Abstract
1. Mechanisms controlling the secretion of [3H]noradrenaline from the noradrenergic nerves of guinea-pig isolated vas deferens, prelabelled by incubation with [3H]noradrenaline, were studied using (a) different modes of (extramural or transmural) electrical nerve stimulation (a total of 300 shocks of varying strength, and a duration of 2 msec) at 1-30 Hz, or (b) depolarizing concentrations of K+ (60-110 mm).
2. The fractional rise in efflux of 3H-labelled material (Δt) was used to measure the secretion of [3H]noradrenaline.
3. The dependence of [3H]noradrenaline secretion on the external Ca2+ concentration (1-8 mm) was essentially hyperbolic. Double reciprocal plot analysis (1/Δt vs. 1/Ca2+) of the data yields that blockade of α-autoinhibition (phentolamine 1 μm) does not increase the maximal secretory velocity, but does enhance the apparent affinity of the secretory mechanism for external Ca2+. Exogenous noradrenaline has (qualitatively) opposite effects. The interaction between α-autoinhibition and external Ca2+ thus shows a `competitive' pattern, indicating that restriction of the utilization of external Ca2+ is a major mechanism in α-autoinhibition of noradrenaline secretion, in this system.
4. Phenoxybenzamine (10 μm) and phentolamine (1 μm) increased the secretion of [3H]noradrenaline evoked by depolarization with K+ much less than that caused by electrical nerve stimulation (frequencies up to 10 Hz). Exogenous noradrenaline (1-5 μm) depressed the secretion evoked by both modes of stimulation. The results indicate that α-autoinhibition of [3H]noradrenaline secretion is mainly operative when the secretory stimulus requires conduction of nerve impulses between varicosities.
5. The frequency dependence of [3H]noradrenaline secretion was hyperbolic, both in the presence and in the absence of α-autoinhibition; at each frequency the secretion (Δt per shock) increased with the Ca2+ concentration in the medium (0·6-8 mm). Double reciprocal plot analysis (1/Δt vs. 1/frequency) of the data yields that the pattern of interaction between external Ca2+ and facilitation depends on the presence or absence of α-autoinhibition (phentolamine 1 μm); in the former case it is `non-competitive', in the latter `competitive'. Similar analysis of the effect of facilitation by increasing the length of stimulus trains (from 5 to 300 pulses) at a constant frequency (5 Hz), on the Ca2+ dependence of Δt (1/Δt vs. 1/Ca2+) in the absence of α-autoinhibition also yields that facilitation promotes utilization of external Ca2+. These results apparently imply that a rise in external Ca2+, in the presence of α-autoinhibition, augments the secretory response to electrical nerve stimulation mainly by promoting recruitment of active units (varicosities?), without markedly altering their `affinity' for facilitation. In the absence of autoinhibition (when all units are already recruited?), the results seem to imply that facilitation promotes depolarization-secretion coupling in each, by more efficient utilization of external Ca2+.
6. The pattern of interaction between α-autoinhibition and facilitation depends on the Ca2+ concentration in the medium. At or below the physiological level of Ca2+ in extracellular fluid (1·2 mm) it is `non-competitive', indicating that α-autoinhibition and facilitation act, at least in part, at separate targets under these conditions. At high (5·4 mm) external Ca2+ the pattern becomes almost purely `competitive', indicating that facilitation can, under suitable conditions, overcome all manifestations of α-autoinhibition.
7. The secretion evoked by electrical nerve stimulation (Δt per shock, at 1 or 10 Hz) increased with the strength of applied shocks, both when applied extra- or transmurally, in the presence or absence of α-autoinhibition. In the former case the rise in (Δt per shock) vs. (current strength) was hyperbolic, in the latter it followed a biphasic pattern. Double reciprocal plot analysis (1/Δt vs. 1/current) of the data yields a `non-competitive' pattern of interaction between facilitation or α-autoinhibition, and exogenous current, when stimulation was extramural. When it was transmural the pattern is `competitive'. The results seem to imply that hyperpolarization, or depolarization, of nerve terminals are major mechanisms whereby α-autoinhibition and facilitation, respectively, exert their effects on the secretory response to electrical nerve stimulation.
