Abstract
Dissimilatory metal-reducing bacteria (DMRB) utilize numerous compounds as terminal electron acceptors, including insoluble iron oxides. The mechanism(s) of insoluble-mineral reduction by DMRB is not well understood. Here we report that extracellular melanin is produced by Shewanella algae BrY. The extracted melanin served as the sole terminal electron acceptor. Upon reduction the reduced, soluble melanin reduced insoluble hydrous ferric oxide in the absence of bacteria, thus demonstrating that melanin produced by S. algae BrY is a soluble Fe(III)-reducing compound. In the presence of bacteria, melanin acted as an electron conduit to Fe(III) minerals and increased Fe(III) mineral reduction rates. Growth of S. algae BrY occurred in anaerobic minimal medium supplemented with melanin extracted from previously grown aerobic cultures of S. algae BrY. Melanin produced by S. algae BrY imparts increased versatility to this organism as a soluble Fe(III) reductant, an electron conduit for iron mineral reduction, and a sole terminal electron acceptor that supports growth.
Dissimilatory metal-reducing bacteria (DMRB) display remarkable versatility in the number of terminal electron acceptors that they use for anaerobic respiration, including insoluble iron minerals (41). Although electron transfer by bacteria to insoluble minerals is known, the molecular mechanism(s) of electron transfer from DMRB to the minerals has yet to be elucidated (11). One model suggests that close physical contact to minerals is required for reduction. For example, the extent of hydrous ferric oxide (HFO) reduction by Shewanella algae BrY is directly related to the degree of attachment (8). Another model postulates the production of soluble electron shuttles by DMRB. Electron shuttling via soluble extracellular cytochromes of Geobacter sulfurreducens may play a role in mineral reduction (36), but the excretion of cytochromes by this organism has been questioned (18). The electron transport properties of humic compounds are directly related to redox cycling capabilities of their quinone moieties (35). With Shewanella oneidensis MR-1 (formerly Shewanella putrefaciens MR-1 [41]), a relation between electron transfer to humic compounds has been linked to uncharacterized, excreted quinones (25).
Since soluble environmental humic compounds mediate electron transfer to iron minerals (19), one plausible biological mechanism by which DMRB can shuttle electrons to minerals is through the production of a polyquinoid humic-like metabolite. The basidiomycete Cryptococcus neoformans uses melanin, a humic-like compound, to reduce soluble Fe(III) to Fe(II) for assimilation (26). Melanin has electrochemical properties which include the ability to act as an amorphous semiconductor, a threshold switch, an electron donor, and an electron acceptor (21, 22, 23, 30). These properties, along with its chemical and functional similarities to other humic compounds (34), led us to hypothesize that melanin is produced by facultatively anaerobic DMRB in the genus Shewanella. Shewanella colwelliana produces melanin by converting tyrosine to homogentisic acid (HGA) with the enzyme p-hydroxyphenylpyruvate dioxygenase (6, 12, 16, 32, 33). HGA is excreted, auto-oxidized, and self-assembled into the quinone-containing polymer, melanin (6, 9, 43). More specifically, this type of melanin is called pyomelanin (43) and is differentiated from other types of melanin because pyomelanin is derived from HGA but not from dihydroxyphenylalanine (DOPA) (43). We hypothesized that melanin is produced by facultatively anaerobic DMRB and could serve as a terminal electron acceptor as well as a soluble electron shuttle to iron minerals, thereby offering a significant growth and energetic advantage to these DMRB. Here we report results that support this hypothesis.
MATERIALS AND METHODS
Organisms and growth conditions.
S. algae BrY (4) (formerly S. alga BrY [38]) was used in this study. Cultures were maintained on tryptic soy agar (Difco Labs, Detroit, Mich.). For bacterial growth experiments, cells were grown in a lactate basal salts medium (LBSM) (19) that contained the following (amounts in parentheses are per liter of distilled water [dH2O]): NH4Cl (1.5 g), KCl (0.1 g), KH2PO4 (0.5 g), PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] (7.57 g), NaCl (10.0 g), 60% sodium lactate (9 ml), vitamin solution (10 ml), mineral solution (10 ml), and tyrosine (2 g). Iron was omitted from the mineral solution above throughout this study so as not to interfere with melanin production or behavior (10, 34). For the pigment production study tyrosine was added at 0, 0.25, 0.5, 1.0, and 2.0 g liter−1. Pigment concentrations in cell-free media were determined spectrophotometrically at a wavelength of 400 nm after 72 h of growth. Duplicate cultures (50 ml) were incubated at 28°C with shaking at 150 rpm. Inocula (10%, vol/vol) were from a 24-h culture grown in LBSM (above), but without tyrosine. Cell density was measured by acridine orange direct counts using an epifluorescence microscope (14). Melanin production in the spent cell culture medium (background subtracted) was determined spectrophotometrically at a wavelength of 400 nm (32).
Melanin production was also evaluated under anaerobic growth conditions according to the previous protocol, except that the medium also contained 30 mM fumarate as a terminal electron acceptor and medium preparation was conducted under anaerobic conditions. After 7 days of incubation the melanin content of the cell supernatant fluid was analyzed spectrophotometrically as above, first under anaerobic conditions and then after vigorous aeration. Aeration was used to facilitate auto-oxidation of any melanin precursors present.
Pigment preparation.
