Abstract
Some unicellular organisms are able to encyst as a protective response to a harmful environment. The cyst wall usually contains chitin as its main structural constituent, but in some cases, as in Acanthamoeba, it consists of cellulose instead. Specific cytochemical differentiation between cellulose and chitin by microscopy has not been possible, due to the similarity of their constituent β-1,4-linked hexose backbones. Thus, various fluorescent brightening agents and lectins bind to both cellulose and chitin. We have used a recombinant cellulose-binding protein consisting of two cellulose-binding domains (CBDs) from Trichoderma reesei cellulases linked together in combination with monoclonal anticellulase antibodies and anti-mouse immunoglobulin fluorescein conjugate to specifically stain cellulose in the cysts of Acanthamoeba strains for fluorescence microscopy imaging. Staining was observed in ruptured cysts and frozen sections of cysts but not in intact mature cysts. No staining reaction was observed with the chitin-containing cyst walls of Giardia intestinalis, Entamoeba dispar, or Pneumocystis carinii. Thus, the recombinant CBD can be used as a marker to distinguish between cellulose and chitin. Thirteen of 25 environmental or clinical isolates of amoebae reacted in the CBD binding assay. All 13 isolates were identified as Acanthamoeba spp. Five isolates of Hartmannella and seven isolates of Naegleria tested negative in the CBD binding assay. Whether cyst wall cellulose really is a unique property of Acanthamoeba spp. among free-living amoebae, as suggested by our findings, remains to be shown in more extensive studies.
Protozoan parasites, which occur in a trophic form, have the ability to protect themselves by forming a cyst wall, which is resistant to environmental stress such as desiccation, lack of nutrients, and variations in temperature and pH. In most pathogenic protozoa studied, chitin is the carbohydrate polymer conveying the required structural toughness to the cyst wall. Acanthamoeba spp. are exceptions, as their endocysts are made up of cellulose (6, 35). The exact composition of ectocyst wall is not well defined. In addition to proteins and lipids, the presence of putative carbohydrate components is suggested by lectin binding studies (7, 29).
Cellulose consists of β-d-glucosyl units linked by β-1,4-glucosidic bonds. Chitin is very similar but contains N-acetylglucosamine as the monomer. Both polymers form very similar crystalline macroscopic structures (5). Specific cytochemical differentiation between cellulose and chitin by microscopy has not been possible due to the similarity of the constituent β-1,4-linked hexose backbones. This is especially true for various fluorescent brightening agents, such as calcofluor white, used as a cytochemical marker in microscopic diagnostics of protozoan and fungal infections, such as intestinal microsporidiosis, Acanthamoeba keratitis and Pneumocystis pneumonia (9, 12, 28).
A two-domain structural organization is often observed in cellulose-degrading enzymes. Most Trichoderma reesei cellulases consist of a catalytic domain and a cellulose-binding domain (CBD) joined by a linker. The catalytic domain contains the active site with the amino acid residues responsible for the hydrolytic mechanism. The role of the CBD is binding to the solid cellulose. The ability of CBDs to attach to cellulose can be utilized in various applications. Individual types of CBDs can vary significantly in their properties, such as affinity, preference for crystalline or amorphous cellulose, and cross-reactivity with other similar carbohydrates (25).
Members of the genus Acanthamoeba have recently received much attention as potential carriers of pathogenic bacteria, with Legionella species being the most intensely studied (14). The present study was initiated as part of our attempts to identify free-living amoebae containing pathogenic bacteria in water samples to which patients with Legionella pneumonia had been exposed (J. Winiecka-Krusnell, E. Linder, M. Lundholm, E. Hjelm, and H. Hallander, presented at the Annual Meeting of Swedish Physicians in Gothenburg, Sweden, 1998).
The current identification method for isolates of free-living amoebae, which is based on morphological and biochemical features, is labor-intensive and requires cloning and axenization. Our objective was to develop a rapid method for the specific identification of Acanthamoeba in samples containing a mixed population of amoebae. In this study we used a recombinant dimeric CBD fusion protein in indirect immunofluorescence to specifically stain the cellulose and visualize its localization in cyst wall.
