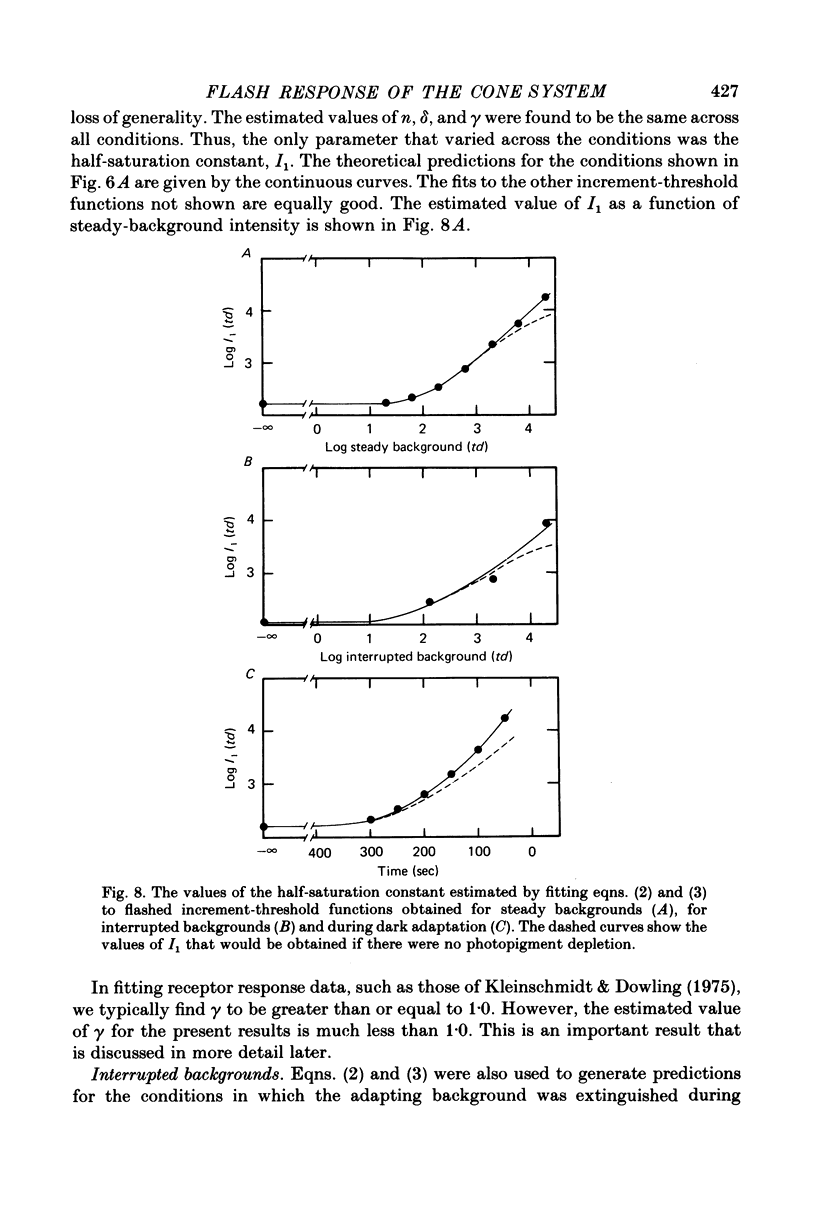
Abstract
1. Increment-threshold functions for flashed backgrounds were measured in the human fovea under several conditions: (1) during dark adaptation following full bleaches, (2) in the presence of steady adapting backgrounds and (3) 500 msec following extinction of adapting backgrounds.