A 5-liter culture of S. algae BrY was grown for 12 days in LBSM supplemented with 2 g of tyrosine liter−1 as described above. Pigment production commenced by 72 h and continued to intensify until day 12, when pigment production stabilized, as measured spectrophometrically as described above. For partial purification of melanin (9, 10), the cell supernatant fluid was acidified with 6 N HCl to a pH of <2.0, and it was allowed to precipitate for 4 h at 20°C. The concentrated melanin was transferred to 8-kDa dialysis tubing and dialyzed for 24 h in dH2O with four changes of water during that time. The dialyzed melanin was then dried at 60°C and powdered prior to storage under nitrogen gas in glass vials which served as the melanin stock. A pure, chemically derived form of melanin (HGA melanin) was used as a control compound in some experiments. This chemically derived melanin was similar to melanin produced by S. algae BrY (see Results). HGA melanin was produced in vitro by auto-oxidation of HGA (Sigma Chemical Co., St. Louis, Mo.) which was stirred constantly for 7 days in dH2O with the pH adjusted to 10 with 10 N NaOH as described previously (32). The HGA melanin was concentrated and dried as described above. Bacterial and HGA melanin (2 g liter−l) demonstrated optical densities (400 nm) of 3.5 and 3.2, respectively. For use in experiments, dried melanins (bacterial and HGA melanin) were dissolved in dH2O with its pH adjusted to 12 with 10 N NaOH. After melanin was in solution, the pH was adjusted to 7.0 by the addition of 1 N HCl and diluted to a volume of 100 ml. Total iron concentrations were <1 μmol g of bacterial and HGA melanin−1, as determined by atomic adsorption spectroscopy. Aliquots of this concentrated stock solution were diluted as needed.
Evaluation of melanin pigment. (i) Chemical characterization of pigment.
Chemical analyses, following the conventional protocol for melanin determination (10, 42), were conducted on solutions and dried bacterial pigment and HGA melanin as described above.
(ii) Quinone assay.
The presence of quinones in the cell-free melanin solutions was determined by the nitroblue tetrazolium-glycinate assay (28). The reagents, which consisted of 300 μl of 2 M potassium glycinate (pH 10) and 1 ml of 0.24 mM nitroblue tetrazolium dissolved in 2 M potassium glycinate (pH 10), were added to 100 μl of cell culture supernatant. Reagents were stored on ice until use. The mixture was incubated at 28°C in the dark for 1 h, and absorbance was read at 530 nm. In addition, concentrated, dried pigment (see below) was dissolved in dilute NaOH (pH 10) and aliquoted into dH2O to concentrations of 0.1 to 1.0 mg liter−1. The response with this assay was linear, with an r2 of 0.9937 and a slope of 0.729, and was consistent with that obtained with other quinoid compounds (28).
(iii) FTIR spectroscopy.
Dried, concentrated, dialyzed bacterial melanin and HGA melanin were lyophilized for 24 h. Fourier transform infrared (FTIR) spectra of lyophilized melanins in KBr disks were recorded with an FTIR spectrophotometer (model 520; Nicolet Instruments, Madison, Wis.) with a mercury-cadmium-telluride detector, against a reference of a nonabsorbing KBr matrix (1, 9).
MW analysis.
Concentrated melanin and HGA melanin (final concentration, 2 g liter−1) were dissolved in dH2O by the addition of 1 N NaOH to a pH of 12, and this solution was neutralized with 1 N HCl to a final pH of 7.0. The molecular weights (MW) of the solutions were determined by high-speed sedimentation (44). Experiments were conducted at 20°C in a Beckman XL-I Ultracentrifuge at 35,000 rpm using absorbance detection. For each sample, three loading concentrations, ranging from an optical density of 0.5 to 1.50 in PIPES buffer and 100 mM NaCl, were examined. Solvent density (1.0017 g ml−1) was calculated using the Sednterp program (17) and an assumed partial specific volume of 0.71 ml g−1 was used in MW calculations. Data were fitted using the NONLIN program (15).
Hydrogen oxidation study.
Aerobic cultures of S. algae BrY were grown in tryptic soy broth (30 g liter−1) for 15 h, at 28°C with shaking (150 rpm). The cells were harvested by centrifugation (8,000 × g, 4°C, 20 min) and washed twice with anaerobic 30 mM sodium bicarbonate buffer under a nitrogen atmosphere. Bacterial melanin was added (final concentration, 2 g liter −1) to 30 mM sodium bicarbonate buffer (pH 7.2), and the mixture was made anaerobic by boiling and cooling under a constant stream of 80% N2-20% CO2. The mixture was transferred anaerobically to serum vials and kept under the same anaerobic atmosphere, after which it was autoclaved. Washed cells, under a nitrogen atmosphere, were added anaerobically to the above mixture to a final density of 109 ml−1. Hydrogen gas (10 kPa) served as an electron donor and was added aseptically to vials with a syringe fitted with a sterile 0.22-μm-pore-size filter. Vials were incubated with agitation (150 rpm) in the dark at 28°C. At different time intervals, samples were removed for analysis.
Melanin reduction assay.
To quantify melanin reduction, 1 ml of cell supernatant fluid was added to 50 μl of 10 mM soluble ferric citrate and the mixture was incubated anaerobically for at least 15 min, as described previously for humic compounds (19). In this procedure, reduced melanin reduced Fe(III) to Fe(II), which was quantified spectrophotometrically with ferrozine (19). The ability of reduced melanin to reduce 1 mM of Fe(III) to Fe(II) is defined as 1 meq of reduced melanin.
Melanin reduction of insoluble Fe(III) oxide.
Bacterial melanin which was previously reduced during the terminal electron acceptor study was centrifuged anaerobically (8,000 × g, 20°C, 20 min) to remove cells. One milliliter of cell-free melanin was added to 200 μl of 6 mM insoluble HFO, and the mixture was incubated at 20°C under anaerobic conditions for 90 min. The amount of Fe(II) produced from the reduction of HFO was determined as described above. HGA melanin, reduced during the growth assay (see below), was also evaluated for HFO reduction ability, as above.
HFO reduction rate as a function of melanin concentration.
HFO reduction over time, by cell suspensions of S. algae BrY and H2 (prepared as described above), was determined with various concentrations of bacterial melanin. HFO reduction to Fe(II) was measured as described above. HFO reduction rates were calculated with the following equation: a = ai (1 − e−kt), where ai is the maximum HFO reduction [as millimolar Fe(II) produced] and k is the HFO reduction rate (hour−1). Fe(II) concentrations were normalized by subtracting Fe(II) values of the control (no added melanin). The normalized Fe(II) values were then fitted to the Michaelis-Menten equation: k = kmax(S/km + S), where k is the HFO reduction rate (hour−1), kmax is the maximum HFO reduction rate (hour−1), S is the melanin concentration (grams liter−1), and km is the half-saturation constant for melanin (grams liter−1). Nonlinear curves were determined with Sigma Plot curve-fitting software (version 7.1; Jandel Co., Chicago, Ill.).
