Abstract
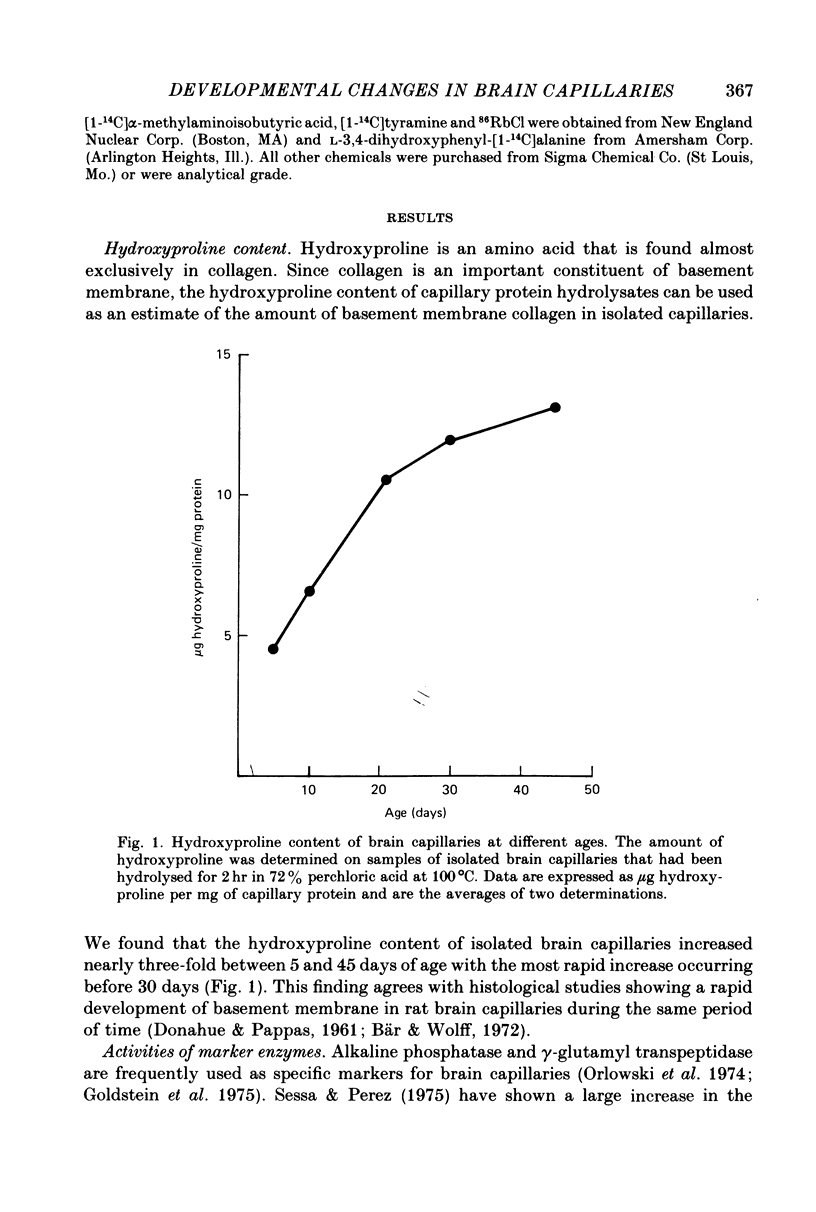
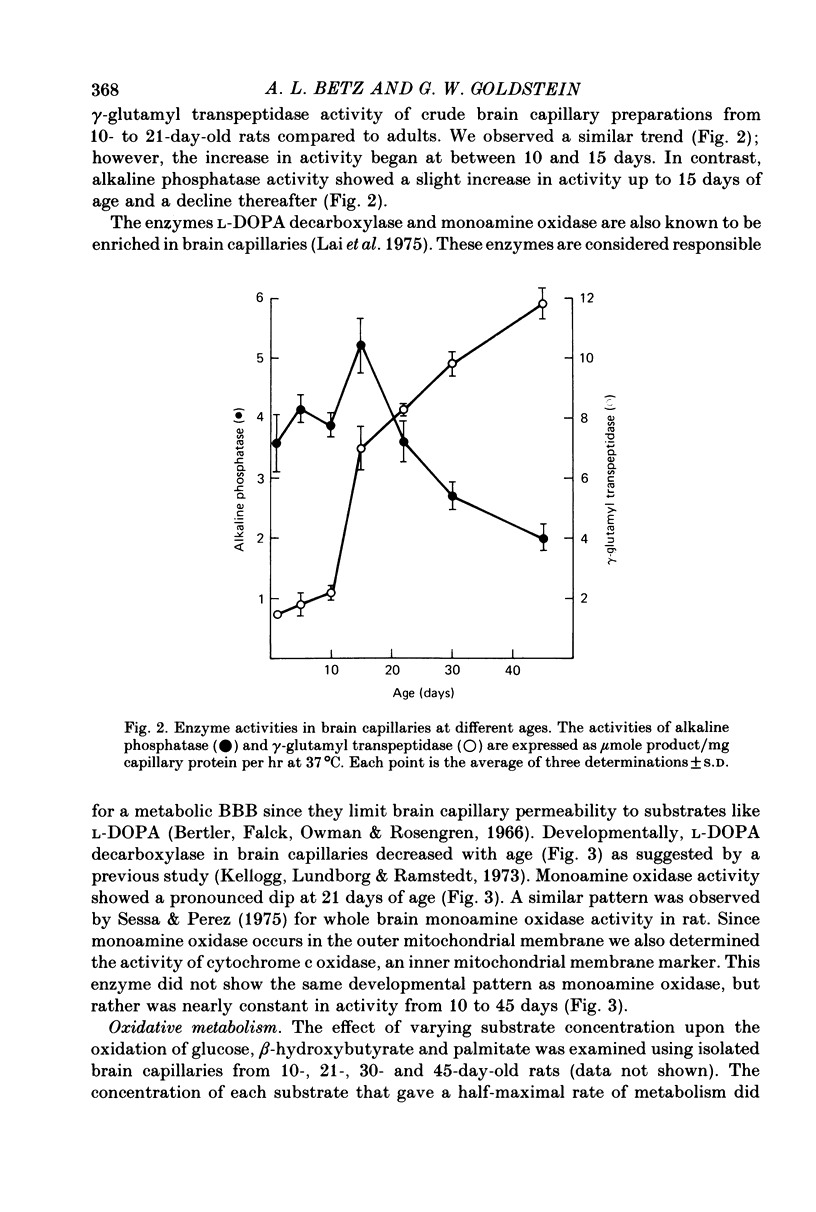
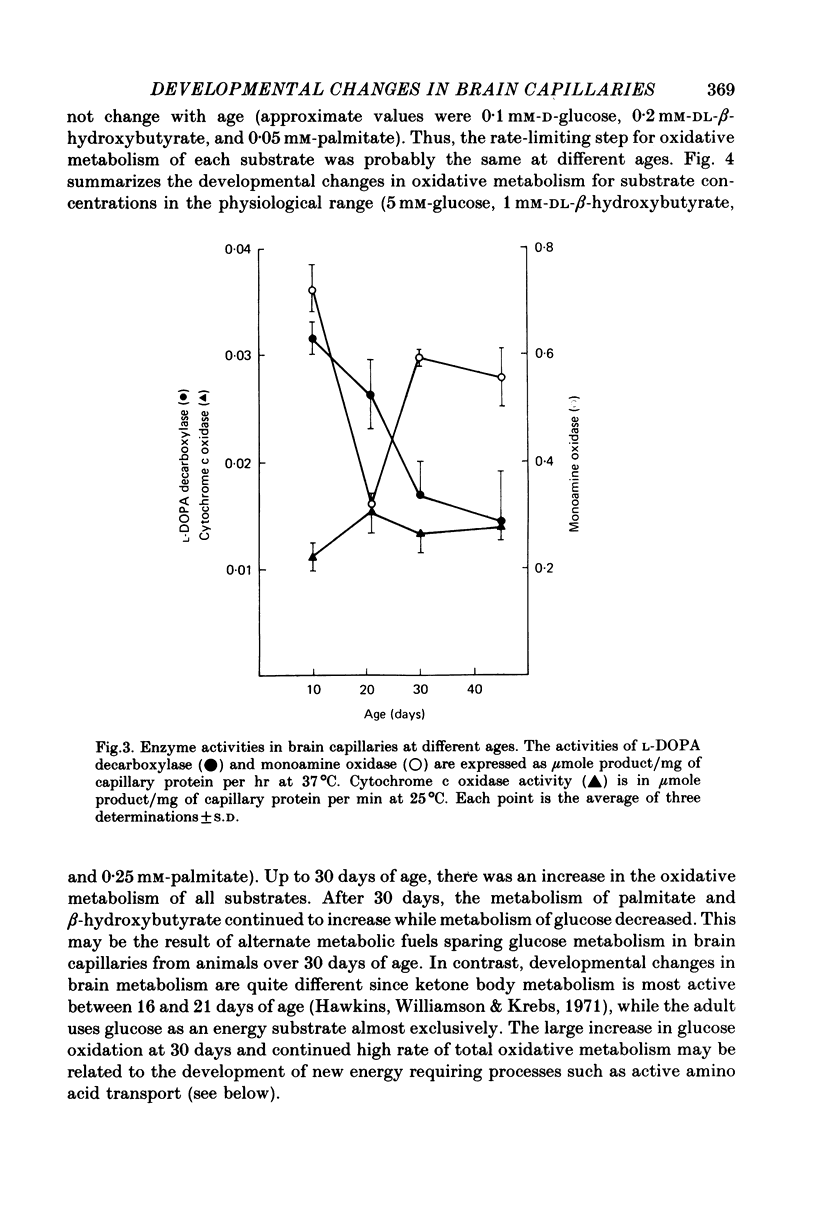
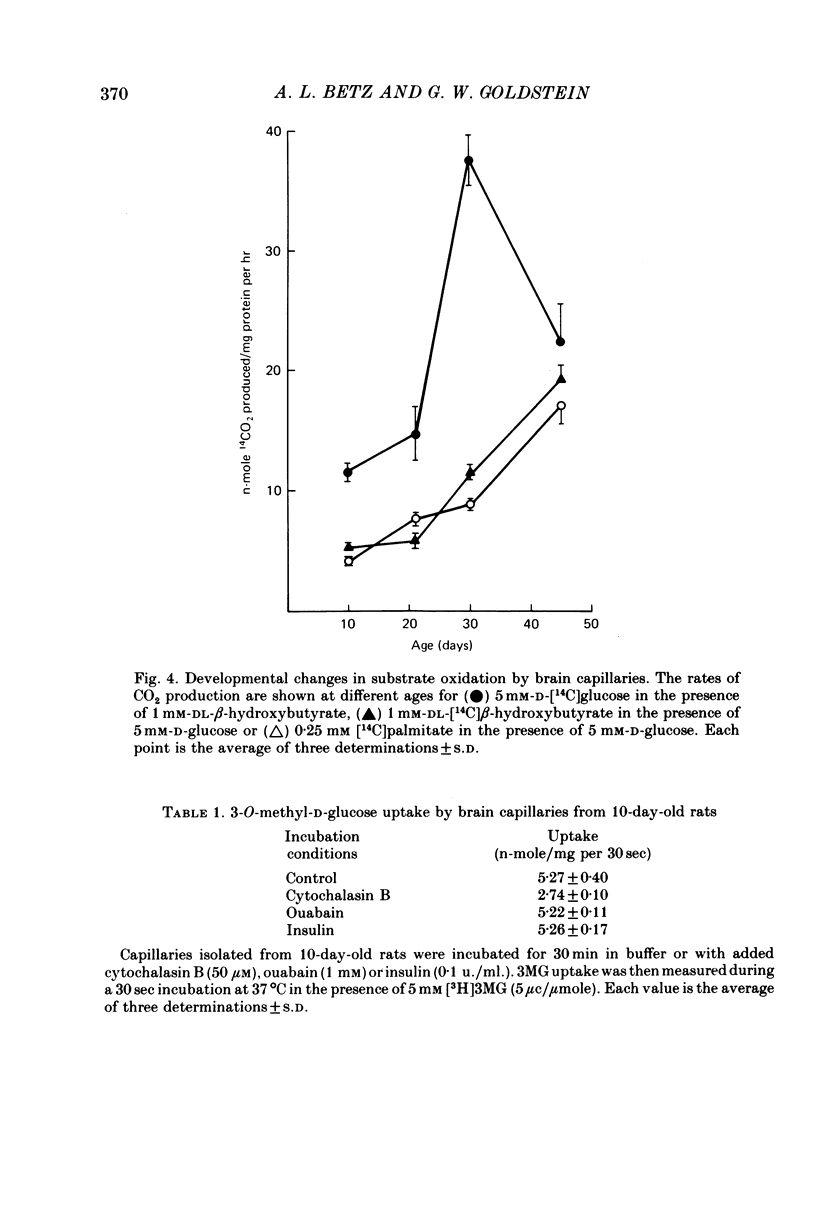
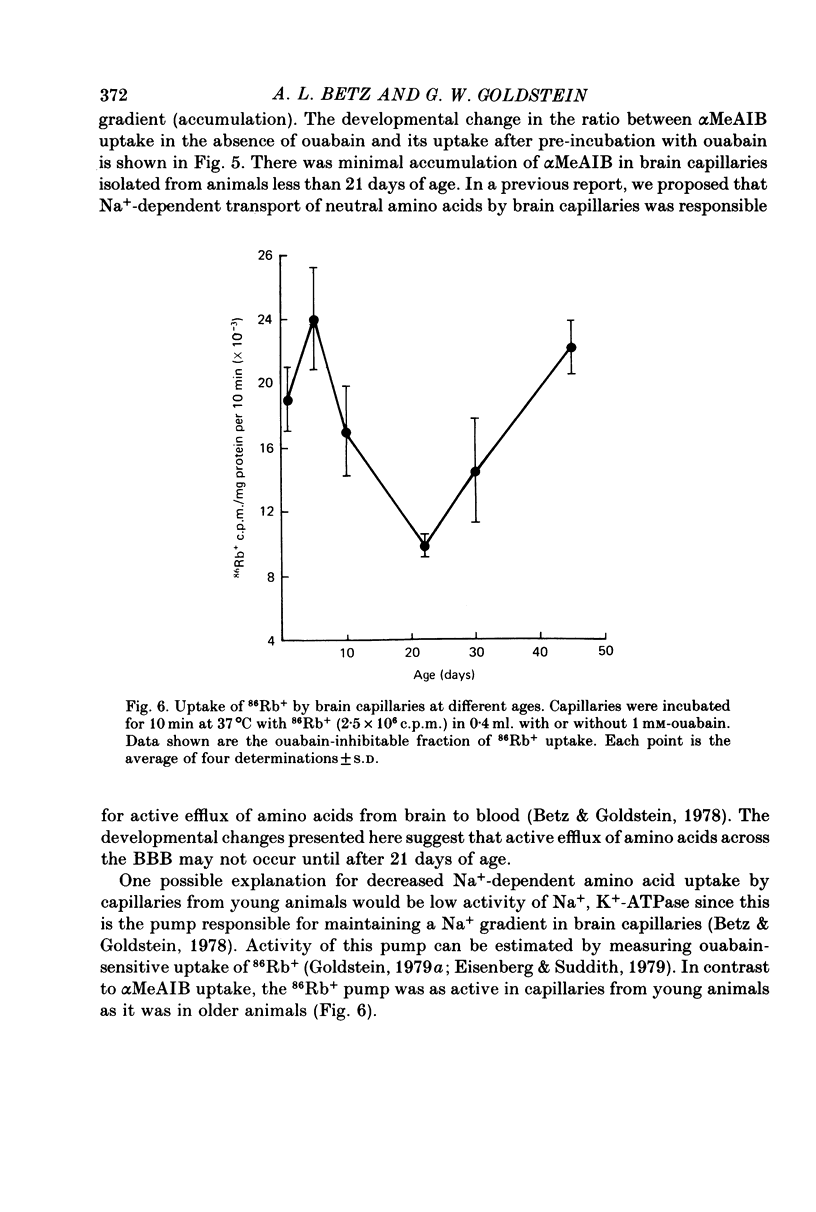
1. Capillaries were isolated from the brains of 1- to 45-day-old rats in order to study the development of metabolic and transport aspects of the blood-brain barrier. 2. The hydroxyproline content of capillary hydrolysates increased nearly threefold between 5 and 45 days of age. This finding is consistent with histological studies showing thickening of capillary basement membrane during development. 3. The activities of L-DOPA decarboxylase and monoamine oxidase were greatest in capillaries from 10-day-old rat brain. Thus, the metabolic blood-brain barrier for amine precursors is present during early development. 4. Capillaries from all ages were able to metabolize glucose, beta-hydroxybutyrate and palmitate. The rate of glucose oxidation more than doubled between 21 and 30 days of age but subsequently decreased. In contrast, beta-hydroxybutyrate and palmitate oxidation increased throughout development. These data suggest a sparing effect by alternate fuels on glucose metabolism. 5. Capillary glucose uptake was similar at 10 and 30 days of age and activity of the ouabain-sensitive K+ pump (measured using 86Rb+) was relatively constant at all ages. In contrast, Na+-dependent neutral amino acid transport was not present until after 21 days of age. Since this transport system may be responsible for the active efflux of neutral amino acids from brain to blood, it is likely that this process does not occur at the immature blood-brain barrier. 