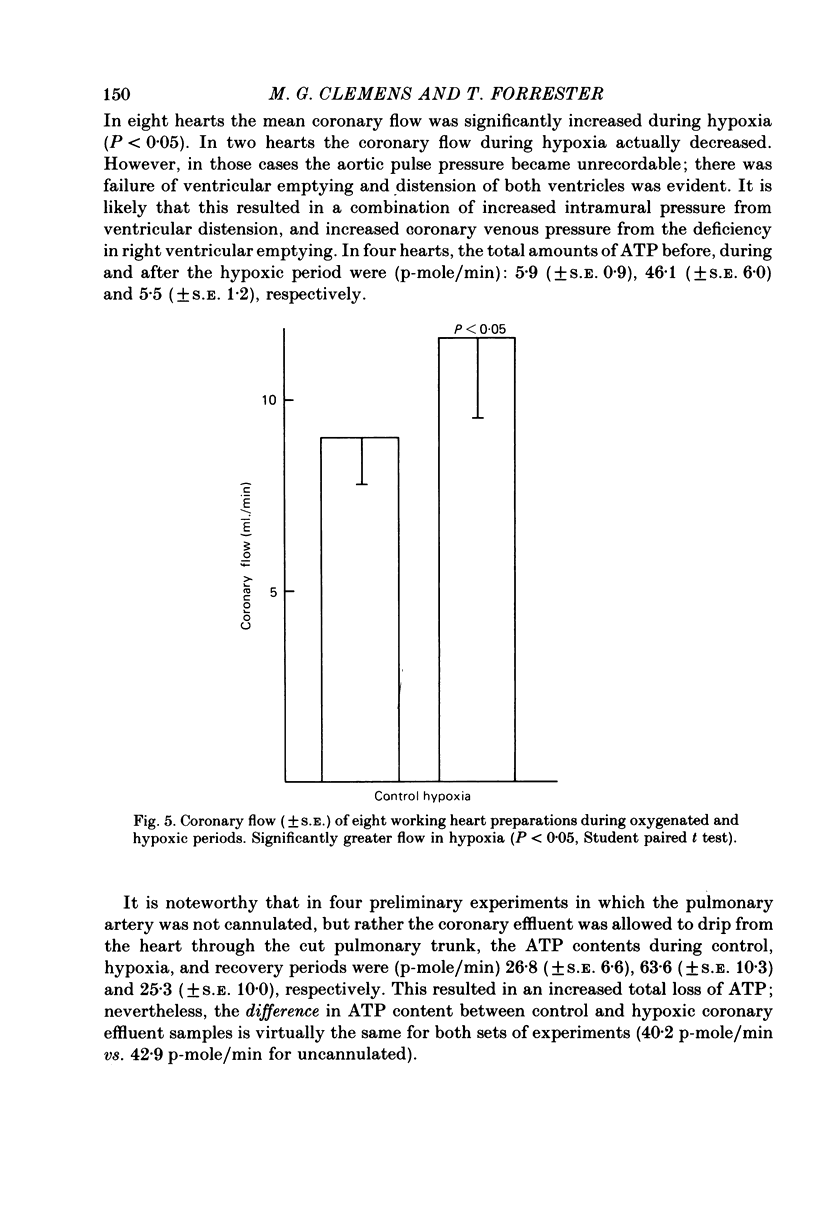
Abstract
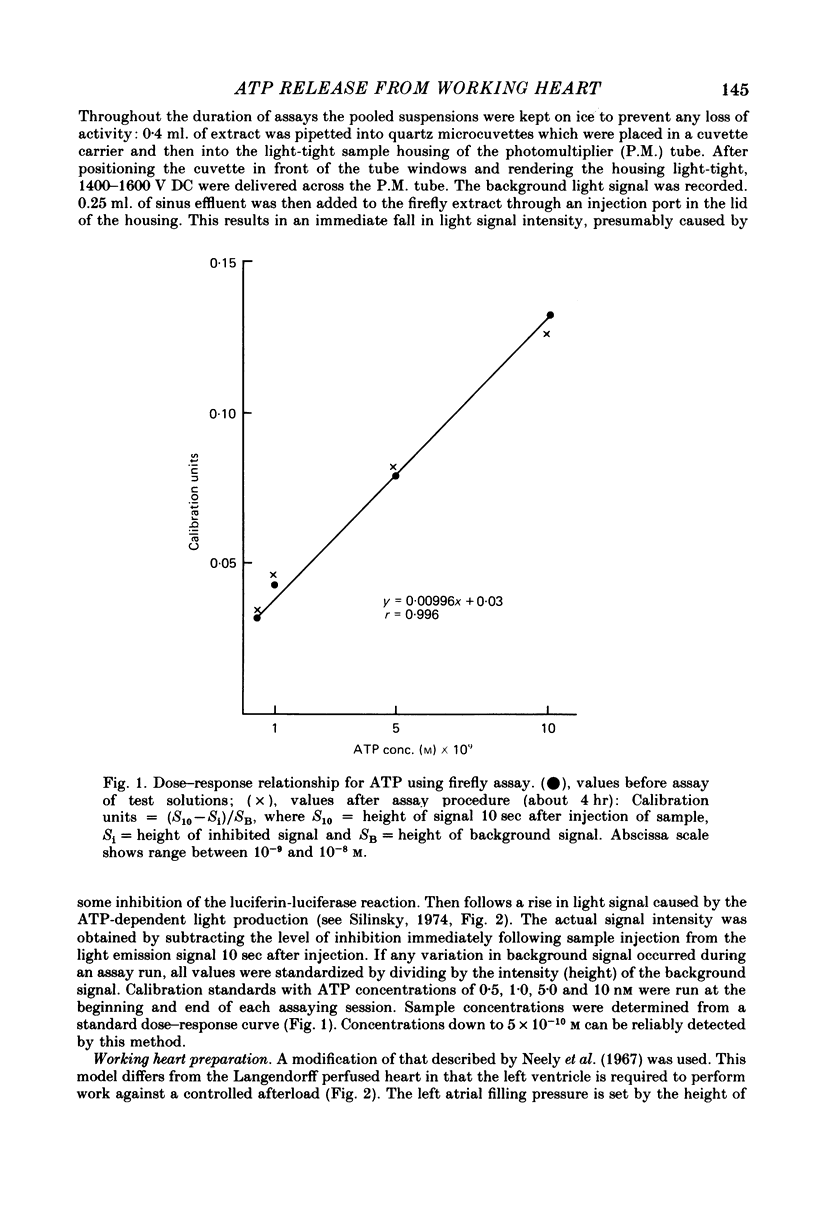
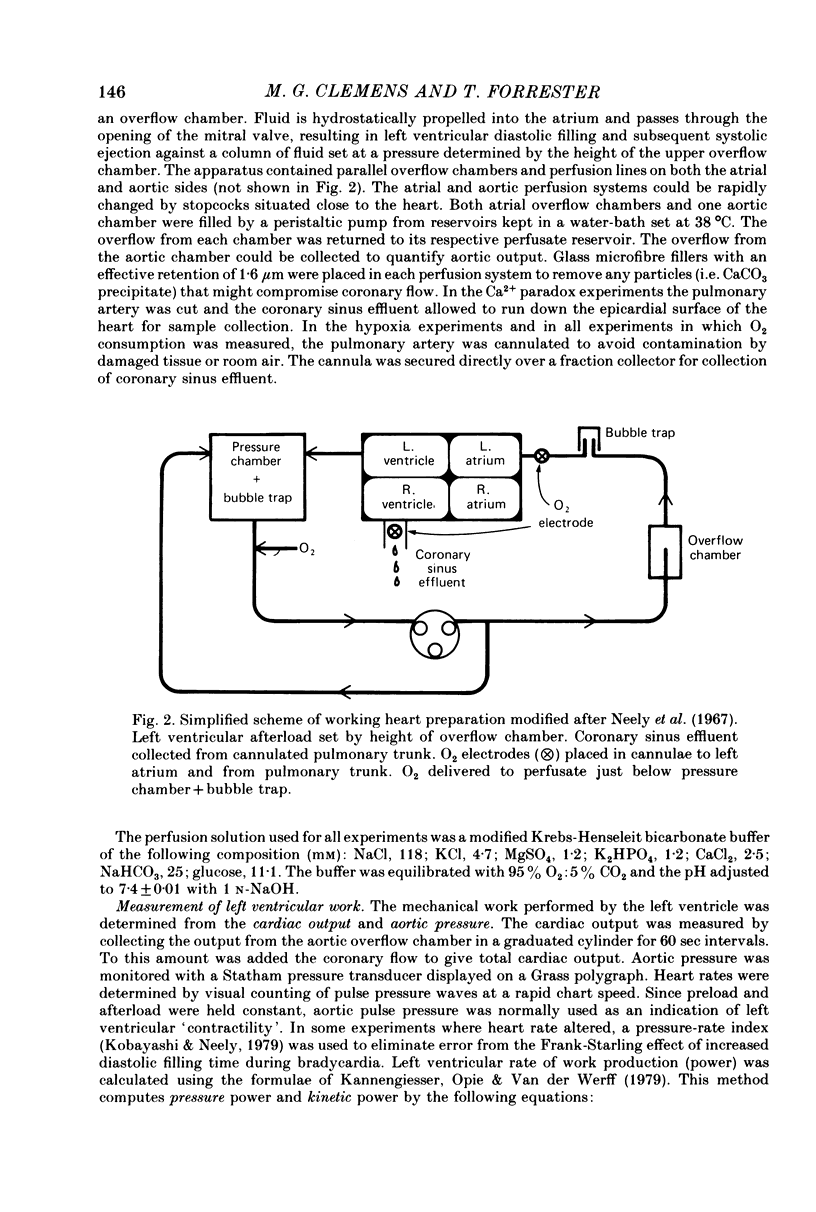
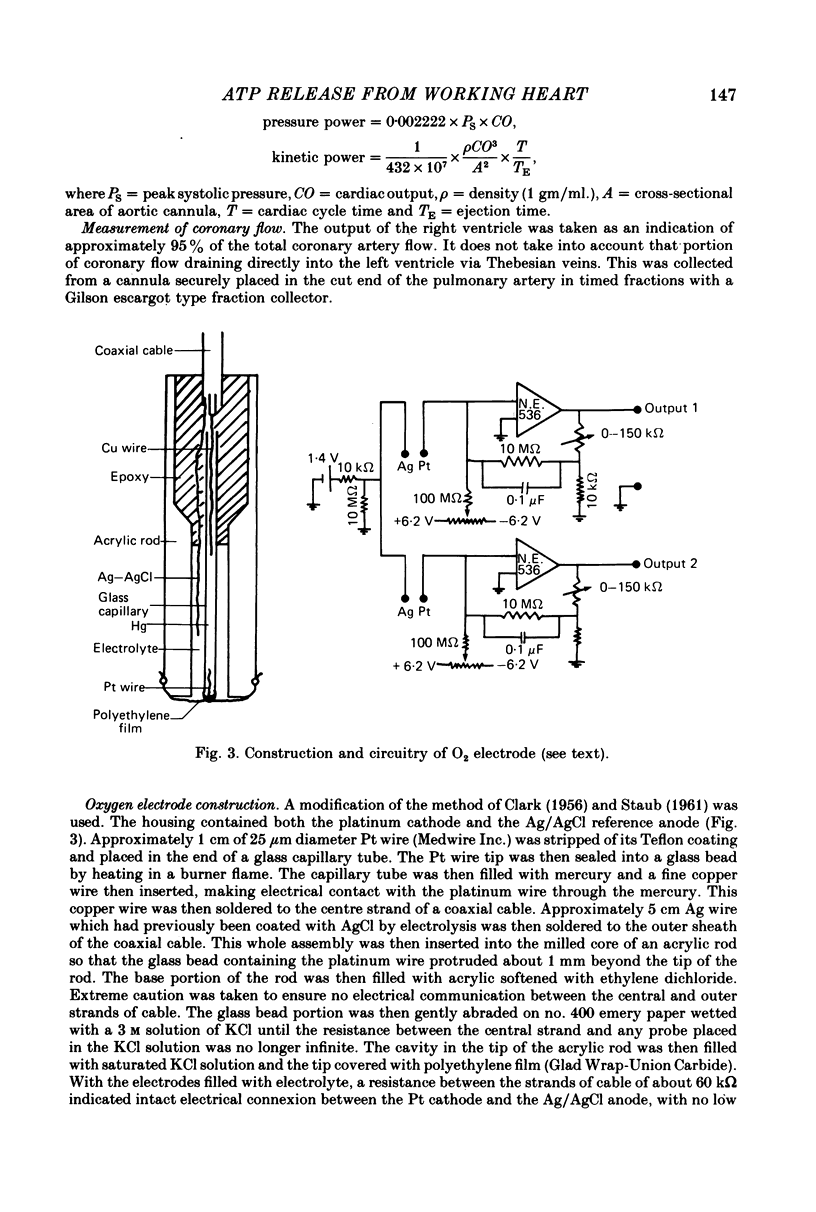
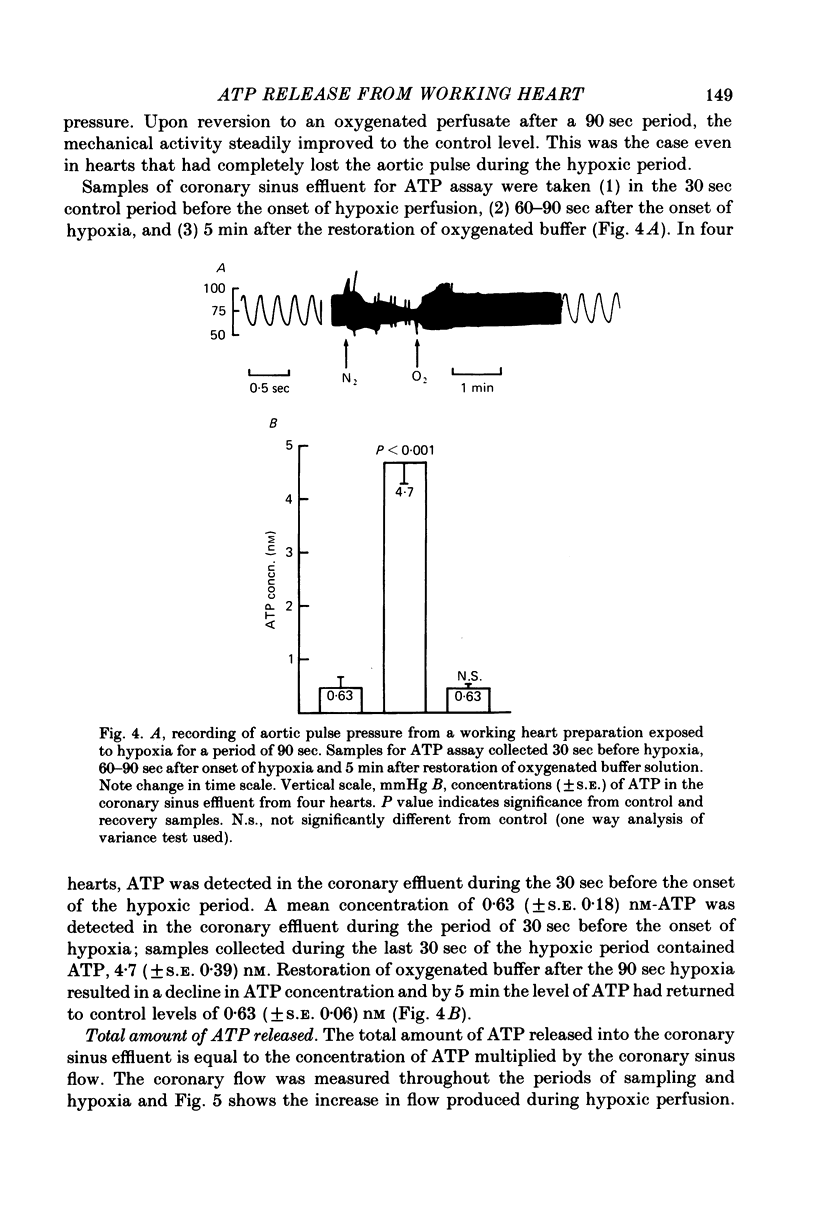
1. A working rat heart preparation was used to study the release of adenosine-5'-triphosphate (ATP) into the coronary sinus effluent in response to hypoxia. 2. The left ventricle was set to pump against an hydrostatic pressure of 65 cm water; the left atrial filling pressure was kept constant at 10 cm water. The power output of the heart at these pressures was estimated to be approximately one half of the maximum power development. 3. Samples for ATP assay were collected (a) 30 sec before onset of hypoxia, (b) 60-90 sec after onset of hypoxia, (c) 5 min after restoration of oxygenated buffer solution. Respective concentrations of ATP were (nM +/- S.E.) 0.63 (+/- 0.18), 4.70 (+/- 0.39) and 0.63 (+/- 0.06). The total amounts of ATP detected were (p-mole/min) 5.9 (+/- 0.9), 46.1 (+/- 6.0) and 5.5 (+/- 1.2) respectively. 4. Viability of the hearts was judged to be satisfactory on the following grounds. Alterations in left atrial filling pressure produced typical Frank-Starling responses of the left