Cytochrome assay.
S. algae BrY cells grown aerobically in LBSM were washed (30 mM sodium bicarbonate buffer) and concentrated under N2 (approximately 109 cells ml−1). Reduced cytochrome scans were generated as previously described (13) by placing an air-oxidized cell suspension in the reference cell of a dual-beam spectrophotometer (Shimadzu UV-2401 PC) with either a dithionite- or H2-reduced cell suspension in the sample cell. For determination of the effect of melanin on the cytochromes, 20 μl of an anaerobic oxidized melanin solution (2 g liter−1) was added anaerobically to 1 ml of the H2-reduced cell suspension (final melanin concentration, 0.04 mg ml−1) and read against an H2-reduced cell suspension without melanin. In order to determine if oxygen impurities interfered with the results, another cytochrome scan was conducted by adding 20 μl of aerobic 30 mM sodium bicarbonate buffer, instead of melanin, to the H2-reduced cell suspension and read against an H2-reduced cell suspension.
Growth assay with melanin as terminal electron acceptor.
Bacterial melanin, prepared as described above, was added to an anaerobic LBSM at final concentrations of 3 and 6 g liter−1. In a separate experiment, HGA melanin (6 g liter−1) was used instead of bacterial melanin. The inocula consisted of anaerobic cells of S. algae BrY that were grown for 48 h at 28°C in the LBSM with fumarate (30 mM) as the terminal electron acceptor and lactate as the carbon and energy source. Cells were harvested by centrifugation (8,000 × g, 4°C, 20 min) and washed twice with anaerobic, 30 mM bicarbonate buffer under a nitrogen atmosphere and inoculated into LBSM with melanin. Melanin reduction was determined as described above. Cell numbers were determined by direct counting with acridine orange staining and an epifluorescence microscope (14).
Data analysis.
Experiments were conducted in duplicate or triplicate. Data are presented as mean values and standard errors. Statistical analyses included Student's t test for determination of P values.
RESULTS
Melanin production.
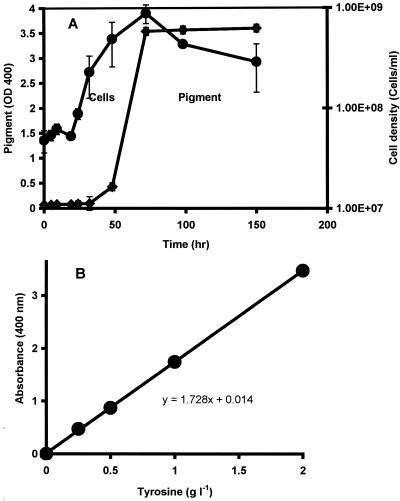
When grown aerobically with tyrosine, S. algae BrY produced a dark, red-brown, extracellular pigment (not shown). Extracellular pigment production by S. algae BrY (Fig. 1A) occurred during the late exponential to early stationary growth phase in tyrosine-supplemented minimal medium, similar to melanin production by S. colwelliana and Vibrio cholerae (32, 42). The amount of soluble pigment produced after 72 h of growth was directly proportional to the initial tyrosine concentration in the medium (Fig. 1B). When grown with 2 g of tyrosine liter−1, the amount of the concentrated, dried pigment (average ± standard error) was 173.9 ± 5.5 mg liter−1. Pigmentation was not detected in anaerobic cultures with supplemental tyrosine (data not shown).
FIG. 1.
Melanin production by S. algae BrY in LBSM supplemented with tyrosine. (A) Melanin production by (⧫) and growth of (•) an aerobic culture of S. algae BrY with 2 g of tyrosine liter−1. OD, optical density. (B) Melanin production by aerobic S. algae BrY cultures was tyrosine dependent. Data are presented as mean values and standard errors (error bars). The absence of error bars indicates that they are smaller than the corresponding symbol for the mean value.
Evaluation of melanin pigment. (i) Chemical characterization of pigment.
The melanin pigment had an absorbance spectrum (300 to 800 nm) similar to that of commercial humic acid (Aldrich Chemical Co.) (data not shown). In addition, the slope of the line for log absorbance versus wavelength (400 to 600 nm) of the spectrum was equal to −0.0035, indicative of melanin (10). At pHs of ≤2, the soluble, cell-free pigment precipitated rapidly, further indicative of melanin and other humic compounds (9, 10, 34). Following acid precipitation, the washed, dialyzed, dried melanin resulted in a black powder with the following properties indicative of melanin (10): (i) insolubility in organic solvents (ethanol, chloroform, and acetone) and (ii) solubility in NaOH solutions with a pH of ≥10. When in solution the pigment was (i) decolorized in H2O2, (ii) precipitated by FeCl3, and (iii) retained by an 8-kDa dialysis membrane. The properties of HGA melanin were consistent with those described above for the purified pigment.
(ii) Quinone assay.
The soluble bacterial pigment and HGA melanin also reduced nitroblue tetrazolium to formazan in the presence of oxygen and excess glycine at pH 10, indicating the existence of quinoid compounds (28).
(iii) FTIR analysis.