MATERIALS AND METHODS
D-CBD and anti-CBD antibodies.
The CBD used in this work is a recombinant fusion protein consisting of the 38-amino-acid CBD from T. reesei cellobiohydrolase II (CBHII) in the N terminus and the 36-amino-acid CBD from cellobiohydrolase I (CBHI) in the C terminus linked by a 22-amino-acid linker (23). The benefits of this double CBD (D-CBD) are that it binds very tightly to cellulose (22) and that a specific monoclonal antibody (CI-89) against the CBHI CBD is obtainable (1). A detailed description of the production and purification of D-CBD has been published (23). Briefly, the coding regions of the pelB signal sequence and the CBHI and CBHII CBD sequences were fused, inserted into a pKK223-3 vector containing the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible tac promoter, and transformed into Escherichia coli. Production was carried out in a 1-liter fermentor, and 1 mM IPTG was used for induction. The D-CBD was purified from the culture supernatant by hydrophobic interaction chromatography and high-pressure liquid chromatography successively. Purified protein was then lyophilized and resuspended in phosphate-buffered saline, pH 7.2 (PBS), to give a concentration of 0.2 g/liter before use in indirect immunofluorescence assays.
The reference material (Table 1) consisted of cellulose and chitin-containing cysts of different protozoa. Cotton fiber swabs obtained from a local pharmacist served as a positive control for cellulose. Strains of Acanthamoeba spp. and Hartmannella vermiformis were maintained either as axenic cultures with PYG medium (3) or as monoxenic cultures carried on nonnutrient agar plates covered with a suspension of E. coli. Cysts and empty cyst walls were collected from prolonged cultures, washed three times with sterile Page's saline (3) and immunostained in suspension or after air drying and fixation in cold acetone for 20 min on eight-well microscope slides (Flow Laboratories, Inc.)
TABLE 1.
Reference protozoan species used in the study
| Parasite | Origin | Cyst wall constituent (reference) |
|---|---|---|
| Acanthamoeba spp. | Cellulose (35) | |
| Acanthamoeba castellaniia | Human cornea | |
| Acanthamoeba rhysodesa | Human cornea | |
| Acanthamoeba sp. strain V 38 | Tissue culture | |
| Hartmannella vermiformis | ATCCb (ATCC 50256) | —c |
| Giardia intestinalis HP88 | Human stool (19) | Chitin (38) |
| Entamoeba dispar | ATCC (ATCC 30931) | Chitin (10) |
| Pneumocystis carinii | Human sputum | Chitin (37) |
Typed at Public Health Laboratory Service, Bath Avon, England.
ATCC, American Type Culture Collection.
No information available.
Entamoeba dispar cysts were obtained from prolonged polyxenic cultures in TYGM-9 medium (3). Cysts of Giardia intestinalis were raised axenically in vitro in modified culture medium as described previously (13). Mature, water-resistant cysts were collected, washed three times in distilled water, and used for immunostaining. Sputum samples from patients with pneumocystis pneumonia were used as source of Pneumocystis carinii organisms (21). Air-dried material was fixed in cold acetone for 20 min and trypsinized for 30 min at 37°C as described previously (11) before staining.
Cysts of free-living amoebae isolates used in the study.
Two amoeba strains isolated from patients with Acanthamoeba keratitis and 23 strains of free-living amoebae isolated from different water sources by filtration of water samples and subsequent cultivation on nonnutrient agar plates covered with the suspension of E. coli (36) were studied. Isolated amoebae were assigned to the genus Acanthamoeba, Naegleria, or Hartmannella using morphological taxonomic criteria as described by Page (30). Tolerance to elevated temperatures, the ability to enter the flagellated stage, and reactivity with rabbit anti-Acanthamoeba antibodies were also tested.