ventricle. Oxygen extraction from the perfusate increased in response to increased workload. Coronary blood flow increased immediately upon introduction of hypoxic conditions and mechanical recovery from hypoxia was always complete within 5 min of restoring oxygen. 5. In view of the marked extracellular ATPase activity it is concluded that significant vasodilatory concentrations of ATP are released into the myocardial extracellular space in response to hypoxia. A scheme is proposed describing the possible role of adenine nucleotides in the local control of myocardial blood flow.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABOOD L. G., KOKETSU K., MIYAMOTO S. Outflux of various phosphates during membrane depolarization of excitable tissues. Am J Physiol. 1962 Mar;202:469–474. doi: 10.1152/ajplegacy.1962.202.3.469. [DOI] [PubMed] [Google Scholar]
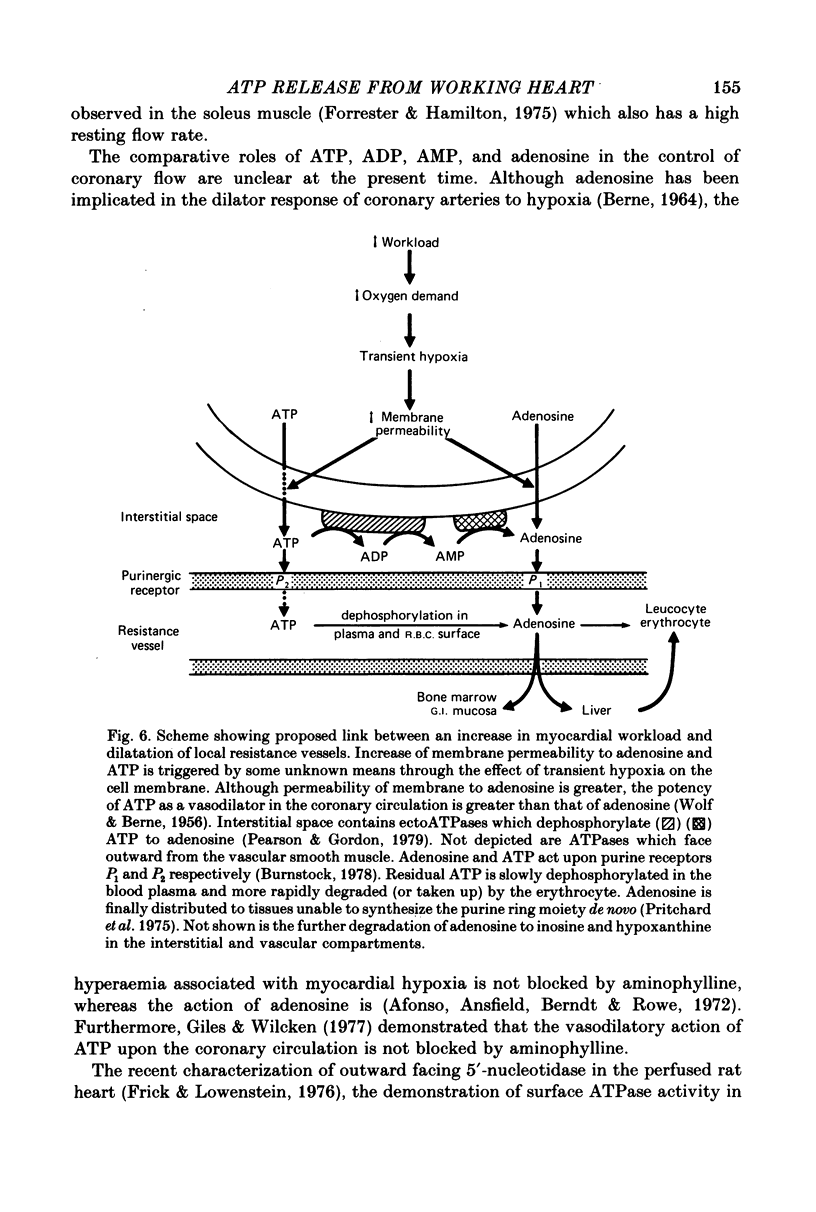
- Afonso S., Ansfield T. J., Berndt T. B., Rowe G. G. Coronary vasodilator responses to hypoxia before and after aminophylline. J Physiol. 1972 Mar;221(3):589–599. doi: 10.1113/jphysiol.1972.sp009769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNE R. M. REGULATION OF CORONARY BLOOD FLOW. Physiol Rev. 1964 Jan;44:1–29. doi: 10.1152/physrev.1964.44.1.1. [DOI] [PubMed] [Google Scholar]