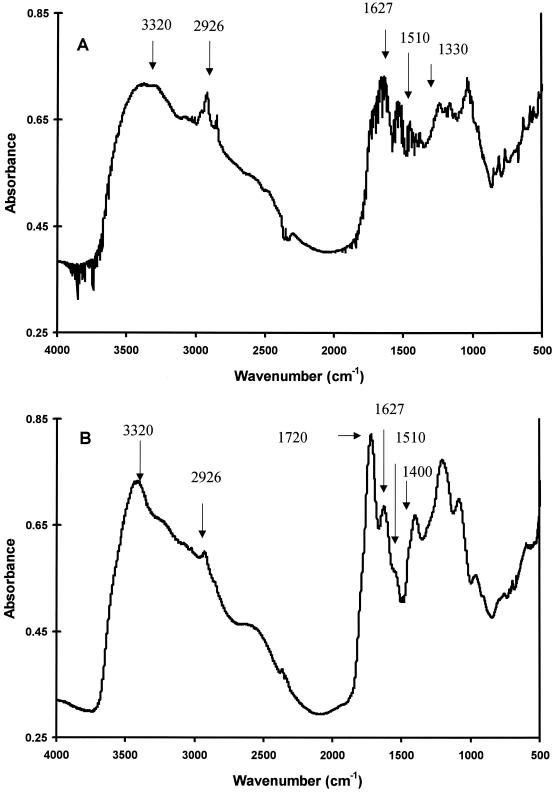
FTIR spectroscopy was chosen to further characterize the bacterial pigment because it is regarded as the most-informative method for well-resolved and detailed structural analysis of melanins (2, 29). Figure 2 shows the FTIR spectra of the concentrated bacterial pigment and HGA melanin, which was used to confirm the pigment's identity (9). The FTIR bands from bacterial pigment (Fig. 2A) showed definite signature peaks previously reported for bacterial pyomelanin and HGA melanin produced in vitro (Fig. 2B) (9). The peaks at the following wave numbers and their corresponding structures include the bands at (i) 3,320 cm−1, a broad band indicative of the OH stretch of polymeric structures (37); (ii) 2,926 cm−1, aliphatic C—H bonds (9, 10, 20, 29); (iii) 1,650 to 1,600 cm−1, aromatic C=C conjugated with C=O and/or COO− groups (20); (iv) 1,510 cm−1, aromatic C=C bonds (20); and (v) 1,410 to 1,310 cm−1, OH groups of phenolic compounds (5).
FIG. 2.
FTIR scans of concentrated, lyophilized extracellular bacterial pigment (A) and HGA melanin (B).
The peak at 1,720 cm−1 from HGA melanin (Fig. 2B) was also present but poorly resolved from bacterial melanin (Fig. 2A) and is ascribed to carbonyl stretching (9). Aromatic C=C conjugated with C=O and/or COO− groups (1,510 cm−1) produced a stronger response from bacterial melanin than from HGA melanin. This stronger response at 1,510 cm−1 for bacterial melanin has been observed in previous studies (9). Overall, these results demonstrate that the bacterial pigment has definite characteristics of humic compounds (10, 34) as well as a high degree of similarity to HGA melanin and bacterial pyomelanin (9).
MW analysis.
The MW of HGA melanin and concentrated extracellular melanin ranged from 11,000 to 17,000 and 12,000 to 14,000, respectively. These values are consistent with previous measurements of pyomelanin (42). In contrast, the MW of DOPA melanin is >1,000,000 (3).
Hydrogen oxidation study.
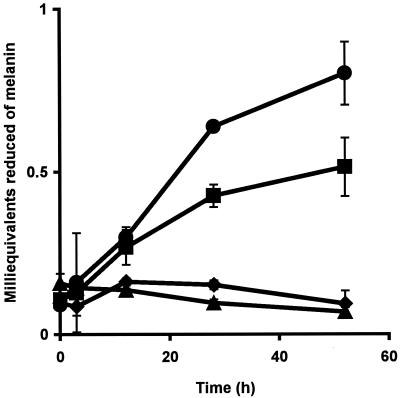
The use of bacterial melanin as an electron acceptor by S. algae BrY was evaluated by monitoring hydrogen-dependent melanin reduction (Fig. 3). Melanin was reduced by resting cell suspensions of S. algae BrY but was not reduced by heat-killed, washed cells with hydrogen or by hydrogen alone (Fig. 3). The amount of melanin reduced by S. algae BrY with hydrogen was significantly greater after 28 h of incubation than the amount reduced by S. algae BrY cells without hydrogen (P < 0.01). Identical results were obtained with pure chemically derived HGA melanin (data not shown). These results are consistent with the hypothesis that S. algae BrY couples the oxidation of H2 to the reduction of melanin.
FIG. 3.
S. algae BrY cell suspensions coupled with H2 oxidation to melanin reduction under anaerobic conditions. Results are shown for melanin reduction in anaerobic 30 mM sodium bicarbonate buffer by suspensions of S. algae BrY cells (109 ml−1) with H2 (•), S. algae BrY cells without H2 (▪), H2 alone (▴), and heat-killed cells with H2 (⧫). Data are presented as mean values and standard errors (error bars). The absence of error bars indicates that they are smaller than the corresponding symbol for the mean value.
Melanin reduction of insoluble Fe(III) oxide.
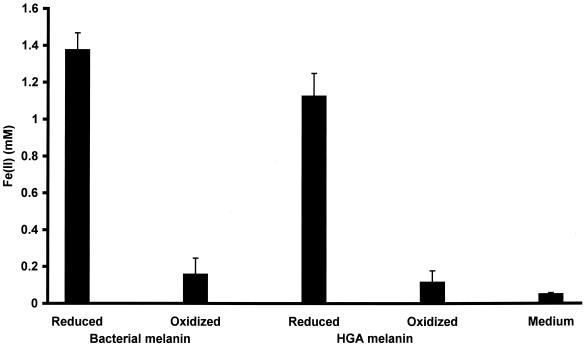
The addition of cell-free, bacterially reduced bacterial melanin and HGA melanin to anaerobic HFO reduced HFO to Fe(II) (Fig. 4). The amount of HFO reduced by reduced melanin was significantly greater than the amount reduced by oxidized melanin (P < 0.01). This is the first demonstration of a soluble metabolite produced by a member of the genus Shewanella that is capable of Fe(III) oxide reduction.
FIG. 4.
Reduced melanin reduces HFO. Melanin reduced by S. algae BrY reduces HFO in the absence of cells, but oxidized melanin and spent, cell culture liquid without melanin do not reduce HFO. Error bars, standard errors.