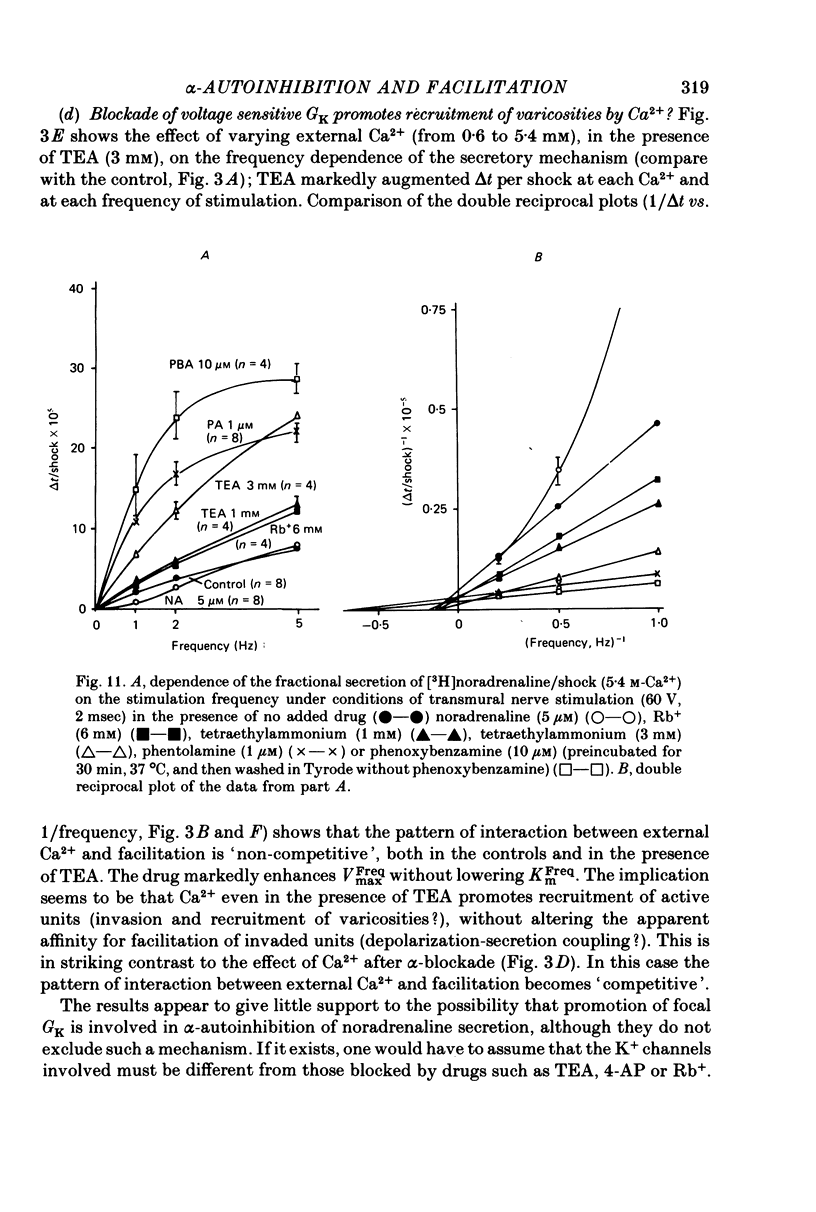
8. Neither activation of Na+, K+-ATPase, nor promotion of GCl appear to be critically involved in α-autoinhibition. Experiments with known blockers of GK (tetraethylammonium, 4-aminopyridine and Rb+) did not give support to the notion that promotion of K+ efflux is a mechanism whereby prejunctional α-adrenoceptors cause (hyperpolarization of nerve terminals and) autoinhibition of secretion. If α-autoinhibition does involve K+ channels in the nerve terminal membrane, then these must be different from the (voltage-sensitive) K+ channels blocked by the above mentioned inhibitors of K+ efflux.
9. The results are discussed in the context of a model that assumes that local control of noradrenaline secretion from noradrenergic nerves may be exerted both by control of invasion of terminals, and by control of depolarization—secretion coupling in each invaded varicosity. Under suitable conditions facilitation and α-autoinhibition may interact at both levels. It proposed that utilization of external Ca2+ plays a pivotal role for both, and that restriction of invasion of nerve terminal varicosities is the main effect of α-autoinhibition, while promotion of depolarization—secretion coupling is the main effect of facilitation, at physiological concentrations of Ca2+ in the medium. For the nerve the role of this dual control system is proposed to be to ensure `rotational' activation of varicosities, and for the effector cell of noradrenergic junctions, to increase the signal/noise ratio.