- Boyd I. A., Forrester T. The release of adenosine triphosphate from frog skeletal muscle in vitro. J Physiol. 1968 Nov;199(1):115–135. doi: 10.1113/jphysiol.1968.sp008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Chaudry I. H., Gould M. K. Evidence for the uptake of ATP by rat soleus muscle in vitro. Biochim Biophys Acta. 1970;196(2):320–326. doi: 10.1016/0005-2736(70)90019-2. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M., Rubin R. P. Efflux of adenine nucleotides from perfused adrenal glands exposed to nicotine and other chromaffin cell stimulants. J Physiol. 1965 Jul;179(1):130–137. doi: 10.1113/jphysiol.1965.sp007652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedelesová M., Ziegelhöffer A., Krause E. G., Wollenberger A. Effect of exogenous adenosine triphosphate on the metabolic state of the excised hypothermic dog heart. Circ Res. 1969 May;24(5):617–627. doi: 10.1161/01.res.24.5.617. [DOI] [PubMed] [Google Scholar]
- Forrester T. An estimate of adenosine triphosphate release into the venous effluent from exercising human forearm muscle. J Physiol. 1972 Aug;224(3):611–628. doi: 10.1113/jphysiol.1972.sp009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T., Hamilton I. J. Proceedings: Functional hyperaemia in soleus muscle of the cat. J Physiol. 1975 Jul;249(1):20P–21P. [PMC free article] [PubMed] [Google Scholar]
- Forrester T., Harper A. M., MacKenzie E. T., Thomson E. M. Effect of adenosine triphosphate and some derivatives on cerebral blood flow and metabolism. J Physiol. 1979 Nov;296:343–355. doi: 10.1113/jphysiol.1979.sp013009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T., Lind A. R. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol. 1969 Oct;204(2):347–364. doi: 10.1113/jphysiol.1969.sp008917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T., Williams C. A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977 Jun;268(2):371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick G. P., Lowenstein J. M. Studies of 5'-nucleotidase in the perfused rat heart. Including measurements of the enzyme in perfused skeletal muscle and liver. J Biol Chem. 1976 Oct 25;251(20):6372–6378. [PubMed] [Google Scholar]
- Giles R. W., Wilcken D. E. Reactive hyperaemia in the dog heart: inter-relations between adenosine, ATP, and aminophylline and the effect of indomethacin. Cardiovasc Res. 1977 Mar;11(2):113–121. doi: 10.1093/cvr/11.2.113. [DOI] [PubMed] [Google Scholar]