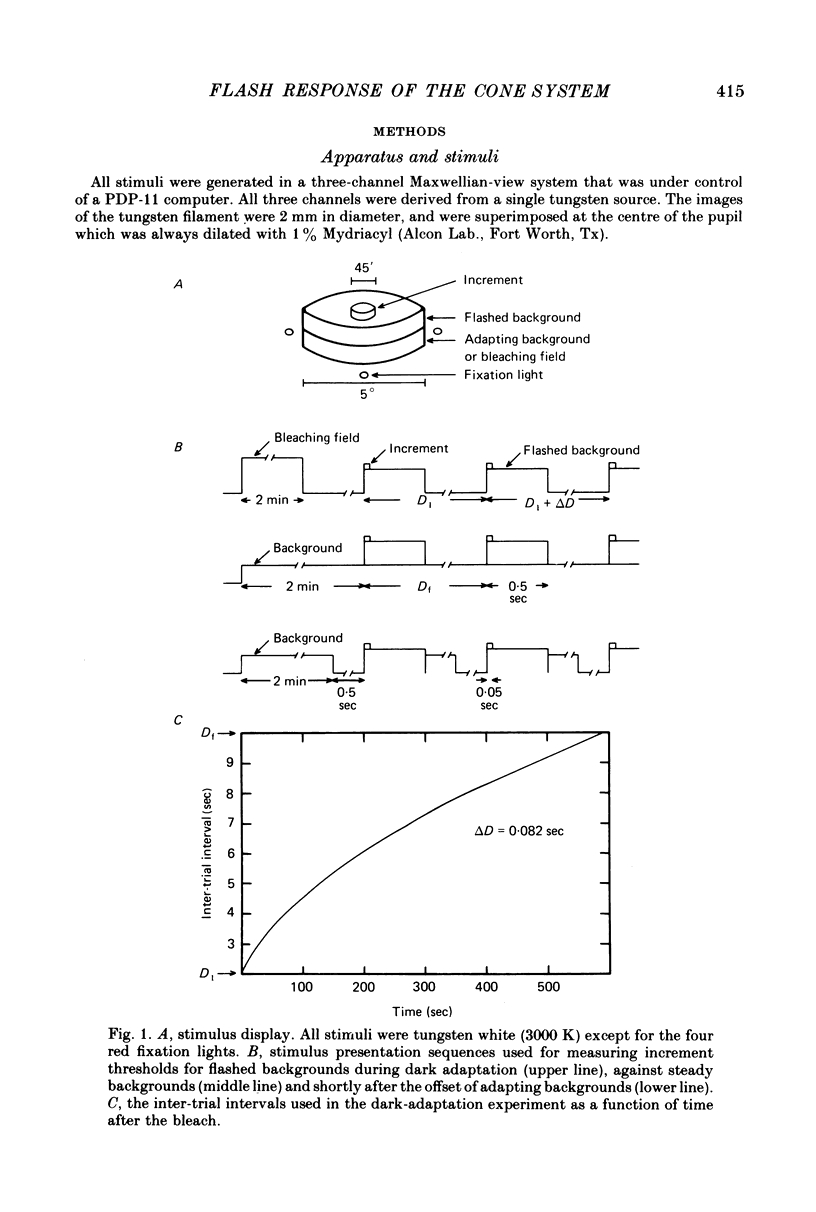
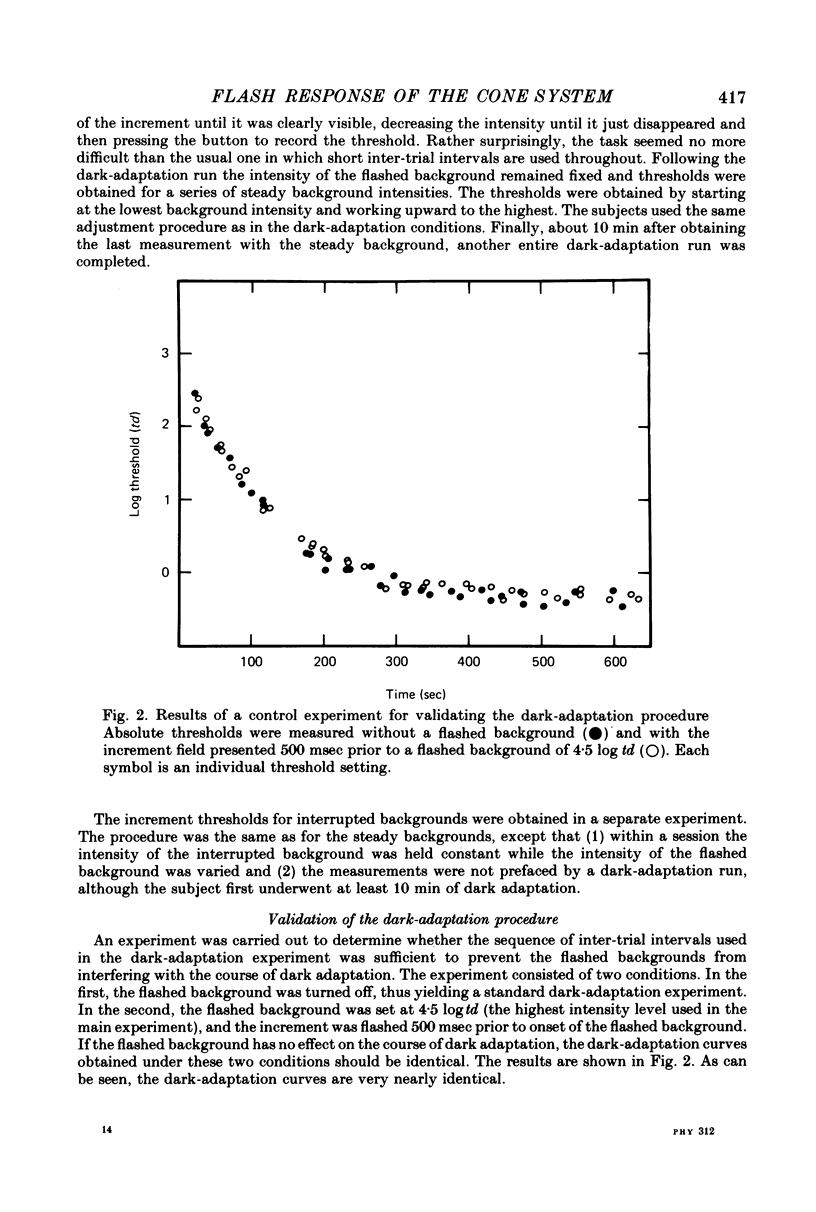
2. To prevent the intense flashed backgrounds from interfering with the course of dark adaptation the inter-trial interval was continuously increased during dark adaptation. This technique may prove generally useful for presenting suprathreshold stimuli during dark adaptation.
