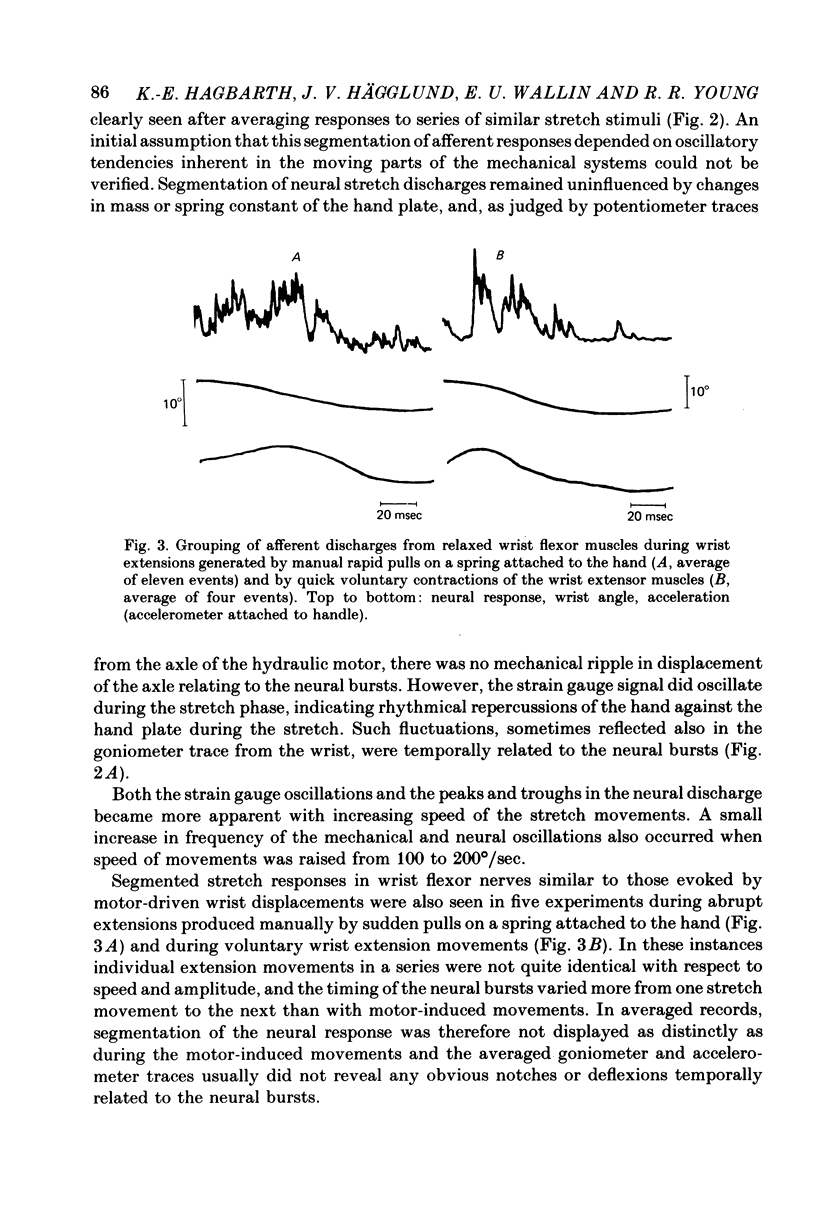
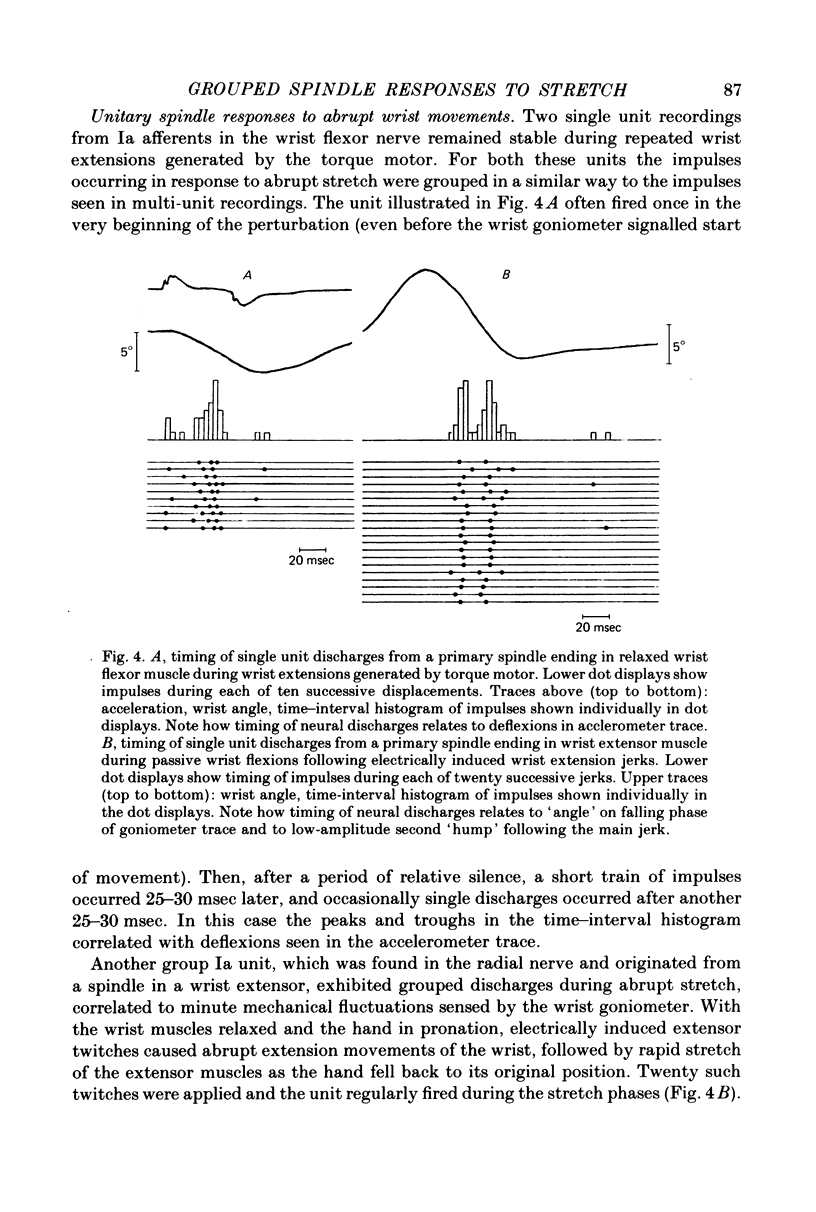
4. Similar segmentation of the neural stretch responses was seen when abrupt displacements were produced by electrically induced muscle twitches, by manual pulls on a spring attached to the hand or by the subject making fast voluntary wrist extensions. This grouping of afferent discharges was seen in both multi-unit and in single-unit recordings from fibres identified as group Ia afferents.
5. It is concluded that mechanical vibrations in the moving parts are initiated by abrupt joint movements and that these vibrations are sensed by the primary endings. With initial background contraction in the stretched muscles, synchronous volleys of spindle discharges produce, via segmental reflex arcs, modulation of the e.m.g. with the appearance of two or three e.m.g. peaks separated by intervals of 20-30 msec. Possible causes for the mechanical oscillations are discussed.
6. For imposed movements with a duration of 60-70 msec the successive e.m.g. peaks caused a fused reflex contraction, appearing as a torque trace deflexion, starting at about the time when the movement ended and reaching its peak within about 40 msec. With longer-lasting movements the mechanical reflex response accompanying the successive e.m.g. bursts, appeared as a decelerative force, starting to oppose the ongoing movement about 60 msec after its start. Mechanical consequences of stretch reflex contractions starting after, rather than during, the stretch movement are discussed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bawa P., Tatton W. G. Motor unit responses in muscles stretched by imposed displacements of the monkey wrist. Exp Brain Res. 1979;37(3):417–437. doi: 10.1007/BF00236815. [DOI] [PubMed] [Google Scholar]
- Burg D., Szumski A. J., Struppler A., Velho F. Afferent and efferent activation of human muscle receptors involved in reflex and voluntary contraction. Exp Neurol. 1973 Dec;41(3):754–768. doi: 10.1016/0014-4886(73)90066-6. [DOI] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976 Oct;261(3):673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D. The role of stretch reflexes during active movements. Brain Res. 1980 Jan 13;181(2):493–497. doi: 10.1016/0006-8993(80)90636-8. [DOI] [PubMed] [Google Scholar]
- Desmedt J. E., Godaux E. Vibration-induced discharge patterns of single motor units in the masseter muscle in man. J Physiol. 1975 Dec;253(2):429–442. doi: 10.1113/jphysiol.1975.sp011198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts E. V., Granit R. Relations of reflexes and intended movements. Prog Brain Res. 1976;44:1–14. doi: 10.1016/S0079-6123(08)60719-0. [DOI] [PubMed] [Google Scholar]