- HOLTON P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J Physiol. 1959 Mar 12;145(3):494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël M., Lesbats B., Meunier F. M., Stinnakre J. Postsynaptic release of adenosine triphosphate induced by single impulse transmitter action. Proc R Soc Lond B Biol Sci. 1976 Jun 30;193(1113):461–468. doi: 10.1098/rspb.1976.0058. [DOI] [PubMed] [Google Scholar]
- KUPERMAN A. S., VOLPERT W. A., OKAMOTO M. RELEASE OF ADENINE NUCLEOTIDE FROM NERVE AXONS. Nature. 1964 Dec 5;204:1000–1001. doi: 10.1038/2041000a0. [DOI] [PubMed] [Google Scholar]
- Kannengiesser G. J., Opie L. H., van der Werff T. J. Impaired cardiac work and oxygen uptake after reperfusion of regionally ischaemic myocardium. J Mol Cell Cardiol. 1979 Feb;11(2):197–207. doi: 10.1016/0022-2828(79)90464-4. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Liebermeister H., Battersby E. J., Morgan H. E. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 1967 Apr;212(4):804–814. doi: 10.1152/ajplegacy.1967.212.4.804. [DOI] [PubMed] [Google Scholar]
- Paddle B. M., Burnstock G. Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels. 1974;11(3):110–119. doi: 10.1159/000158005. [DOI] [PubMed] [Google Scholar]