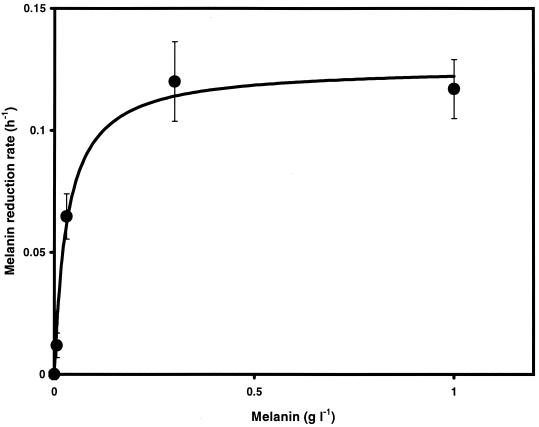
Melanin also accelerated the rate of bacterial HFO reduction during anaerobic respiration. The rates of Fe(III) oxide reduction by cell suspensions of S. algae BrY increased as a function of oxidized melanin concentration (Fig. 5). Fe(II) adsorption to Fe(III) oxides limits the rate and extent of bacterial Fe(III) oxide reduction (31). Since melanin complexes with soluble iron (26), one possible explanation for these results is that melanin influenced the increased Fe(III) reduction rates by forming a complex with Fe(II), which would normally have bound to the oxide surface. However, an analysis of the kinetics of iron reduction showed that the data did not fit a Langmuir isotherm (40) (r2 = 0.54) (data not shown) but were more precisely described by a Michaelis-Menten model (r2 = 0.98) (Fig. 5). These results demonstrate that melanin acted directly to increase HFO reduction rates rather than just to impede Fe(II) sorption to HFO. The Michaelis-Menten kinetic analysis yielded a kmax for HFO of 0.12 h−1, a half-saturation constant (km) of 32 mg of melanin liter−1, and an optimum concentration of approximately 200 mg of melanin liter−1. These results demonstrate that melanin increased the rate of bacterial Fe(III) oxide reduction. One possible mechanism for this effect is electron shuttling through quinone moieties as previously described for other humic compounds (35).
FIG. 5.
The rate of HFO reduction by cell suspensions of S. algae BrY and H2 increases with increasing melanin concentrations. HFO reduction rates of S. algae BrY as a function of melanin concentration followed Michaelis-Menten kinetics (r2 = 0.98). Maximum HFO reduction rate (kmax) = 0.12 h−1; km = 0.032 g liter−1. Error bars, standard errors.
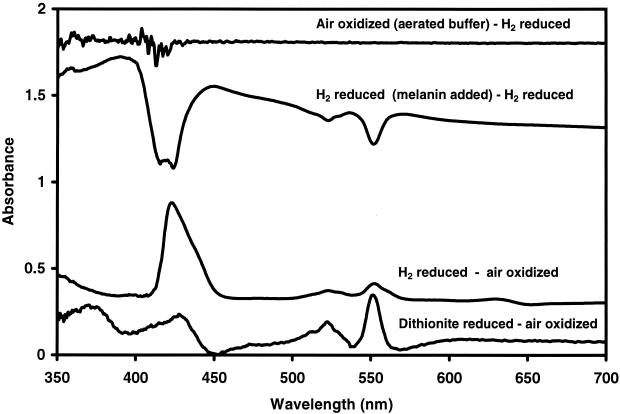
Cytochrome assay.
For melanin to transfer electrons to HFO, the electrons must first be transferred from the cell to melanin. In order to determine if cytochromes were involved in electron transport to oxidized, soluble melanin, whole-cell cytochrome scans were conducted. Difference scans of dithionite-reduced or H2-reduced cells minus air-oxidized cells revealed peaks indicative of bacterial cytochromes (27) Whole-cell absorbance scans from H2-reduced cells minus H2-reduced cells plus anaerobic, oxidized melanin revealed oxidized cytochromes (Fig. 6). The addition of 20 μl of aerated buffer to the cell suspension did not yield cytochrome scans similar to those of melanin-oxidized cells. This indicates that oxygen impurities from the anaerobic melanin would not contribute significantly to the scans. These results illustrate that the reduced cytochromes of S. algae BrY are capable of oxidization by soluble, anaerobic, oxidized melanin.
FIG. 6.
Difference spectra for whole cells of S. algae BrY. The scans from bottom to top include differences for dithionite-reduced cells minus air-oxidized cells, H2-reduced cells minus air-oxidized cells, and H2-reduced cells (with oxidized, anaerobic melanin) minus H2-reduced cells. The top scan shows an H2-reduced cell suspension (plus 20 μl of aerobic bicarbonate buffer) minus an H2-reduced cell suspension.
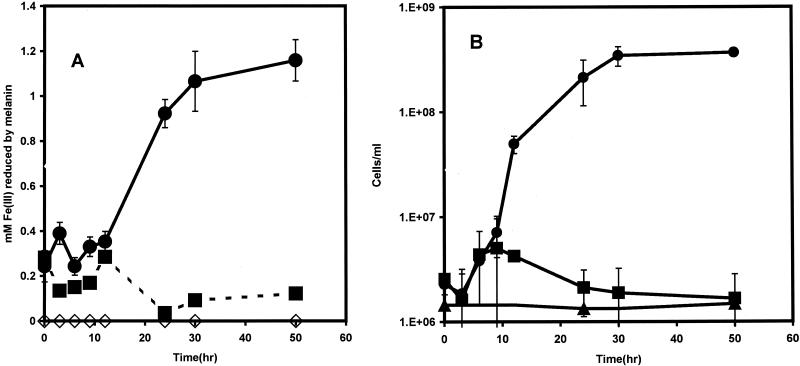
Growth assay with melanin as terminal electron acceptor.