Full text
PDF




































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. THE RUBIDIUM AND POTASSIUM PERMEABILITY OF FROG MUSCLE MEMBRANE. J Physiol. 1964 Dec;175:134–159. doi: 10.1113/jphysiol.1964.sp007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian G. K., Bunney B. S. Dopamine"autoreceptors": pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch Pharmacol. 1977 Mar;297(1):1–7. doi: 10.1007/BF00508803. [DOI] [PubMed] [Google Scholar]
- Aghajanian G. K., Cedarbaum J. M., Wang R. Y. Evidence for norepinephrine-mediated collateral inhibition of locus coeruleus neurons. Brain Res. 1977 Nov 18;136(3):570–577. doi: 10.1016/0006-8993(77)90083-x. [DOI] [PubMed] [Google Scholar]
- BROWN G. L., GILLESPIE J. S. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957 Aug 29;138(1):81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN G. L., HOLMES O. The effects of activity on mammalian nerve fibres of low conduction velocity. Proc R Soc Lond B Biol Sci. 1956 Mar 27;144(918):1–14. doi: 10.1098/rspb.1956.0013. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E., KURIYAMA H. FACILITATION OF TRANSMISSION FROM AUTONOMIC NERVE TO SMOOTH MUSCLE OF GUINEA-PIG VAS DEFERENS. J Physiol. 1964 Jul;172:31–49. doi: 10.1113/jphysiol.1964.sp007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bell C., Vogt M. Release of endogenous noradrenaline from an isolated muscular artery. Release of endogenous noradrenaline from an isolated muscular artery. J Physiol. 1971 Jun;215(2):509–520. doi: 10.1113/jphysiol.1971.sp009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. An electrophysiological analysis of the storage and release of noradrenaline at sympathetic nerve terminals. J Physiol. 1973 Mar;229(2):515–531. doi: 10.1113/jphysiol.1973.sp010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T. An electrophysiological analysis of the effect of Ca ions on neuromuscular transmission in the mouse vas deferens. Br J Pharmacol. 1975 Sep;55(1):97–104. doi: 10.1111/j.1476-5381.1975.tb07616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Middleton J. An electrophysiological analysis of the effects of amine-uptake blockers and alpha-adrenoceptor blockers on adrenergic neuromuscular transmission. Br J Pharmacol. 1975 Sep;55(1):87–95. doi: 10.1111/j.1476-5381.1975.tb07615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley A. G., Cunnane T. C. Is the vesicle the quantum of sympathetic transmission? [proceedings]. J Physiol. 1978 Jul;280:30P–31P. [PubMed] [Google Scholar]
- Blakeley A. G., Cunnane T. C. The packeted release of transmitter from the sympathetic nerves of the guinea-pig vas deferens: an electrophysiological study. J Physiol. 1979 Nov;296:85–96. doi: 10.1113/jphysiol.1979.sp012992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Johnson E. M., Jr, Needleman P. Calcium-dependent norepinephrine release from presynaptic nerve endings in vitro. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2237–2240. doi: 10.1073/pnas.69.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Caulfield M. P. Hyperpolarizing 'alpha 2'-adrenoceptors in rat sympathetic ganglia. Br J Pharmacol. 1979 Mar;65(3):435–445. doi: 10.1111/j.1476-5381.1979.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The generation of impulses in motoneurones. J Physiol. 1957 Dec 3;139(2):232–249. doi: 10.1113/jphysiol.1957.sp005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum J. M., Aghajanian G. K. Catecholamine receptors on locus coeruleus neurons: pharmacological characterization. Eur J Pharmacol. 1977 Aug 15;44(4):375–385. doi: 10.1016/0014-2999(77)90312-0. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Kalsner S. An examination of the negative feedback function of presynaptic adrenoceptors in a vascular tissue. Br J Pharmacol. 1979 Nov;67(3):401–407. doi: 10.1111/j.1476-5381.1979.tb08694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J. POTENTIAL CHANGES IN THE CRAYFISH MOTOR NERVE TERMINAL DURING REPETITIVE STIMULATION. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Jan 11;282:323–337. doi: 10.1007/BF00412507. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Volle R. L. The actions of the catecholamines on transmission in the superior cervical ganglion of the cat. J Pharmacol Exp Ther. 1966 Oct;154(1):1–13. [PubMed] [Google Scholar]