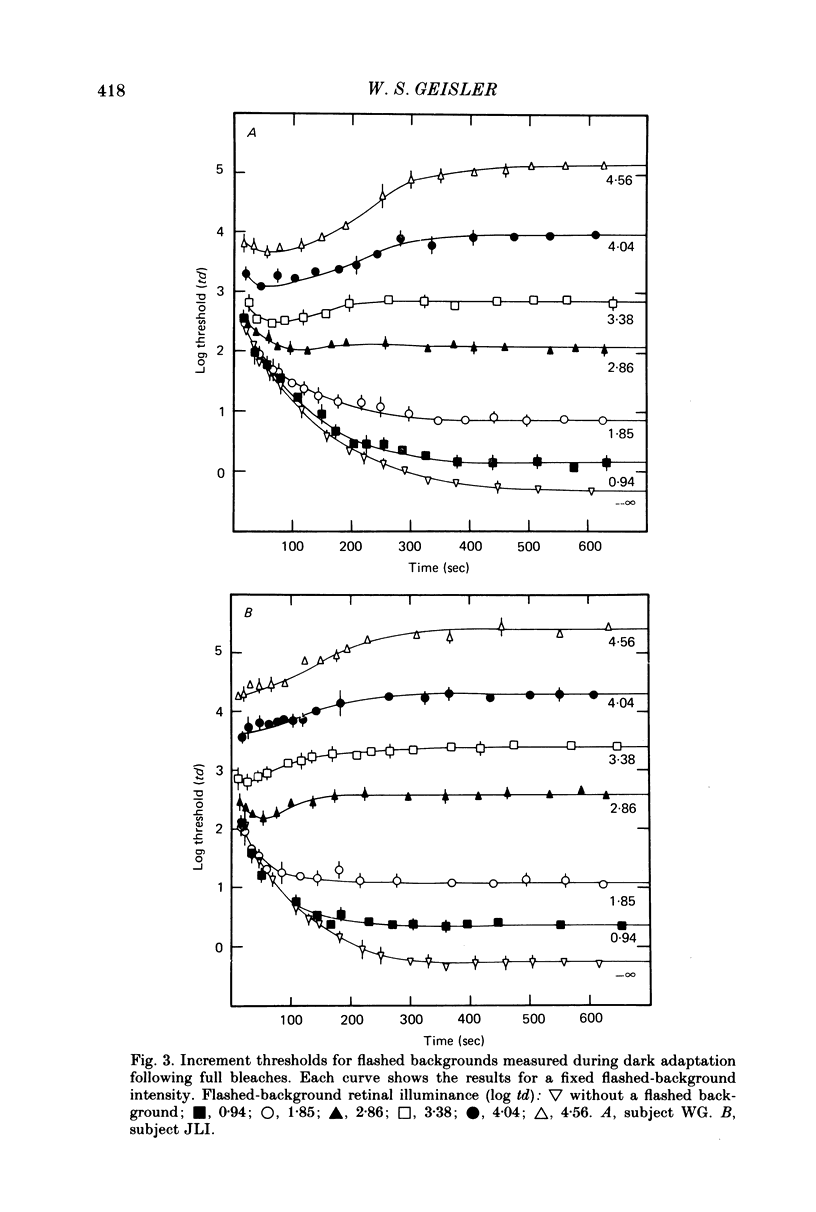
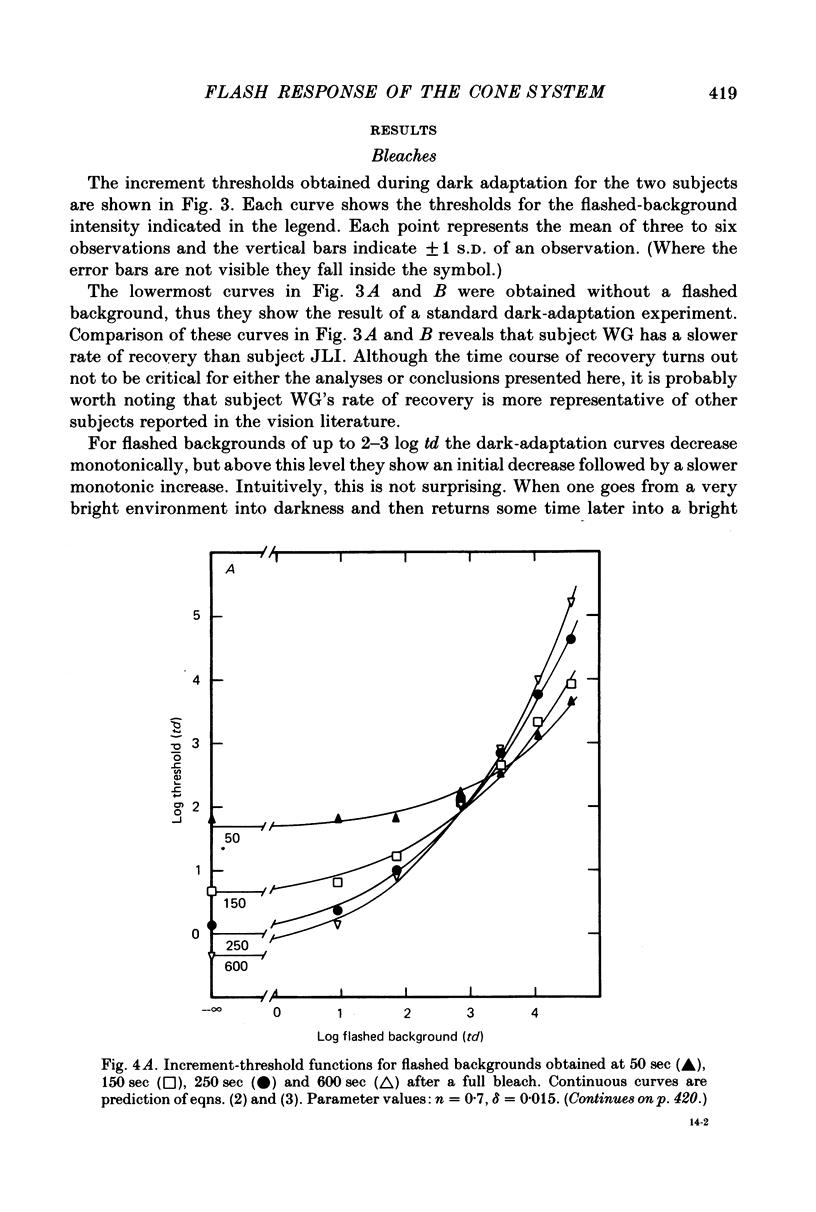
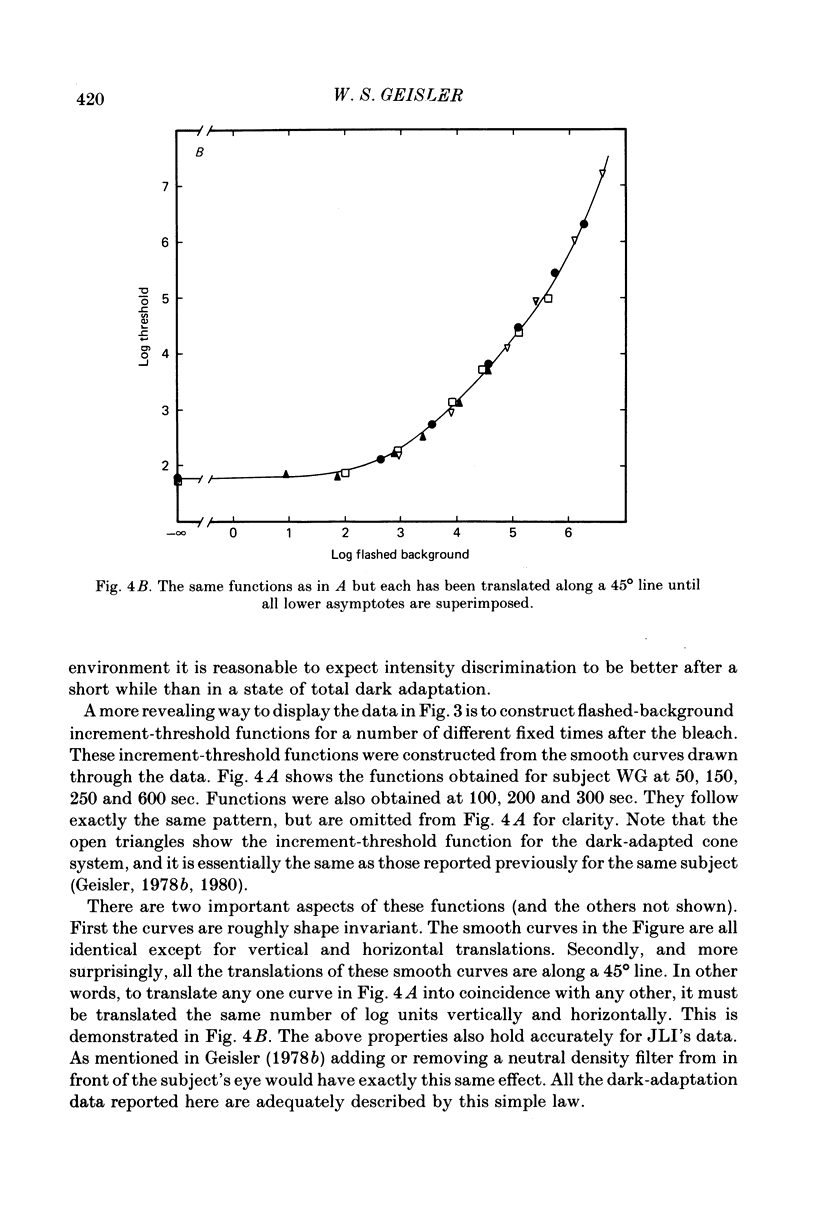
3. All the increment-threshold functions measured during dark adaptation were found to be roughly shape invariant and continuously accelerating when plotted in log—log co-ordinates. Furthermore, in order to translate a function