6. We conclude that various aspects of brain capillary functions show distinct developmental patterns which may be related to changes in blood-brain barrier permeability during development.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baños G., Daniel P. M., Pratt O. E. The effect of age upon the entry of some amino acids into the brain, and their incorporation into cerebral protein. Dev Med Child Neurol. 1978 Jun;20(3):335–346. doi: 10.1111/j.1469-8749.1978.tb15223.x. [DOI] [PubMed] [Google Scholar]
- Bertler A., Falck B., Owman C., Rosengrenn E. The localization of monoaminergic blood-brain barrier mechanisms. Pharmacol Rev. 1966 Mar;18(1):369–385. [PubMed] [Google Scholar]
- Betz A. L., Csejtey J., Goldstein G. W. Hexose transport and phosphorylation by capillaries isolated from rat brain. Am J Physiol. 1979 Jan;236(1):C96–102. doi: 10.1152/ajpcell.1979.236.1.C96. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Firth J. A., Goldstein G. W. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res. 1980 Jun 16;192(1):17–28. doi: 10.1016/0006-8993(80)91004-5. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Goldstein G. W. Polarity of the blood-brain barrier: neutral amino acid transport into isolated brain capillaries. Science. 1978 Oct 13;202(4364):225–227. doi: 10.1126/science.211586. [DOI] [PubMed] [Google Scholar]
- Braun L. D., Cornford E. M., Oldendorf W. H. Newborn rabbit blood-brain barrier is selectively permeable and differs substantially from the adult. J Neurochem. 1980 Jan;34(1):147–152. doi: 10.1111/j.1471-4159.1980.tb04633.x. [DOI] [PubMed] [Google Scholar]