S. algae BrY cells used melanin as a sole terminal electron acceptor to support cellular growth (Fig. 7A). Melanin reduction coincided with a >100-fold increase in cell numbers (Fig. 7B), and the reduced cell-free melanin was capable of reducing 1.2 mM soluble Fe(III) to Fe(II). In a parallel experiment, an initial melanin concentration of 3 g liter−1, with lactate as the electron donor, produced a 50-fold increase in bacterial density and an Fe(III) reduction potential of 0.6 mM from the reduced melanin (data not shown). No growth occurred with melanin in the absence of an electron donor. S. algae BrY grew anaerobically with 6 g of HGA melanin liter−1 as a sole terminal electron acceptor and had a >50-fold increase in cell numbers after 48 h of incubation (data not shown). The reduced HGA melanin from these experiments was also capable of reducing HFO (Fig. 4).
FIG. 7.
Melanin (6 g liter−1) as a sole terminal electron acceptor supported growth of S. algae BrY in LBSM. (A) Melanin reduction during growth of S. algae BrY cells with melanin and LBSM (•), S. algae BrY cells without melanin (▪), and sterile LBSM with melanin (◊). (B) Cell growth measurements of S. algae BrY with melanin as the terminal electron acceptor (•), S. algae BrY cells without melanin (▪), and S. algae BrY cells with melanin but without lactate or hydrogen (▴). Data are presented as mean values and standard errors (error bars). The absence of error bars indicates that they are smaller than the corresponding symbol for the mean value.
DISCUSSION
Melanin production has been documented for the family Vibrionaceae, including S. colwelliana and V. cholerae (7, 16, 32, 42). The work presented here is the first demonstration that another member of the Vibrionaceae, S. algae BrY, also produces the pigment melanin, as determined through conventional chemical characterization for melanin and FTIR evaluation. In MW comparisons S. algae BrY melanin was similar to HGA melanin but not to DOPA melanin (3). In addition previous work demonstrated that HGA, but not DOPA, is excreted by S. algae BrY and melanin production by S. algae BrY is inhibited by sulcotrione, a specific inhibitor of the enzyme p-hydroxyphenylpyruvate dioxygenase, which produces HGA (39). In contrast, sulcotrione does not inhibit the enzyme tyrosinase, which produces DOPA (39). Therefore, melanin produced by S. algae BrY is more characteristic of HGA melanin (pyomelanin) and similar to that produced by S. colwelliana and V. cholerae. Because of the similarities of melanin produced by S. algae BrY to HGA melanin and not DOPA melanin, pure HGA melanin was produced in vitro and used as a surrogate to bacterial melanin in some of the following studies.
Although melanin production by microorganisms is well documented, the physiological significance of microbial melanogenesis is not completely understood. Previous work on bacterial melanin of S. colwelliana and V. cholerae focused on settlement of oyster larvae (42) and virulence (7). The present study concentrated on the electrochemical properties of melanin and its relation to bioenergetics. Various types of environmental humic compounds serve as electron acceptors and electron shuttles for DMRB (19, 35). Because melanin is characterized as a humic compound (10, 34), it is therefore consistent that it possesses similar properties. Thus, melanin is well suited to function as an electron acceptor for DMRB. The production of melanin by S. algae BrY potentially offers a considerable survival advantage. S. algae BrY is a facultative anaerobe with no fermentative ability (4, 41). Its survival in the oxic-anoxic interface of marine sediments is due to the variety of compounds it uses as terminal electron acceptors during anaerobic respiration (4, 41). Because potential electron acceptors are often in the form of insoluble minerals, their bioavailability to S. algae BrY is low.
Melanin may offer several survival advantages to S. algae BrY in its natural habitat. The initiation of melanin production during late- to postexponential growth is theorized to be a response to environmental stress (7, 16). In the presence of mineral oxides, electron shuttling by even minute quantities of melanin significantly enhanced the rate of mineral reduction by S. algae BrY.
The results of this study indicate that S. algae BrY, a facultative anaerobe, can utilize extracellular melanin for increased rates of insoluble iron mineral reduction. Very low quantities of melanin are required. For instance, approximately 3 fg/cell was required to achieve 10% of the maximum HFO reduction rate. Considering the redox cycling behavior of melanin (19, 35), bacterial cells may only require trace amounts of tyrosine and oxygen to produce physiologically effective quantities of melanin. Other humic compounds also significantly enhance Fe(III) reduction, even in quantities as low as 50 μg kg of sediment−1 (24). Subsequently, when oxygen concentrations diminish, melanin could play a vital role in Fe(III) mineral reduction by S. algae BrY in the environment. Reduced melanin can also serve as a soluble metabolite capable of mineral reduction in the absence of bacteria. Since humic compounds are recalcitrant, bacterial melanin may have a long-term role in biogeochemical cycling and the physiological ecology of S. algae BrY.
These results also provide the first example of a compound produced and used as a sole terminal electron acceptor for anaerobic respiration and growth by the same organism. This portion of the study was conducted primarily to determine the bioenergetic potential of the melanin. In comparison, the melanin examined in this study performed as well as or better than other humic compounds (19). The possible presence of other bioenergetic components such as low-MW quinones or cytochromes in the partially purified bacterial melanin cannot be discounted. If present, these compounds may have contributed to electron transfer to some degree. However, pure HGA melanin served as a sole terminal electron acceptor and supported growth of S. algae BrY. The low iron concentration of bacterial and HGA melanin (<1 μM g−1) would not contribute significantly to anaerobic growth. In addition, bacterially reduced HGA melanin reduced more than 80% of HFO in comparison to that reduced by the bacterial melanin (Fig. 4). This is further evidence of the bioenergetic properties of melanin produced by S. algae BrY.
Specific quantification of the bioenergetic properties of melanin will be conducted in later studies. The high concentrations of tyrosine used in this study were to facilitate melanin production in sufficient quantities for analysis. In the natural environment tyrosine concentrations would of course be substantially lower, as would be the expected degrees of melanin production.