obtained at any given time into coincidence with a function obtained at any other time, it had to be translated vertically and horizontally the same number of log units. This is equivalent to adding or removing neutral density filters from in front of the eye.
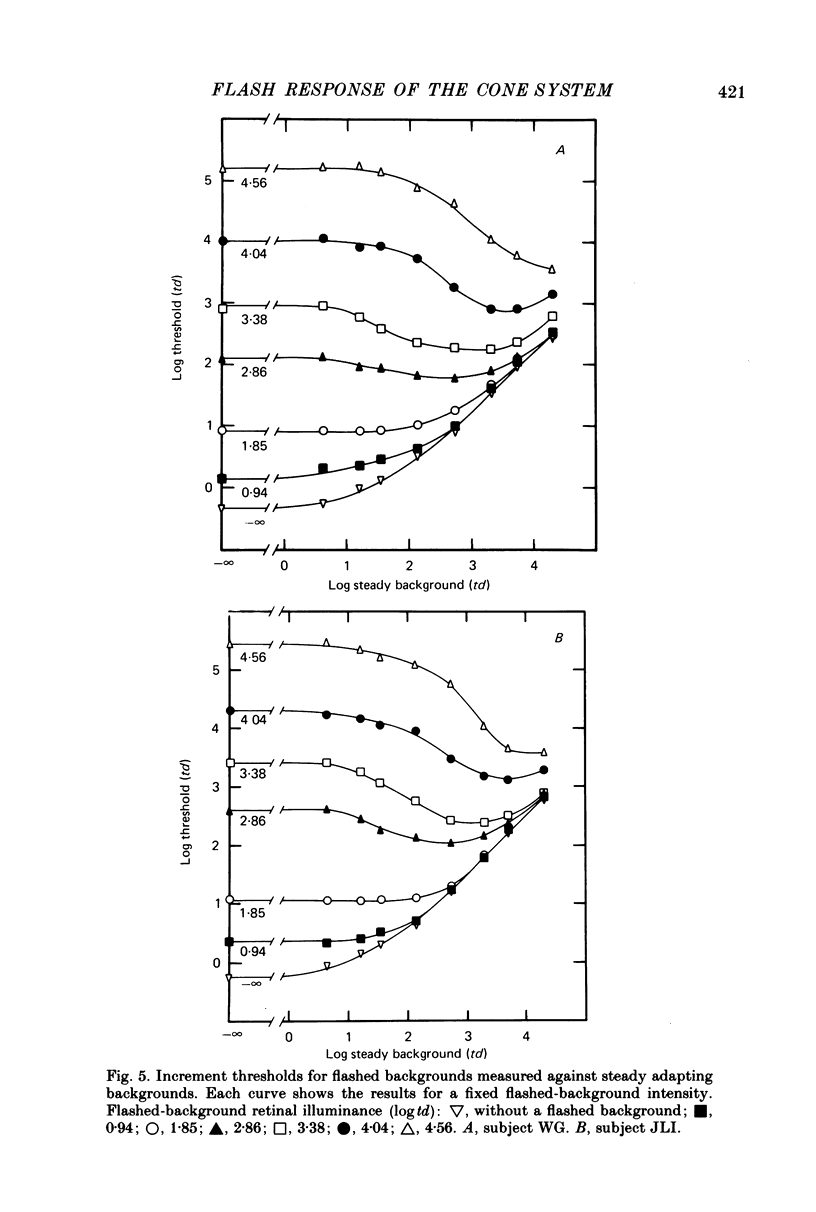
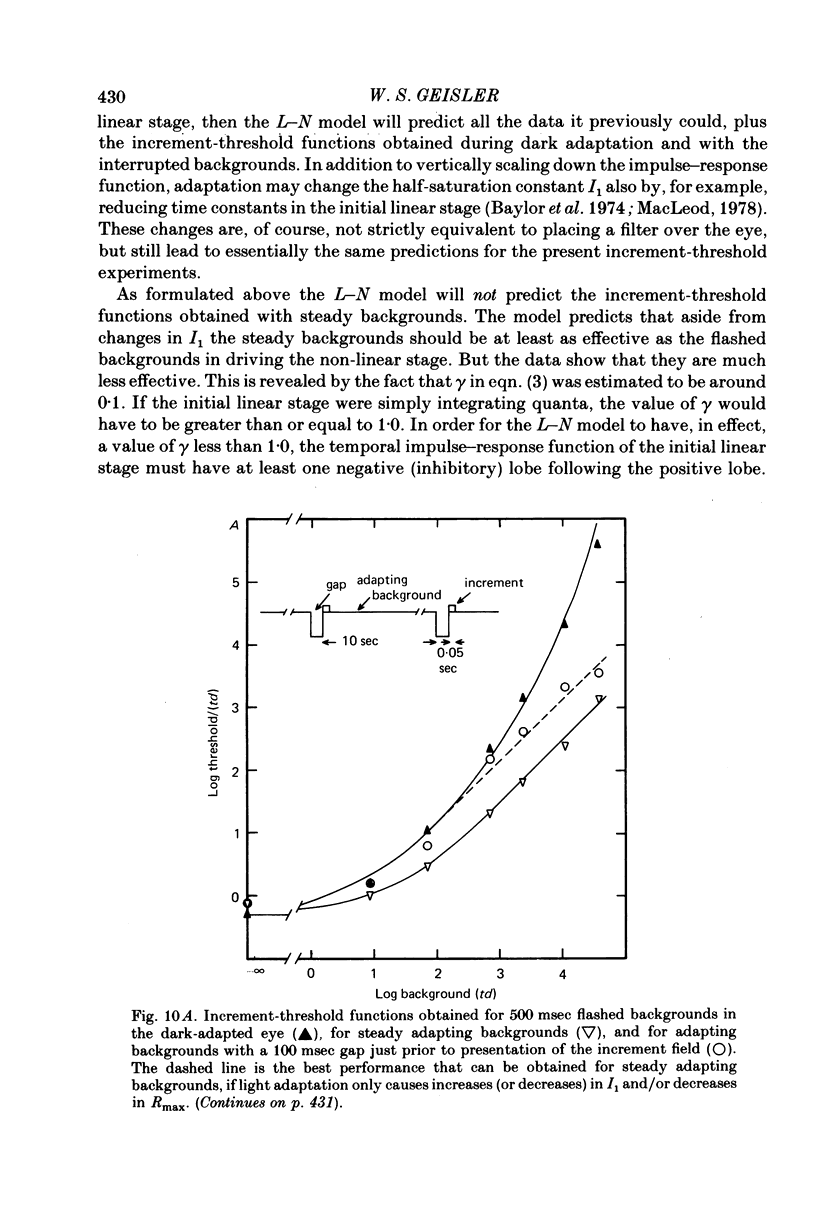