- Brendel K., Meezan E., Carlson E. C. Isolated brain microvessels: a purified, metabolically active preparation from bovine cerebral cortex. Science. 1974 Sep 13;185(4155):953–955. doi: 10.1126/science.185.4155.953. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär T., Wolff J. R. The formation of capillary basement membranes during internal vascularization of the rat's cerebral cortex. Z Zellforsch Mikrosk Anat. 1972;133(2):231–248. doi: 10.1007/BF00307145. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Christensen H. N. On the development of amino acid transport systems. Fed Proc. 1973 Jan;32(1):19–28. [PubMed] [Google Scholar]
- Cremer J. E., Braun L. D., Oldendorf W. H. Changes during development in transport processes of the blood-brain barrier. Biochim Biophys Acta. 1976 Nov 2;448(4):633–637. doi: 10.1016/0005-2736(76)90120-6. [DOI] [PubMed] [Google Scholar]
- Cremer J. E., Cunningham V. J., Pardridge W. M., Braun L. D., Oldendorf W. H. Kinetics of blood-brain barrier transport of pyruvate, lactate and glucose in suckling, weanling and adult rats. J Neurochem. 1979 Aug;33(2):439–445. doi: 10.1111/j.1471-4159.1979.tb05173.x. [DOI] [PubMed] [Google Scholar]
- DONAHUE S., PAPPAS G. D. The fine structure of capillaries in the cerebral cortex of the rat at various stages of development. Am J Anat. 1961 May;108:331–347. doi: 10.1002/aja.1001080307. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Love E. R., Pratt O. E. The effect of age upon the influx of glucose into the brain. J Physiol. 