Cysts collected from the surface of the agar were washed three times in Page's saline and distributed on eight-well Teflon-coated microscope slides (Flow Laboratories Inc.) or embedded in freezing medium (Tissue-Tek; Miles, Naperville, Ill.) for cryosections. Five-micrometer-thick sections were dried on slides at room temperature overnight, fixed in cold acetone for 20 min, and used for immunostaining.
Detection of cellulose using D-CBD and immunofluorescence.
Acetone-fixed smears or cryosections of reference material and from isolated amoebae prepared as described above, were immersed briefly in PBS and incubated for 30 min at room temperature with D-CBD at a concentration of 0.2 g/liter. After three washings in PBS, the monoclonal mouse antibody CI-89 diluted 1/200 in PBS was added, and slides were incubated for 30 min at room temperature. Three subsequent washings with PBS were followed by a third incubation (30 min, room temperature) with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin A (IgA)-IgG-IgM (Sigma Chemical Co.) diluted 1/50 in 0.0001% Evans blue. For some experiments, antigen slides were incubated with 0.25% trypsin for 30 min at 37°C prior to the incubation with D-CBD. For microscopy a DMRB fluorescence microscope (Leica Mikroskopie und Systeme GmbH) equipped with dichroic mirror filter combinations for UV excitation light (A) and blue FITC excitation light (L4). For photography, a Nikon-Kodak (Eastman Kodak Inc.) digital camera was used.
Calcofluor white staining was performed essentially as previously described for paraffin sections of tissue containing microsporidia (33). In short, a 0.05% aqueous solution was overlaid for about 5 min at room temperature on preparations of cells fixed to microscope slides. As an alternative, a Calcofluor solution diluted 10 times more was used as staining medium for cells in suspension. The staining reactions obtained with commercial preparations of Calcofluor white (Sigma Chemical Co) and Fungi Fluor Pneumocystis kit (Polysciences, Inc.) (2) were similar. 4′,6′-Diamidino-2-phenylindole (DAPI) was used for DNA staining.
RESULTS AND DISCUSSION
We showed that cellulose can easily and efficiently be stained and detected by immunofluorescence using T. reesei CBDs in combination with anti-CBD and fluorescein-labeled antibodies. We further showed that this staining is applicable to the detection of cellulose in the inner cyst wall of Acanthamoeba spp. The reference strains of Acanthamoeba spp. and 13 Acanthamoeba strains isolated from water and from keratitis patients reacted in a D-CBD-anti-CBD immunofluorescent test (Table 2). All Naegleria and Hartmannella isolates (Table 3) gave negative reactions. The strongest reaction was obtained with empty cyst shells of Acanthamoeba spp. left after excystation, while trophozoites were negative (Fig. 1). In contrast to the D-CBD, calcofluor bound to both cellulose-containing Acanthamoeba spp. (Fig. 1D) and chitin-containing P. carinii (Fig. 1I). Calcofluor and related optical brightening agents with broad absorption and emission spectra have been used for diagnostic purposes to detect not only Pneumocystis but also Entamoeba, Chilomastix, and Microsporidia (9). Calcofluor does stain both cellulose and chitin, making it impossible to distinguish between them, and the putative chitin-specific fluorescent probe Fungalase-F-FITC (Anomeric, Inc.) was shown also to react with cellulose (34). Both cellulose and chitin are recognized by N-acetylglucosamine-specific lectins such as wheat germ agglutinin and tomato lectin (20, 38). Furthermore, in a carefully performed study using other fluorescein-conjugated CBDs (family II CEX from Cellulomonas fimi and CBHII from T. reesei single domains), it was not possible to distinguish between cellulose and chitin (34).
TABLE 2.