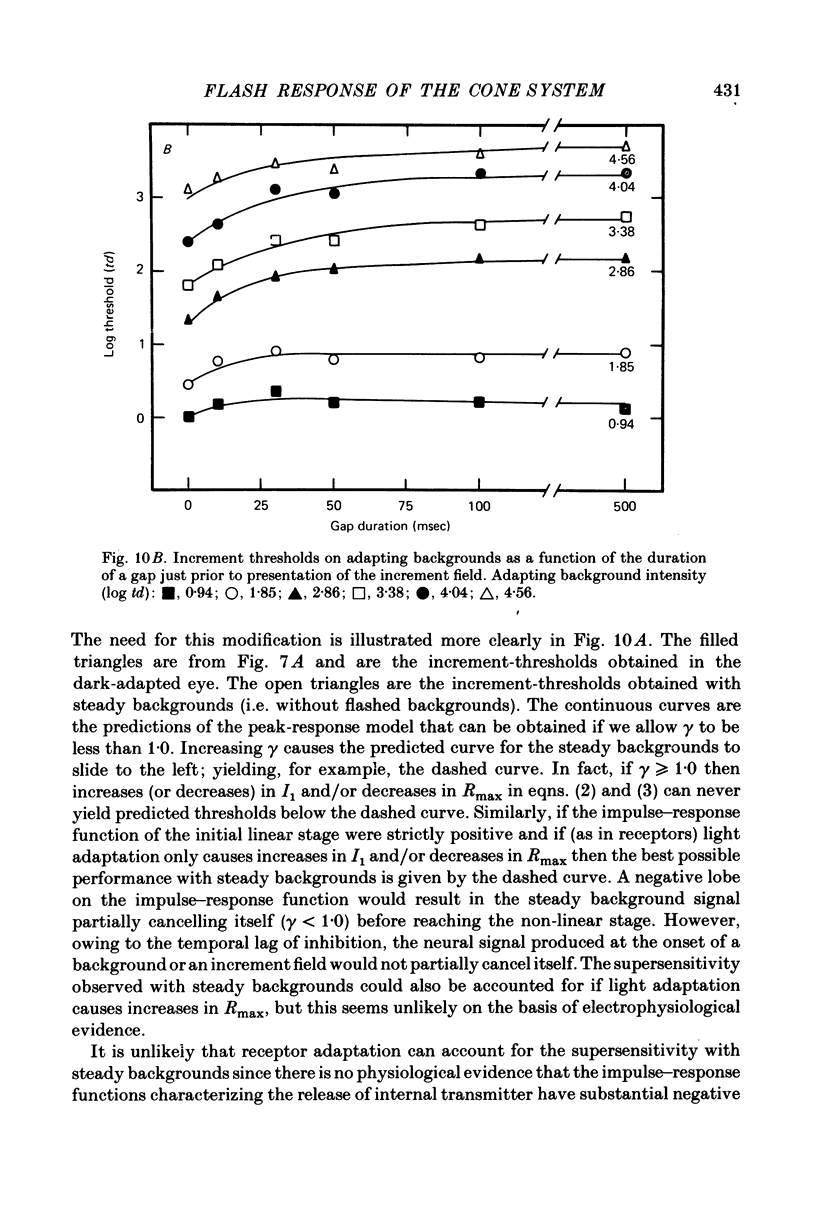
4. The increment-threshold functions obtained with steady adapting backgrounds were also continuously accelerating, but could not be brought into coincidence by equal vertical and horizontal translation. However, this became possible again if the adapting background was extinguished during presentation of the flashed background.