- Evarts E. V. Motor cortex reflexes associated with learned movement. Science. 1973 Feb 2;179(4072):501–503. doi: 10.1126/science.179.4072.501. [DOI] [PubMed] [Google Scholar]
- Evarts E. V., Tanji J. Reflex and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol. 1976 Sep;39(5):1069–1080. doi: 10.1152/jn.1976.39.5.1069. [DOI] [PubMed] [Google Scholar]
- Ghez C., Shinoda Y. Spinal mechanisms of the functional stretch reflex. Exp Brain Res. 1978 May 12;32(1):55–68. doi: 10.1007/BF00237390. [DOI] [PubMed] [Google Scholar]
- HALLIDAY A. M., REDFEARN J. W. An analysis of the frequencies of finger tremor in healthy subjects. J Physiol. 1956 Dec 28;134(3):600–611. doi: 10.1113/jphysiol.1956.sp005668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMMOND P. H. Involuntary activity in biceps following the sudden application of velocity to the abducted forearm. J Physiol. 1955 Feb 28;127(2):23–5P. [PMC free article] [PubMed] [Google Scholar]
- HAMMOND P. H., MERTON P. A., SUTTON G. G. Nervous gradation of muscular contraction. Br Med Bull. 1956 Sep;12(3):214–218. doi: 10.1093/oxfordjournals.bmb.a069553. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E. Exteroceptive, proprioceptive, and sympathetic activity recorded with microelectrodes from human peripheral nerves. Mayo Clin Proc. 1979 Jun;54(6):353–365. [PubMed] [Google Scholar]
- Hagbarth K. E., Hellsing G., Löfstedt L. TVR and vibration-induced timing of motor impulses in the human jaw elevator muscles. J Neurol Neurosurg Psychiatry. 1976 Aug;39(8):719–728. doi: 10.1136/jnnp.39.8.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Wallin G., Löfstedt L., Aquilonius S. M. Muscle spindle activity in alternating tremor of Parkinsonism and in clonus. J Neurol Neurosurg Psychiatry. 1975 Jul;38(7):636–641. doi: 10.1136/jnnp.38.7.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Young R. R., Hägglund J. V., Wallin E. U. Segmentation of human spindle and EMG responses to sudden muscle stretch. Neurosci Lett. 1980 Sep;19(2):213–217. doi: 10.1016/0304-3940(80)90197-4. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Young R. R. Participation of the stretch reflex in human physiological tremor. Brain. 1979 Sep;102(3):509–526. doi: 10.1093/brain/102.3.509. [DOI] [PubMed] [Google Scholar]
- Hasan Z., Houk J. C. Transition in sensitivity of spindle receptors that occurs when muscle is stretched more than a fraction of a millimeter. J Neurophysiol. 1975 May;38(3):673–689. doi: 10.1152/jn.1975.38.3.673. [DOI] [PubMed] [Google Scholar]
- Homma S., Kanda K., Watanabe S. Tonic vibration reflex in human and monkey subjects. Jpn J Physiol. 1971 Aug;21(4):419–430. doi: 10.2170/jjphysiol.21.419. [DOI] [PubMed] [Google Scholar]
- Houk J. C. Regulation of stiffness by skeletomotor reflexes. Annu Rev Physiol. 1979;41:99–114. doi: 10.1146/annurev.ph.41.030179.000531. [DOI] [PubMed] [Google Scholar]