Isolates of free-living amoebae belonging to the genus Acanthamoeba which tested positive for the presence of cellulose in the cyst walla
| Isolate | Source |
|---|---|
| Ac 3172 | Human cornea, Sweden |
| Ac 896 | Human cornea, Sweden |
| MTC 4a | Tap water from eye shower, Sweden |
| SMI 2 | Tap water from ice machine, Sweden |
| I 3 | Tap water from hospital, Sweden |
| BMH18 | Tap water from hospital, Germany |
| B 6 | Biowaste, hospital, Germany |
| E 46 | Tap water, Nicaragua |
| B 77 | Tap water, Nicaragua |
| B 99 | Well, Nicaragua |
| E 52 | Well, Nicaragua |
| I 10 | Geyser, Iceland |
| I 4 | Geyser, Iceland |
Staining was performed on cryosections.
TABLE 3.
Free-living amoebae of the genera Hartmannella and Naegleria which tested negative for the presence of cellulose in the cyst walla
| Isolate | Source |
|---|---|
| Hartmannella | |
| U1 | Tap water, hospital, Sweden |
| UJ3 | Tap water, hospital, Sweden |
| HSVA 997 | Tap water, hospital, Sweden |
| MTC 2b | Tap water, eye shower, Sweden |
| I 8.2 | Geyser, Iceland |
| Naegleria | |
| I 8.1 | Geyser, Iceland |
| SMI1 | Tap water, ice machine, Sweden |
| E 14 | Tap water, Nicaragua |
| E 12 | Well, Nicaragua |
| E 51 | Well, Nicaragua |
| E 55 | Well, Nicaragua |
| E 57 | Well, Nicaragua |
Staining was performed on cryosections.
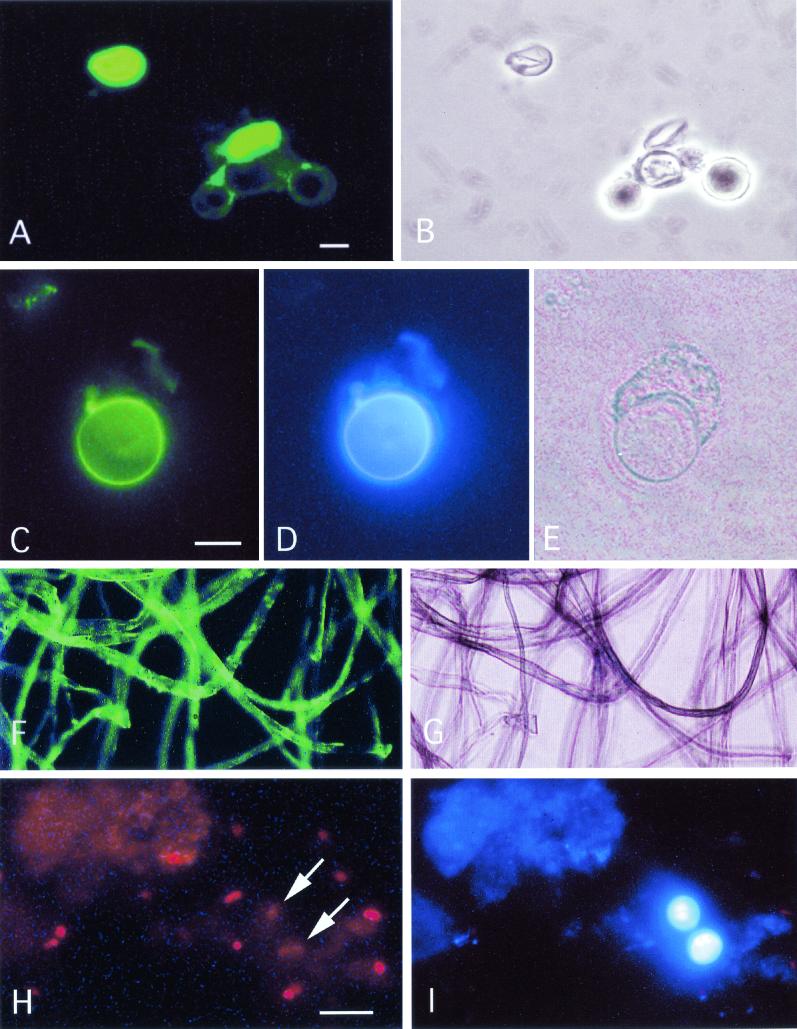
FIG.1.