5. These results contradict the equivalent-background hypothesis. None the less, they suggest that under present conditions the effects of bleaches and backgrounds may be similar except that steady backgrounds provide additional quanta which drive the visual system part of the way up its intensity—response function.
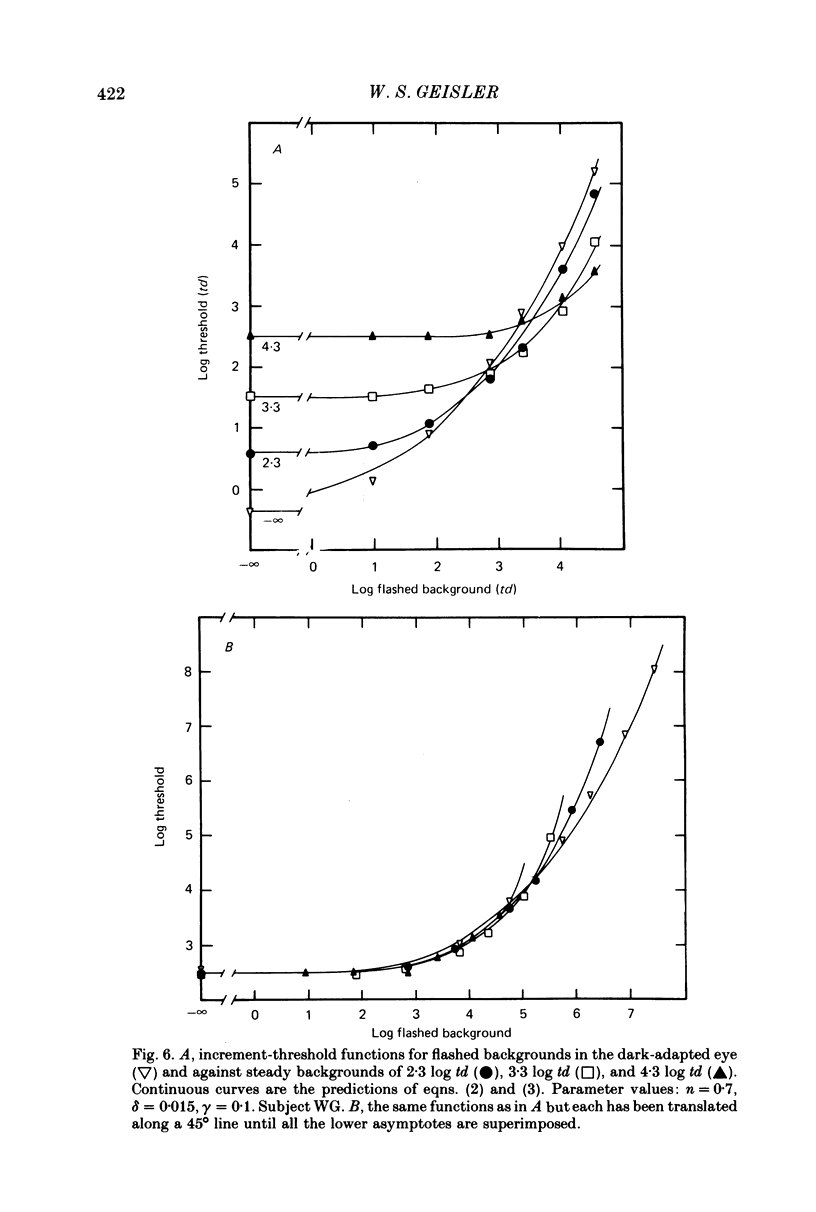