- Jones G. M., Watt D. G. Observations on the control of stepping and hopping movements in man. J Physiol. 1971 Dec;219(3):709–727. doi: 10.1113/jphysiol.1971.sp009684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Westbury D. R. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol. 1969 Oct;204(2):461–474. doi: 10.1113/jphysiol.1969.sp008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. G., Tatton W. G. Motor responses to sudden limb displacements in primates with specific CNS lesions and in human patients with motor system disorders. Can J Neurol Sci. 1975 Aug;2(3):285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in human voluntary movement. Nature. 1972 Jul 21;238(5360):140–143. doi: 10.1038/238140a0. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in the human thumb. J Physiol. 1976 May;257(1):1–44. doi: 10.1113/jphysiol.1976.sp011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Stretch reflex and servo action in a variety of human muscles. J Physiol. 1976 Jul;259(2):531–560. doi: 10.1113/jphysiol.1976.sp011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969 Feb;200(3):723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T. R., Houk J. C. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol. 1976 Jan;39(1):119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Phillips C. G. The Ferrier lecture, 1968. Motor apparatus of the baboon's hand. Proc R Soc Lond B Biol Sci. 1969 May 20;173(1031):141–174. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Bowman R. J. Quantitative description of linear behavior of mammalian muscle spindles. J Neurophysiol. 1970 Jan;33(1):59–72. doi: 10.1152/jn.1970.33.1.59. [DOI] [PubMed] [Google Scholar]
- Pringle J. W. The Croonian Lecture, 1977. Stretch activation of muscle: function and mechanism. Proc R Soc Lond B Biol Sci. 1978 May 5;201(1143):107–130. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- Tatton W. G., Bawa P. Input-output properties of motor unit responses in muscles stretched by imposed displacements of the monkey wrist. Exp Brain Res. 1979;37(3):439–457. doi: 10.1007/BF00236816. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E. Impulses recorded with micro-electrodes in human muscle nerves during stimulation of mechanoreceptors and voluntary contractions. Electroencephalogr Clin Neurophysiol. 1967 Oct;23(4):392–392. [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E., Torebjörk H. E., Wallin B. G. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979 Oct;59(4):919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Young R. R., Hagbarth K. E. Physiological tremor enhanced by manoeuvres affecting the segmental stretch reflex. J Neurol Neurosurg Psychiatry. 1980 Mar;43(3):248–256. doi: 10.1136/jnnp.43.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]