(A) Cellulose of Acanthamoeba castellanii stained by immunofluorescence using recombinant T. reesei D-CBD in combination with monoclonal mouse anti-CBD antibodies and FITC-labeled anti-mouse immunoglobulin antibodies. (A) Strong reaction with two ruptured amoeba cysts but not with three intact cysts. (B) Corresponding area seen by phase-contrast microscopy. (C and D) Double fluorescence staining for cellulose in A. castellanii in suspension using the CBD procedure (C) and calcofluor (D). Note the similar distribution of cyst wall staining with the two methods and absence of staining of the excysted trophozoite. (E) The same area seen by phase contrast illumination. (F) Control CBD staining of cotton fibers showing cellulose-specific fluorescent labeling. (G) Corresponding area seen by phase-contrast illumination. (H) The CBD assay fails to detect P. carinii organisms (arrows) in bronchoalveolar lavage fluid from patient with pneumonia. The red dots represent nuclear material visualized with DAPI. (I) P. carinii cysts are readily detected after double staining with calcofluor. Bar, 10 μm.
The failure of the D-CBD to react with mature, intact cysts of Acanthamoeba spp. is apparently due to lack of access of the D-CBD to cellulose present at the inner aspect of the cyst wall. No reaction was seen with a H. vermiformis reference strain or with G. intestinalis, E. dispar, or P. carinii cysts known to contain chitin (10, 37, 38).
The D-CBD used in the present study was shown previously by other methods to have some affinity for chitin but not for other insoluble carbohydrates, such as mannan or xylan (23), or any significant binding to soluble carbohydrates. The reason for the observed selective staining of cellulose in the present study is consistent with previous observations on the behavior of different variants of the CBD. The CBHI CBD alone is not very tightly bound to cellulose and does not significantly bind to chitin (23, 26). Because it is easily washed away from cellulose, it is not by itself useful as a probe, as noted previously (22, 34). However, the D-CBD is, mainly due to the CBHII CBD, tightly bound to cellulose (22), although it is easily washed away from chitin (8). The D-CBD has the additional benefit that it can be produced heterologously rather efficiently in E. coli. Detection of chitin using antichitin antibodies seems to be possible (20, 37). In analogy with the demonstrated cellulase-based immunocytochemical detection of cyst wall cellulose, the possibility of detecting chitin with chitinase is obvious. Chitinases occur widely in nature and appear to be efficient antifungal agents (16). Interestingly, a highly sensitive assay to detect alpha-chitin has been developed based on the binding of a unique protein from Streptomyces olivaceoviridis, which interacts specifically with crystalline alpha-chitin (39).
Lack of D-CBD binding to the chitinous cyst walls however, is not a definite proof of cellulose specificity or of lack of chitin reactivity, since some intact Acanthamoeba cysts failed to stain. Apparently mature cysts pose a diffusion barrier. Such a masking of cellulose by noncellulose materials has also been suggested in previous studies (17, 35). We tried trypsinization prior to incubation with D-CBD and anti-CBD antibody without success. Drastic chemical treatment may be necessary, as shown for the chitinous layer of Neurospora crassa hyphae (17), but preliminary experiments using 1 M sodium hydroxide caused severe morphological damage and nonspecific background staining. We therefore prepared frozen sections, which yielded reproducible results (Fig. 2). A positive staining reaction was seen only with Acanthamoeba cysts. No reactivity of D-CBD was seen on sections of G. intestinalis, E. dispar, or H. vermiformis.