- Dismukes K., de Boer A. A., Mulder A. H. On the mechanism of alpha-receptor mediated modulation of 3H-noradrenaline release from slices of rat brain neocortex. Naunyn Schmiedebergs Arch Pharmacol. 1977 Sep;299(2):115–122. doi: 10.1007/BF00498553. [DOI] [PubMed] [Google Scholar]
- EDWARDS C., OTTOSON D. The site of impulse initiation in a nerve cell of a crustacean stretch receptor. J Physiol. 1958 Aug 29;143(1):138–148. doi: 10.1113/jphysiol.1958.sp006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Drug-induced changes in the release of ( 3 H)-noradrenaline from field stimulated rat iris. Br J Pharmacol. 1971 Sep;43(1):97–106. doi: 10.1111/j.1476-5381.1971.tb07160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O., Malamfors T. 3 H-noradrenaline release and mechanical response in the field stimulated mouse vas deferens. Acta Physiol Scand Suppl. 1971;371:1–18. doi: 10.1111/j.1748-1716.1971.tb05210.x. [DOI] [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M., Pascual R. Release of noradrenaline from cat spleen slices by potassium. Br J Pharmacol. 1978 Feb;62(2):207–211. doi: 10.1111/j.1476-5381.1978.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. An electrophysiological investigation of mammalian motor nerve terminals. J Physiol. 1963 Apr;166:145–167. doi: 10.1113/jphysiol.1963.sp007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefely W. E. Effects of catecholamines in the cat superior cervical ganglion and their postulated rôle as physiological modulators of ganglionic transmission. Prog Brain Res. 1969;31:61–72. doi: 10.1016/S0079-6123(08)63228-8. [DOI] [PubMed] [Google Scholar]
- Harper B., Hughes I. E. The effect of stimulus intensity on alpha-adrenoceptor-mediated feedback control of noradrenaline release. Br J Pharmacol. 1978 Aug;63(4):689–691. doi: 10.1111/j.1476-5381.1978.tb17284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G., Hughes J. Proceedings: Modulation of frequency-dependent noradrenaline release by calcium, angiotensin and morphine. Br J Pharmacol. 1974 Nov;52(3):455P–456P. [PMC free article] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Alpha-drenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol. 1980 Apr;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. Differential labelling of intraneuronal noradrenaline stores with different concentrations of (-)-3H-noradrenaline. Br J Pharmacol. 1973 Feb;47(2):428–430. doi: 10.1111/j.1476-5381.1973.tb08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. Evaluation of mechanisms controlling the release and inactivation of the adrenergic transmitter in the rabbit portal vein and vas deferens. Br J Pharmacol. 1972 Mar;44(3):472–491. doi: 10.1111/j.1476-5381.1972.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H., MORTON I. K. EFFECTS OF NORADRENALINE AND ISOPRENALINE ON THE PERMEABILITY OF DEPOLARIZED INTESTINAL SMOOTH MUSCLE TO INORGANIC IONS. Nature. 1965 Jan 30;205:505–506. doi: 10.1038/205505a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsner S. Single pulse stimulation of guinea-pig vas deferens and the presynaptic receptor hypothesis. Br J Pharmacol. 1979 Jun;66(2):343–349. doi: 10.1111/j.1476-5381.1979.tb13686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., McCullough J. R. Electrophysiological properties of splenic nerve in relation to norepinephrine overflow. J Pharmacol Exp Ther. 1973 Apr;185(1):49–59. [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of local blockage of motor nerve terminals. J Physiol. 1968 Dec;199(3):729–741. doi: 10.1113/jphysiol.1968.sp008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. R., Edman K. A. Effects of 4-aminopyridine on the excitation-contraction coupling in frog and rat skeletal muscle. Acta Physiol Scand. 1979 Apr;105(4):443–452. doi: 10.1111/j.1748-1716.1979.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Kirpekar M., Kirpekar S. M., Prat J. C. Effect of 4-aminopyridine on release of noradrenaline from the perfused cat spleen by nerve stimulation. J Physiol. 1977 Nov;272(3):517–528. doi: 10.1113/jphysiol.1977.sp012057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Puig M., Wakade A. R. Modification of the evoked release of noradrenaline from the perfused cat spleen by various ions and agents. J Physiol. 