1978 Jan;274:141–148. doi: 10.1113/jphysiol.1978.sp012139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davson H. Review lecture. The blood-brain barrier. J Physiol. 1976 Feb;255(1):1–28. doi: 10.1113/jphysiol.1976.sp011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Evans C. A., Malinowska D. H., Møllgård K., Reynolds J. M., Reynolds M. L., Saunders N. R. Studies of the development of brain barrier systems to lipid insoluble molecules in fetal sheep. J Physiol. 1979 Jul;292:207–231. doi: 10.1113/jphysiol.1979.sp012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg H. M., Suddith R. L. Cerebral vessels have the capacity to transport sodium and potassium. Science. 1979 Nov 30;206(4422):1083–1085. doi: 10.1126/science.227060. [DOI] [PubMed] [Google Scholar]
- Gałasiński W., Gadek A., Ratkiewicz A., Rzeczycki W. A convenient modification of the method for hydroxyproline determination in proteins. Anal Biochem. 1978 Apr;85(2):550–555. doi: 10.1016/0003-2697(78)90253-1. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W. Pathogenesis of brain edema and hemorrhage: role of the brain capillary. Pediatrics. 1979 Sep;64(3):357–360. [PubMed] [Google Scholar]
- Goldstein G. W. Relation of potassium transport to oxidative metabolism in isolated brain capillaries. J Physiol. 1979 Jan;286:185–195. doi: 10.1113/jphysiol.1979.sp012613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. W., Wolinsky J. S., Csejtey J., Diamond I. Isolation of metabolically active capillaries from rat brain. J Neurochem. 1975 Nov;25(5):715–717. doi: 10.1111/j.1471-4159.1975.tb04395.x. [DOI] [PubMed] [Google Scholar]