The ability to produce melanin and use it as a sole terminal electron acceptor for growth may allow these organisms to respire under anoxic conditions in the absence of other terminal electron acceptors. While small quantities of melanin are required for enhanced iron oxide reduction, the amount of melanin required as a sole terminal electron acceptor is much higher, considering that in this case the melanin would not likely be reoxidized and hence would not be reused. Continuous production of melanin may be possible when S. algae BrY encounters an abundance of carbon and energy, such as when it functions as a saprophyte. The availability of abundant carbon and the subsequent decrease in oxygen concentration present a physiological dilemma for a nonfermenting, facultative anaerobe. However, these environmental conditions may provide the right circumstances for melanin production to proceed. Although the complete catabolism of tyrosine provides more carbon and energy to the cell than melanin production would, in the absence of any other terminal electron acceptor, the conversion of tyrosine, ultimately to melanin, would permit respiration to continue under conditions of abundant organic carbon.
While this study has demonstrated the production and use of melanin by the DMRB S. algae BrY, the extent of this phenomenon has yet to be established in the environment. However, our data indicate that only minute amounts of melanin (femtogram quantities per cell) are required to significantly enhance HFO reduction rates. This suggests that only minute quantities of tyrosine are required to produce a functional concentration of melanin for Fe(III) oxide reduction. Possibly, tyrosine may be obtained from the environment or the de novo production of tyrosine may also contribute to melanin production. Determination of the environmental significance of melanin production on bacterial physiology and biogeochemistry offers an interesting challenge, primarily due to the low concentrations of melanin that may be produced in situ.
Acknowledgments
This research was supported in part by AES Hatch grant 389 and by the College of Life Science and Agriculture, The University of New Hampshire—Durham.
We thank D. R. Lovley, J. D. Coates, and Y. A. Gorby for helpful discussions; R.P. Blakemore for thoughtful insight and review of the manuscript; Thomas Laue and Kari Hartman for MW analyses; D. Brad Martin and Carol Stork for total iron determinations; and Sterling Tommilini for assistance with FTIR analyses.
Footnotes
Scientific Contribution number 2091 from the NH Agricultural Experimental Station.
REFERENCES
- 1.Abu, G. O., R. M. Weiner, J. Rice, and R. R. Colwell. 1991. Properties of an extracellular adhesive polymer from the marine bacterium Shewanella colwelliana. Biofouling 3:69-84. [Google Scholar]
- 2.Biliñska, B. 1996. Progress of infrared investigations of melanin structures. Spectrochim. Acta Part A 52:1157-1162. [Google Scholar]
- 3.Bridelli, M. G. 1998. Self-assembly of melanin studied by laser light scattering. Biophys. Chem. 73:227-239. [DOI] [PubMed] [Google Scholar]
- 4.Caccavo, F., Jr., R. P. Blakemore, and D. R. Lovley. 1992. A hydrogen-oxidizing, Fe(III)-reducing microorganism from the Great Bay estuary, New Hampshire. Appl. Environ. Microbiol. 58:3211-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conley, R. T. 1966. Infrared spectroscopy, p. 87-175. Allyn and Bacon Inc., Boston, Mass.
- 6.Coon, S. L., S. Kotob, B. B. Jarvis, S. Wang, W. C. Fuqua, and R. M. Weiner. 1994. Homogentisic acid is the product of MelA, which mediates melanogenesis in the marine bacterium Shewanella colwelliana D. Appl. Environ. Microbiol. 60:3006-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne, V. E., and L. Al-Harthi. 1992. Induction of melanin biosynthesis in Vibrio cholerae. Appl. Environ. Microbiol. 58:2861-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das, A., and F. Caccavo, Jr. 2000. Fe(III) oxide reduction by Shewanella alga BrY requires adhesion. Curr. Microbiol. 40:344-347. [DOI] [PubMed] [Google Scholar]
- 9.David, C., A. Daro, E. Szalai, T. Atarhouch, and M. Mergeay. 1996. Formation of polymeric pigments in the presence of bacteria and comparison with chemical oxidative coupling. II. Catabolism of tyrosine and hydroxyphenylacetic acid by Alcaligenes eutrophus CH34 and mutants. Eur. Polym. J. 32:669-679. [Google Scholar]
- 10.Ellis, D. H., and D. A. Griffiths. 1974. The location and analysis of melanins in cell walls of some soil fungi. Can. J. Microbiol. 20:1379-1386. [Google Scholar]
- 11.Fredrickson, J. K., and Y. A. Gorby. 1996. Environmental processes mediated by iron-reducing bacteria. Curr. Opin. Biotechnol. 7:287-294. [DOI] [PubMed] [Google Scholar]
- 12.Fuqua, W. C., V. E. Coyne, D. C. Stein, C.-M. Lin, and R. M. Weiner. 1991. Characterization of melA: a gene encoding melanin biosynthesis from the marine bacterium Shewanella colwelliana. Gene 109:131-136. [DOI] [PubMed] [Google Scholar]
- 13.Gorby, Y. A., and D. R. Lovley. 1991. Electron transport in the dissimilatory iron reducer, GS-15. Appl. Environ. Microbiol. 57:867-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, M. L., J. J. Correria, D. A. Yphantis, and H. R. Halvorson. 1981. Analysis of data from the analytical ultracentrifuge by non-linear least-squares techniques. Biophys. J. 36:575-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotob, S. I., S. L. Coon, E. J. Quintero, and R. M. Weiner. 1995. Homogentisic acid is the primary precursor of melanin synthesis in Vibrio cholera, a Hyphomonas strain, and Shewanella colwelliana. Appl. Environ. Microbiol. 61:1620-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laue, T. M., B. D. Shah, T. M. Ridgeway, and S. L. Pelletier. 1992. Computer-aided interpretation of analytical sedimentation data for proteins, p. 90-125. In S. E. Harding, A. J. Rowe, and J. C. Horton (ed.), Analytical ultracentrifugation in biochemistry and polymer science. Royal Society of Chemistry, Cambridge, United Kingdom.