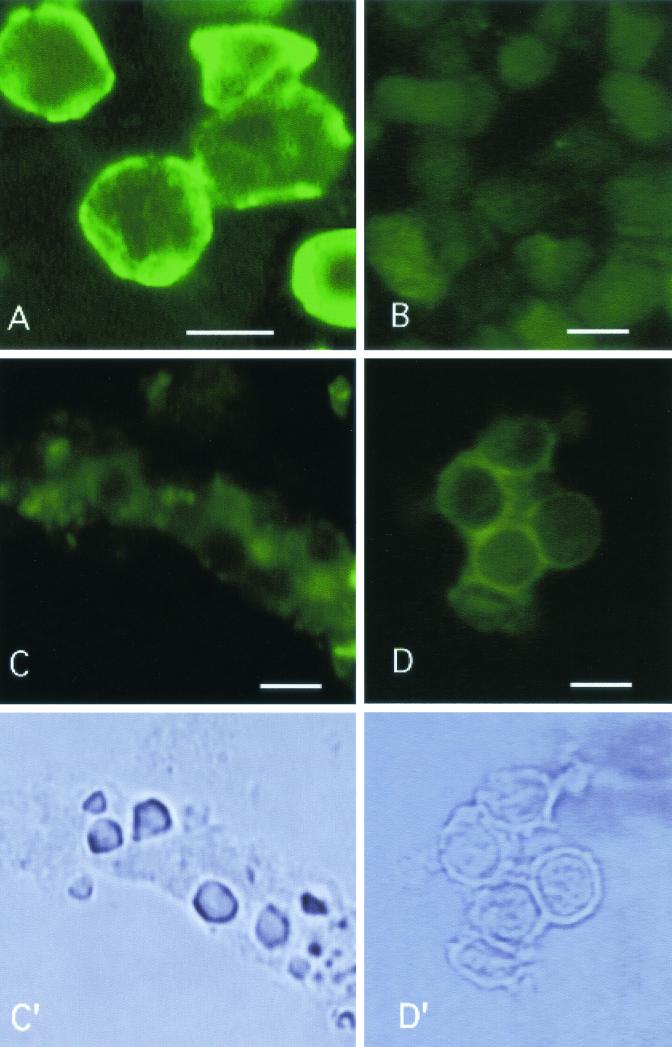
FIG. 2.
Staining for cyst wall cellulose in frozen sections of different protozoan cysts using the D-CBD procedure. (A) Control sections of A. castellanii show a distinct linear cyst wall reaction, whereas G. intestinalis (B), E. dispar (C), and H. vermiformis (D) fail to react. (C′ and D′) The latter two organisms shown by phase-contrast illumination. Bar, 10 μm.
Free-living amoebae represent a large, nontaxonomic group comprising various genera which commonly inhabit all types of aquatic environments. Several different species can be detected in drinking water distribution systems (15). Three frequently found genera, which also have medical importance, were included in our study. H. vermiformis has been identified as the most dominant amoeba in water distribution systems, often associated with the presence of Legionella pneumophila (31, 32), while Acanthamoeba and Naegleria, in addition to supporting growth of pathogenic bacteria, are potential human pathogens (4, 18). There is very little information on the constituents of cyst walls of free-living amoebae. Among the genera tested here, D-CBD binds only to cysts of Acanthamoeba, suggesting that the others do not contain cellulose. However, if the presence of cellulose is a unique characteristic of Acanthamoeba spp., distinguishing this genus from other free-living amoebae will require further investigation of other species in this group.
The described method has potential as a diagnostic tool for the detection of cellulose-containing protozoa, which also seems to be useful for tissue sections, as shown recently (27). The problems encountered in studies on environmental samples were (i) exposing the cyst wall cellulose in order to make it accessible to the CBDs, (ii) getting rid of nonspecific background fluorescence, and (iii) making CBDs readily available to interested workers in the field.
We are addressing these points in ongoing studies. It should be possible to solve the problem of exposure of cellulose by using different extraction methods eliminating the barrier formed by the outer cyst membrane. A novel D-CBD, produced as a hydrophobin I fusion protein in the homologous host T. reesei, which can be easily purified by two-phase separation at production levels of grams per liter (24), also selectively recognizes cellulose. The problem of nonspecific background fluorescence due to autofluorescence of protozoan cyst walls can be eliminated by using immunocytological staining methods for light microscopy. Furthermore, direct labeling of the D-CBD obviously should be a practical improvement.