- Goridis C., Neff N. H. Monoamine oxidase: an approximation of turnover rates. J Neurochem. 1971 Sep;18(9):1673–1682. doi: 10.1111/j.1471-4159.1971.tb03740.x. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H., Krebs H. A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelle J. T., Baird-Lambert J., Cardinale G., Specor S., Udenfriend S. Isolated microvessels: the blood-brain barrier in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4544–4548. doi: 10.1073/pnas.75.9.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg C., Lundborg P., Ramstedt L. Analysis of capillary and parenchymal aromatic L-amino acid decarboxylase activity in regional brain areas during ontogenic development in the rat. Brain Res. 1973 Feb 28;50(2):369–378. doi: 10.1016/0006-8993(73)90738-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai F. M., Udenfriend S., Spector S. Presence of norepinephrine and related enzymes in isolated brain microvessels. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4622–4625. doi: 10.1073/pnas.72.11.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou L. A. Uptake of monoamines into central neurones and the blood-brain barrier in the infant rat. Br J Pharmacol. 1970 Dec;40(4):800–813. doi: 10.1111/j.1476-5381.1970.tb10656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. J., Lione A. P., Regen D. M., Tarpley H. L., Raines P. L. Brain glucose metabolism in the newborn rat. Am J Physiol. 1971 Dec;221(6):1746–1753. doi: 10.1152/ajplegacy.1971.221.6.1746. [DOI] [PubMed] [Google Scholar]