- 18.Lloyd, J. R., E. L. Blunt-Harris, and D. R. Lovley. 1999. The periplasmic 9.6-kilodalton c-type cytochrome is not an electron shuttle to Fe(III). J. Bacteriol. 181:7647-7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovley, D. R., J. D. Coates, E. L. Blunt-Harris, E. J. P. Phillips, and J. C. Woodward. 1996. Humic substances as electron acceptors for microbial respiration. Nature 382:445-448. [Google Scholar]
- 20.MacCarthy, P., and J. A. Rice. 1985. Spectroscopic methods (other than NMR) for determining functionality in humic substances, p. 527-560. In G. R. Aiken, D. M. McKnight, R. L. Wershaw, and P. MacCarthy (ed.), Humic substances in soil, sediment, and water. Wiley-Interscience, New York, N.Y.
- 21.McGinness, J. E. 1972. Mobility gaps: a mechanism for band gaps in melanins. Science 177:896-897. [DOI] [PubMed] [Google Scholar]
- 22.McGinness, J., P. Corry, and P. Proctor. 1974. Amorphous semiconductor switching in melanins. Science 183:853-855. [DOI] [PubMed] [Google Scholar]
- 23.Menter, J. M., and I. Willis. 1997. Electron transfer and photoprotective properties of melanins in solution. Pigment Cell Res. 10:214-217. [DOI] [PubMed] [Google Scholar]
- 24.Nevin, K. P., and D. R. Lovley. 2000. Potential for nonenzymatic reduction of Fe(III) via electron shuttling in subsurface environments. Environ. Sci. Technol. 34:2472-2478. [Google Scholar]
- 25.Newman, D. K., and R. Kolter. 2000. A role for excreted quinones in extracellular electron transfer. Nature 405:94-97. [DOI] [PubMed] [Google Scholar]
- 26.Nyhus, K. J., A. T. Wilborn, and E. S. Jacobson. 1997. Ferric iron reduction by Cryptococcus neoformans. Infect. Immun. 65:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obuekwe, C. O., and D. S. W. Westlake. 1982. Effects of medium composition on cell pigmentation, cytochrome content, and ferric iron reduction in a Pseudomonas sp. isolated from crude oil. Can. J. Microbiol. 28:989-992. [DOI] [PubMed] [Google Scholar]
- 28.Paz, M. A. R. A. Fluckiger, A. Boak, H. M. Kagan, and P. M. Gallop. 1991. Specific detection of quinoproteins by redox-cycling staining. J. Biol. Chem. 266:689-692. [PubMed] [Google Scholar]
- 29.Pierce, J. A., and D. M. Rast. 1995. A comparison of native and synthetic mushroom melanins by Fourier-transform infrared spectroscopy. Phytochemistry 39:49-55. [Google Scholar]
- 30.Pullman, A., and B. Pullman. 1961. The band structure of melanins. Biochim. Biophys. Acta 54:384-385. [DOI] [PubMed] [Google Scholar]
- 31.Roden, E. E., and M. M. Urrutia. 1999. Ferrous iron removal promotes microbial reduction of crystalline iron(III) oxides. Environ. Sci. Technol. 33:1847-1853. [Google Scholar]
- 32.Ruzafa, C., A. Sanchez-Amat, and F. Solano. 1995. Characterization of the melanogenic system in Vibrio cholerae ATCC 14035. Pigment Cell Res. 8:147-152. [DOI] [PubMed] [Google Scholar]
- 33.Ruzafa, C., F. Solano, and A. Sanchez-Amat. 1994. The protein encoded by the Shewanella colwelliana melA gene is p-hydroxyphenylpyruvate dioxygenase. FEMS Microbiol. Lett. 124:179-184. [DOI] [PubMed] [Google Scholar]
- 34.Scott, D. E., and J. P. Martin. 1990. Synthesis and degradation of natural and synthetic humic material in soil, p. 37-58. In P. MacCarthy, C. E. Clapp, R. L. Malcolm, and P. R. Bloom (ed.), Humic substances in soil and crop sciences: selected readings. Soil Science Society of America, Inc., Madison, Wis.
- 35.Scott, D. T., D. M. McKnight, E. L. Blunt-Harris, S. E. Kolesar, and D. R. Lovley. 1998. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 32:2984-2989. [Google Scholar]
- 36.Seeliger, S., R. Cord-Ruwisch, and B. A. Schink. 1998. A periplasmic and extracellular c-type cytochrome of Geobacter sulfurreducens acts as a ferric iron reductase and as an electron carrier in other acceptors or to partner bacteria. J. Bacteriol. 180:3686-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman, R. M. 1991. Infrared spectra, p. 111. In R. M. Silverman, G. C. Bassler, and T. C. Morrill (ed.), Spectrometric identification of organic compounds. J. Wiley and Sons, Inc., New York, N.Y.
- 38.Truper, H. G., and L. de'Clari. 1997. Taxonomic note: necessary correction of specific epithets formed as substantives (nouns) “in apposition.” Int. J. Syst. Bacteriol. 47:908-909. [Google Scholar]
- 39.Turick, C. E. 2001. Ph.D. thesis. University of New Hampshire, Durham.
- 40.Veith, J. A., and G. Sposito. 1977. The use of the Langmuir equation in the interpretation of “adsorption” phenomena. Soil Sci. Soc. Am. J. 41:697-702. [Google Scholar]
- 41.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burgardt, E. Stackebrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49:705-724. [DOI] [PubMed] [Google Scholar]
- 42.Weiner, R. M., A. M. Segall, and R. R. Colwell. 1985. Characterization of a marine bacterium associated with Crassostrea virginica (the eastern oyster). Appl. Environ. Microbiol. 49:83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yabuuchi, E., and A. Omyama. 1972. Characterization of “pyomelanin”-producing strains of Pseudomonas aeruginosa. Int. J. Syst. Bacteriol. 22:53-64. [Google Scholar]
- 44.Yphantis, D. A. 1960. Rapid determination of molecular weights of peptides and proteins. Ann. N. Y. Acad. Sci. 88:586-601. [DOI] [PubMed] [Google Scholar]