- Parkinson P. I. Proceedings: The effect of graduated exercise on the concentration of adenine nucleotides in plasma. J Physiol. 1973 Oct;234(2):72P–74P. [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Pritchard J. B., O'Connor N., Oliver J. M., Berlin R. D. Uptake and supply of purine compounds by the liver. Am J Physiol. 1975 Oct;229(4):967–972. doi: 10.1152/ajplegacy.1975.229.4.967. [DOI] [PubMed] [Google Scholar]
- Pull I., McIlwain H. Metabolism of ( 14 C)adenine and derivatives by cerebral tissues, superfused and electrically stimulated. Biochem J. 1972 Feb;126(4):965–973. doi: 10.1042/bj1260965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok T. J., Boink A. B., Spies F., Blok F. J., Maas A. H., Zimmerman A. N. Energy dependence of the calcium paradox. J Mol Cell Cardiol. 1978 Nov;10(11):991–1002. doi: 10.1016/0022-2828(78)90395-4. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. A simple, rapid method for detecting the efflux of small quantities of adenosine triphosphate from biological tissues. Comp Biochem Physiol A Comp Physiol. 1974 Jul 1;48(3):561–571. doi: 10.1016/0300-9629(74)90739-7. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON J. R., DIPIETRO D. L. EVIDENCE FOR EXTRACELLULAR ENZYMIC ACTIVITY OF THE ISOLATED PERFUSED RAT HEART. Biochem J. 1965 Apr;95:226–232. doi: 10.1042/bj0950226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINBURY M. M., PAPIERSKI D. H., HEMMER M. L., HAMBOURGER W. E. Coronary dilator action of the adenine-ATP series. J Pharmacol Exp Ther. 1953 Nov;109(3):255–260. [PubMed] [Google Scholar]
- WOLF M. M., BERNE R. M. Coronary vasodilator properties of purine and pyrimidine derivatives. Circ Res. 1956 May;4(3):343–348. doi: 10.1161/01.res.4.3.343. [DOI] [PubMed] [Google Scholar]
- Ziegelhöffer A., Fedelesová M., Siska K. The influence of exogenous ATP on cardiac metabolism in acute hypoxia. Cardiology. 1971;56(1):136–142. doi: 10.1159/000169355. [DOI] [PubMed] [Google Scholar]
- Zimmer H. G., Trendelenburg C., Kammermeier H., Gerlach E. De novo synthesis of myocardial adenine nucleotides in the rat. Acceleration during recovery from oxygen deficiency. Circ Res. 1973 May;32(5):635–642. doi: 10.1161/01.res.32.5.635. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. N., Hülsmann W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966 Aug 6;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]