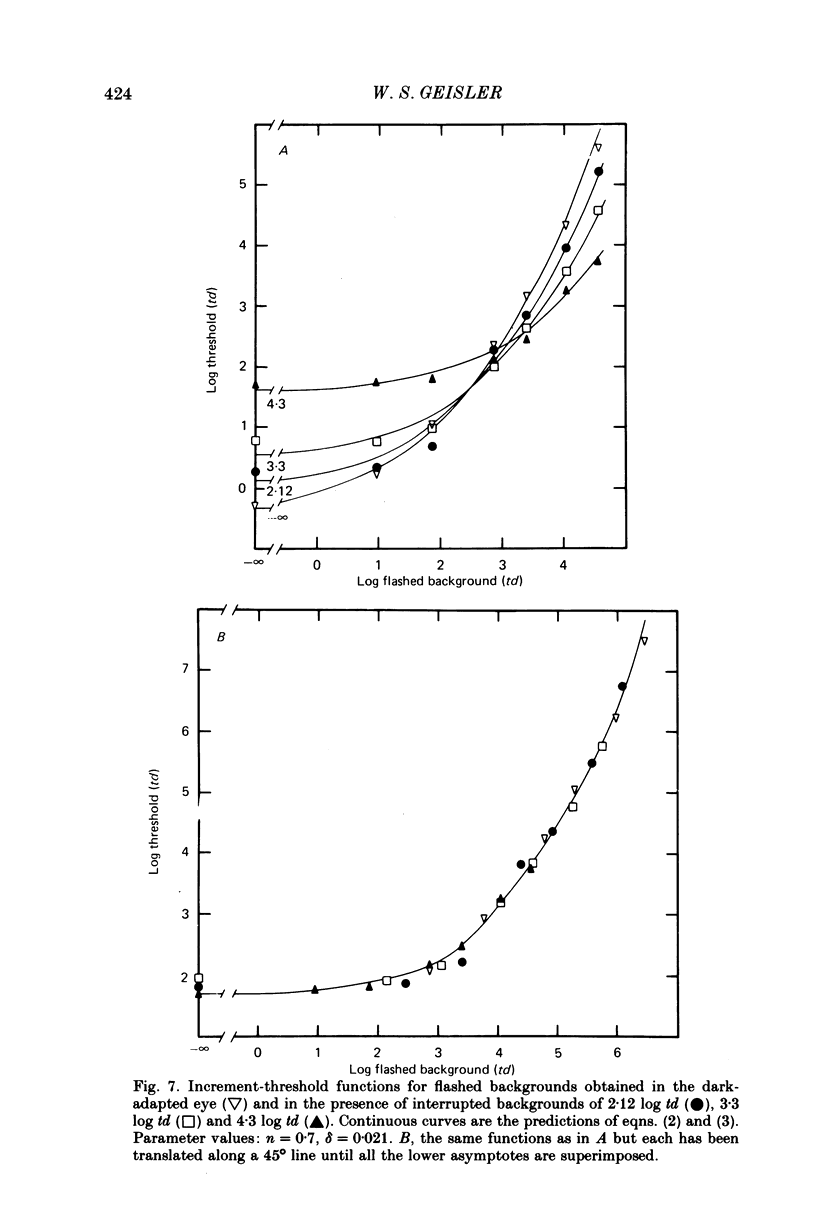
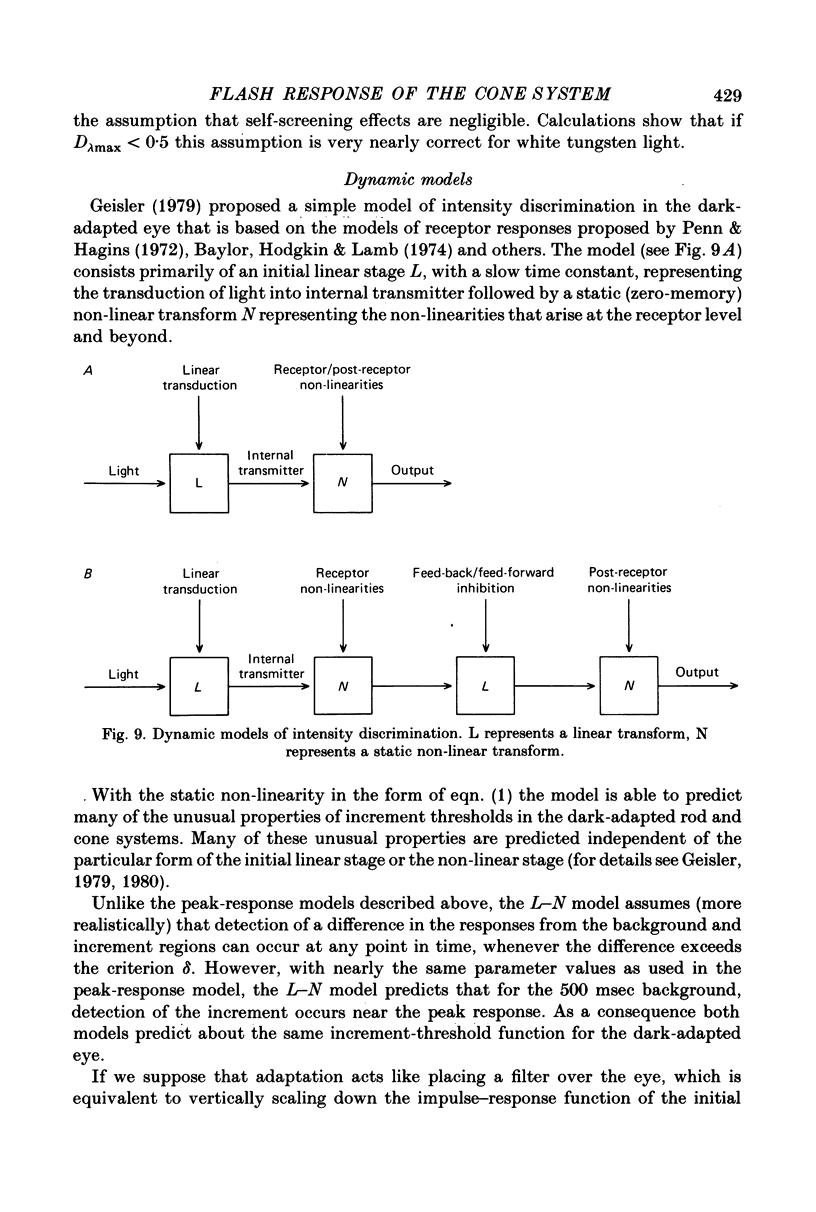
6. The conclusions above were supported by applying a simple model based on the equation R = Rmax. In / (In + I1n), which has frequently been used to describe the peak responses of retinal neurones to flashed stimuli. Virtually all of the data reported here were fitted by this simple model with Rmax held constant.