1972 Mar;221(3):601–615. doi: 10.1113/jphysiol.1972.sp009770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Wakade A. R. Effect of calcium on the relationship between frequency of stimulation and release of noradrenaline from the perfused spleen of the cat. Naunyn Schmiedebergs Arch Pharmacol. 1975;287(2):205–212. doi: 10.1007/BF00510451. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Puig M. Effect of flow-stop on noradrenaline release from normal spleens and spleens treated with cocaine, phentolamine or phenoxybenzamine. Br J Pharmacol. 1971 Oct;43(2):359–369. [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R. Release of noradrenaline from the cat spleen by potassium. J Physiol. 1968 Feb;194(3):595–608. doi: 10.1113/jphysiol.1968.sp008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh H., Leander S., Thesleff S. Antagonism of the paralysis produced by botulinum toxin in the rat. The effects of tetraethylammonium, guanidine and 4-aminopyridine. J Neurol Sci. 1977 May;32(1):29–43. doi: 10.1016/0022-510x(77)90037-5. [DOI] [PubMed] [Google Scholar]
- Lundh H., Thesleff S. The mode of action of 4-aminopyridine and guanidine on transmitter release from motor nerve terminals. Eur J Pharmacol. 1977 Apr 21;42(4):411–412. doi: 10.1016/0014-2999(77)90176-5. [DOI] [PubMed] [Google Scholar]
- MERRILLEES N. C., BURNSTOCK G., HOLMAN M. E. CORRELATION OF FINE STRUCTURE AND PHYSIOLOGY OF THE INNERVATION OF SMOOTH MUSCLE IN THE GUINEA PIG VAS DEFERENS. J Cell Biol. 1963 Dec;19:529–550. doi: 10.1083/jcb.19.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Bártfai T. Discrimination between mathematical models of biological systems exemplified by enzyme steady state kinetics. Acta Biol Med Ger. 1973;31(2):203–215. [PubMed] [Google Scholar]
- McCulloch M. W., Bevan J. A., Su C. Effects of phenoxybenzamine and norepinephrine on transmitter release in the pulmonary artery of the rabbit. Blood Vessels. 1975;12(2):122–123. doi: 10.1159/000158044. [DOI] [PubMed] [Google Scholar]
- Merrillees N. C. The nervous environment of individual smooth muscle cells of the guinea pig vas deferens. J Cell Biol. 1968 Jun;37(3):794–817. doi: 10.1083/jcb.37.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgó J., Lundh H., Thesleff S. Potency of 3,4-diaminopyridine and 4-aminopyridine on mammalian neuromuscular transmission and the effect of pH changes. Eur J Pharmacol. 1980 Jan 11;61(1):25–34. doi: 10.1016/0014-2999(80)90378-7. [DOI] [PubMed] [Google Scholar]
- Mulder A. H., de Langen C. D., de Regt V., Hogenboom F. Alpha-receptor-mediated modulation of 3H-noradrenaline release from rat brain cortex synaptosomes. Naunyn Schmiedebergs Arch Pharmacol. 1978 Jun;303(2):193–196. doi: 10.1007/BF00508068. [DOI] [PubMed] [Google Scholar]
- Parnas I., Segev I. A mathematical model for conduction of action potentials along bifurcating axons. J Physiol. 1979 Oct;295:323–343. doi: 10.1113/jphysiol.1979.sp012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelhate M., Pichon Y. Proceedings: Selective inhibition of potassium current in the giant axon of the cockroach. J Physiol. 1974 Oct;242(2):90P–91P. [PubMed] [Google Scholar]
- RICHARDSON K. C. The fine structure of autonomic nerve endings in smooth muscle of the rat vas deferens. J Anat. 1962 Oct;96:427–442. [PMC free article] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Starke K. Influence of -receptor stimulants on noradrenaline release. Naturwissenschaften. 1971 Aug;58(8):420–420. doi: 10.1007/BF00591535. [DOI] [PubMed] [Google Scholar]
- Starke K. Influence of extracellular noradrenaline on the stimulation-evoked secretion of noradrenaline from sympathetic nerves: evidence for an -receptor-mediated feed-back inhibition of noradrenaline release. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(1):11–23. doi: 10.1007/BF00505064. [DOI] [PubMed] [Google Scholar]