- Moore T. J., Lione A. P., Sugden M. C., Regen D. M. Beta-hydroxybutyrate transport in rat brain: developmental and dietary modulations. Am J Physiol. 1976 Mar;230(3):619–630. doi: 10.1152/ajplegacy.1976.230.3.619. [DOI] [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. ISOLATION OF GAMMA-GLUTAMYL TRANSPEPTIDASE FROM HOG KIDNEY. J Biol Chem. 1965 Jan;240:338–347. [PubMed] [Google Scholar]
- Orlowski M., Sessa G., Green J. P. Gamma-glutamyl transpeptidase in brain capillaries: possible site of a blood-brain barrier for amino acids. Science. 1974 Apr 5;184(4132):66–68. doi: 10.1126/science.184.4132.66. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders N. R. Ontogeny of the blood-brain barrier. Exp Eye Res. 1977;25 (Suppl):523–550. doi: 10.1016/s0014-4835(77)80046-8. [DOI] [PubMed] [Google Scholar]
- Sershen H., Lajtha A. Capillary transport of amino acids in the developing brain. Exp Neurol. 1976 Nov;53(2):465–474. doi: 10.1016/0014-4886(76)90086-8. [DOI] [PubMed] [Google Scholar]
- Sessa G., Perez M. M. Biochemical changes in rat brain associated with the development of the blood-brain barrier. J Neurochem. 1975 Dec;25(6):779–782. doi: 10.1111/j.1471-4159.1975.tb04407.x. [DOI] [PubMed] [Google Scholar]
- Seta K., Sershen H., Lajtha A. Cerebral amino acid uptake in vivo in newborn mice. Brain Res. 1972 Dec 12;47(2):415–425. doi: 10.1016/0006-8993(72)90649-x. [DOI] [PubMed] [Google Scholar]
- Vaughan G. L., Cook J. S. Regeneration of cation-transport capacity in HeLa cell membranes after specific blockade by ouabain. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2627–2631. doi: 10.1073/pnas.69.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]