7. The parameters estimated from the model imply that the flash responses measured in the present experiments differ in at least one fundamental way from receptor responses. Even after taking into account changes in the half saturation constant I1, steady backgrounds were found to be much less effective than flashed backgrounds in driving the visual system up its intensity—response function. A subtractive inhibitory network prior to the non-linear stages responsible for threshold saturation could explain this result.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern M., Rushton W. A., Torii S. Signals from cones. J Physiol. 1970 Apr;207(2):463–475. doi: 10.1113/jphysiol.1970.sp009073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern M., Rushton W. A., Torii S. The attenuation of rod signals by backgrounds. J Physiol. 1970 Jan;206(1):209–227. doi: 10.1113/jphysiol.1970.sp009007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern M., Rushton W. A., Torii S. The attenuation of rod signals by bleachings. J Physiol. 1970 Apr;207(2):449–461. doi: 10.1113/jphysiol.1970.sp009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINDLEY G. S. The discrimination of after-images. J Physiol. 1959 Jun 23;147(1):194–203. doi: 10.1113/jphysiol.1959.sp006234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Craik K. J. The effect of adaptation on differential brightness discrimination. J Physiol. 1938 May 14;92(4):406–421. doi: 10.1113/jphysiol.1938.sp003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWLING J. E. Chemistry of visual adaptation in the rat. Nature. 1960 Oct 8;188:114–118. doi: 10.1038/188114a0. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Adaptation in skate photoreceptors. J Gen Physiol. 1972 Dec;60(6):698–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. S-potentials in the skate retina. Intracellular recordings during light and dark adaptation. J Gen Physiol. 1971 Aug;58(2):163–189. doi: 10.1085/jgp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler W. S. Adaptation, afterimages and cone saturation. Vision Res. 1978;18(3):279–289. doi: 10.1016/0042-6989(78)90162-1. [DOI] [PubMed] [Google Scholar]
- Geisler W. S. Initial-image and afterimage discrimination in the human rod and cone systems. J Physiol. 1979 Sep;294:165–179. doi: 10.1113/jphysiol.1979.sp012923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler W. S. The effects of photopigment depletion on brightness and threshold. Vision Res. 1978;18(3):269–278. doi: 10.1016/0042-6989(78)90161-x. [DOI] [PubMed] [Google Scholar]
- Grabowski S. R., Pinto L. H., Pak W. L. Adaptation in retinal rods of axolotl: intracellular recordings. Science. 1972 Jun 16;176(4040):1240–1243. doi: 10.1126/science.176.4040.1240. [DOI] [PubMed] [Google Scholar]
- Green D. G., Dowling J. E., Siegel I. M., Ripps H. Retinal mechanisms of visual adaptation in the skate. J Gen Physiol. 1975 Apr;65(4):483–502. doi: 10.1085/jgp.65.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood D. C., Finkelstein M. A., Buckingham E. Psychophysical tests of models of the response function. Vision Res. 1979;19(4):401–406. doi: 10.1016/0042-6989(79)90104-4. [DOI] [PubMed] [Google Scholar]
- Hood D. C., Ilves T., Maurer E., Wandell B., Buckingham E. Human cone saturation as a function of ambient intensity: a test of models of shifts in the dynamic range. Vision Res. 1978;18(8):983–993. doi: 10.1016/0042-6989(78)90026-3. [DOI] [PubMed] [Google Scholar]
- KELLY D. H. Visual response to time-dependent stimuli. I. Amplitude sensitivity measurements. J Opt Soc Am. 1961 Apr;51:422–429. doi: 10.1364/josa.51.000422. [DOI] [PubMed] [Google Scholar]
- Kelly D. H. Theory of flicker and transient responses. I. Uniform fields. J Opt Soc Am. 1971 Apr;61(4):537–546. doi: 10.1364/josa.61.000537. [DOI] [PubMed] [Google Scholar]
- King-Smith P. E., Webb J. R. The use of photopic saturation in determining the fundamental spectral sensitivity curves. Vision Res. 1974 Jun;14(6):421–429. doi: 10.1016/0042-6989(74)90240-5. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D. I. Visual sensitivity. Annu Rev Psychol. 1978;29:613–645. doi: 10.1146/annurev.ps.29.020178.003145. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from luminosity units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):587–599. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R. A., Perlman I. The effects of background illumination on the photoresponses of red and green cones. J Physiol. 1979 Jan;286:491–507. doi: 10.1113/jphysiol.1979.sp012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Mollon J. D. A theory of the pi1 and pi3 color mechanisms of Stiles. Vision Res. 1979;19(3):293–312. doi: 10.1016/0042-6989(79)90175-5. [DOI] [PubMed] [Google Scholar]
- Rashbass C. The visibility of transient changes of luminance. J Physiol. 1970 Sep;210(1):165–186. doi: 10.1113/jphysiol.1970.sp009202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roufs J. A. Dynamic properties of vision. I. Experimental relationships between flicker and flash thresholds. Vision Res. 1972 Feb;12(2):261–278. doi: 10.1016/0042-6989(72)90117-4. [DOI] [PubMed] [Google Scholar]
- Roufs J. A. Dynamic properties of vision. II. Theoretical relationships between flicker and flash thresholds. Vision Res. 1972 Feb;12(2):279–292. doi: 10.1016/0042-6989(72)90118-6. [DOI] [PubMed] [Google Scholar]
- Roufs J. A. Dynamic properties of vision. IV. Thresholds of decremental flashes, incremental flashes and doublets in relation to flicker fusion. Vision Res. 1974 Sep;14(9):831–851. doi: 10.1016/0042-6989(74)90148-5. [DOI] [PubMed] [Google Scholar]
- Rushton W. A., Henry G. H. Bleaching and regeneration of cone pigments in man. Vision Res. 1968 Jun;8(6):617–631. doi: 10.1016/0042-6989(68)90040-0. [DOI] [PubMed] [Google Scholar]
- Shevell S. K. Saturation in human cones. Vision Res. 1977;17(3):427–434. doi: 10.1016/0042-6989(77)90035-9. [DOI] [PubMed] [Google Scholar]
- Wandell B. A. On the analysis of nerve signals deduced from metacontrast experiments with human observers. J Physiol. 1976 Dec;263(3):321–329. doi: 10.1113/jphysiol.1976.sp011633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Control of retinal sensitivity. II. Lateral interactions at the outer plexi form layer. J Gen Physiol. 1974 Jan;63(1):62–87. doi: 10.1085/jgp.63.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. The Maxwellian view. Vision Res. 1966 Dec;6(12):669–682. doi: 10.1016/0042-6989(66)90078-2. [DOI] [PubMed] [Google Scholar]
- Williams T. P., Gale J. G. A critique of an incremental threshold function. Vision Res. 1977;17(7):881–882. doi: 10.1016/0042-6989(77)90133-x. [DOI] [PubMed] [Google Scholar]