- Starke K., Montel H. Influence of drugs with affinity for alpha-adrenoceptors on noradrenaline release by potassium, tyramine and dimethylphenylpiperazinium. Eur J Pharmacol. 1974 Aug;27(3):273–280. doi: 10.1016/0014-2999(74)90001-6. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Bartfai T., Alberts P. The influence of 8-Br 3', 5'-cyclic nucleotide analogs and of inhibitors of 3', 5'-cyclic nucleotide phosphodiesterase, on noradrenaline secretion and neuromuscular transmission in guinea-pig vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1979 Aug;308(2):99–105. doi: 10.1007/BF00499050. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Brundin J. Frequency-dependence of 3H-noradrenaline secretion from human vasoconstrictor nerves: modification by factors interfering with alpha-or beta-adrenoceptor or prostaglandin E2 mediated control. Acta Physiol Scand. 1977 Oct;101(2):199–210. doi: 10.1111/j.1748-1716.1977.tb05999.x. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Comparison of secretion of sympathetic neurotransmitter induced by nerve stimulation with that evoked by high potassium, as triggers of dual alpha-adrenoceptor mediated negative feed-back control of noradrenaline secretion. Prostaglandins. 1973 Apr;3(4):421–426. doi: 10.1016/0090-6980(73)90149-4. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Differences in secretory excitability between short and long adrenergic neurons: comparison of 3H-noradrenaline secretion evoked by field stimulation of guinea-pig vas deferens and human blood vessels. Acta Physiol Scand. 1977 Jun;100(2):264–266. doi: 10.1111/j.1748-1716.1977.tb05947.x. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Facilitation and receptor-mediated regulation of noradrenaline secretion by control of recruitment of varicosities as well as by control of electro-secretory coupling. Neuroscience. 1978;3(12):1147–1155. doi: 10.1016/0306-4522(78)90135-5. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Frequency dependence of dual negative feedback control of secretion of sympathetic neurotransmitter in guinea-pig vas deferens. Br J Pharmacol. 1973 Oct;49(2):358–360. doi: 10.1111/j.1476-5381.1973.tb08383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L. Inhibitory effects of noradrenaline and prostaglandin E2 on neurotransmitter secretion evoked by single shocks or by short trains of nerve stimuli. Acta Physiol Scand. 1978 Feb;102(2):251–253. doi: 10.1111/j.1748-1716.1978.tb06070.x. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Presynaptic alpha-receptors do not depress the secretion of 3H-noradrenaline induced by veratridine. Acta Physiol Scand. 1979 Jul;106(3):379–380. doi: 10.1111/j.1748-1716.1979.tb06415.x. [DOI] [PubMed] [Google Scholar]
- Swedin G. Postnatal development of the mechanical response of the isolated rat vas deferens to nerve stimulation. Acta Physiol Scand. 1972 Feb;84(2):217–223. doi: 10.1111/j.1748-1716.1972.tb05172.x. [DOI] [PubMed] [Google Scholar]
- Thoa N. B., Wooten G. F., Axelrod J., Kopin I. J. On the mechanism of release of norepinephrine from sympathetic nerves induced by depolarizing agents and sympathomimetic drugs. Mol Pharmacol. 1975 Jan;11(1):10–18. [PubMed] [Google Scholar]
- Thoenen H., Haefely W., Staehelin H. Potentiation by tetraethylammonium of the response of the cat spleen to postganglionic sympathetic nerve stimulation. J Pharmacol Exp Ther. 1967 Sep;157(3):532–540. [PubMed] [Google Scholar]
- Vizi E. S. Na+-K+-activated adenosinetriphosphatase as a trigger in transmitter release. Neuroscience. 1978;3(4-5):367–384. doi: 10.1016/0306-4522(78)90040-4. [DOI] [PubMed] [Google Scholar]
- Vizi E. S. Presynaptic modulation of neurochemical transmission. Prog Neurobiol. 1979;12(3-4):181–290. doi: 10.1016/0301-0082(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Somogyi G. T., Hadházy P., Knoll J. Effect of duration and frequency of stimulation on the presynaptic inhibition by alpha-adrenoceptor stimulation of the adrenergic transmission. Naunyn Schmiedebergs Arch Pharmacol. 1973;280(1):79–91. doi: 10.1007/BF00505357. [DOI] [PubMed] [Google Scholar]
- Westfall T. C. Local regulation of adrenergic neurotransmission. Physiol Rev. 1977 Oct;57(4):659–728. doi: 10.1152/physrev.1977.57.4.659. [DOI] [PubMed] [Google Scholar]
- Younkin S. G. An analysis of the role of calcium in facilitation at the frog neuromuscular junction. J Physiol. 1974 Feb;237(1):1–14. doi: 10.1113/jphysiol.1974.sp010466. [DOI] [PMC free article] [PubMed] [Google Scholar]