Acknowledgments
Acanthamoeba strains BMH18 and B6 were a kind gift from Ute Rohr, Institute for Hygiene and Microbiology, Ruhr University, Bochum, Germany, and rabbit anti-Acanthamoeba serum was donated by Jan-Åke Liljeqvist, Department of Clinical Virology, University of Gothemburg, Sweden.
M.L. acknowledges support from the Academy of Finland.
REFERENCES
- 1.Aho, S., V. Olkkonen, T. Jalava, M. Paloheimo, R. Buhler, M.-L. Niku-Paavola, D. Bamford, and M. Korhola. 1991. Monoclonal antibodies against core and cellulose-binding domains of Trichoderma reesei cellobiohydrolases I and II and endoglucanase I. Eur. J. Biochem. 200:643-649. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, F., V. Bandi, C. Stager, and K. K. Guntupalli. 1997. Detection of Pneumocystis carinii in tracheal aspirates of intubated patients using calcofluor-white (Fungi-Fluor) and immunofluorescence antibody (Genetic Systems) stains. Crit. Care Med. 25:948-952. [DOI] [PubMed] [Google Scholar]
- 3.American Type Culture Collection. 1993. Catalogue of protists, p. 68. American Type Culture Collection, Manassas, Va.
- 4.Barker, J., and M. R. W. Brown. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253-1259. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell, J. 1988. Physical methods for the determination of chitin structure and conformation. Methods Enzymol. 161:435-442. [Google Scholar]
- 6.Blanton, W. E., and C. L. Villemez. 1978. Molecular size and chain length distribution in Acanthamoeba cellulose. J. Protozool. 25:264-267. [Google Scholar]
- 7.Bose, K., D. K. Ghosh, and A. Bhattacharya. 1989. Membrane carbohydrate characterization of Acanthamoeba astronyxis, A. castellanii and Naegleria fowleri by fluorescein-conjugated lectins. Int. J. Parasitol. 19:737-741. [DOI] [PubMed] [Google Scholar]
- 8.Carrard, G., and M. Linder. 1999. Widely different off-rates of two closely related cellulose-binding domains from Trichoderma reesei. Eur. J. Biochem. 262:637-643. [DOI] [PubMed] [Google Scholar]
- 9.Conteas, C. N., T. Sowerby, G. W. Berlin, F. Dahlan, A. Nguyen, R. Porschen, J. Donovan, M. LaRiviere, and J. M. Orenstein. 1996. Fluorescence techniques for diagnosing intestinal microsporidiosis in stool, enteric fluid, and biopsy specimens from acquired immunodeficiency syndrome patients with chronic diarrhea. Arch. Pathol. Lab. Med. 120:847-853. [PubMed] [Google Scholar]
- 10.Eichinger, D. 1997. Encystation of Entamoeba parasites. Bioessays 19:633-639. [DOI] [PubMed] [Google Scholar]
- 11.Elvin, K. M., A. Bjorkman, E. Linder, N. Heurlin, and A. Hjerpe. 1988. Pneumocystis carinii pneumonia: detection of parasites in sputum and bronchoalveolar lavage fluid by monoclonal antibodies. BMJ 297:381-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraire, A. E., B. Kemp, S. D. Greenberg, H. S. Kim, R. Estrada, and R. A. McBride. 1996. Calcofluor white stain for the detection of Pneumocystis carinii in transbronchial lung biopsy specimens: a study of 68 cases. Mod. Pathol. 9:861-864. [PubMed] [Google Scholar]
- 13.Gillin, F. D., S. E. Boucher, S. S. Rossi, and D. S. Reiner. 1989. Giardia lamblia: the roles of bile, lactic acid and pH in the completion of the life cycle in vitro. Exp. Parasitol. 69:164-174. [DOI] [PubMed] [Google Scholar]
- 14.Harb, O. S., L.-Y. Gao, and Y. Abu Kwaik. 2000. From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Environ. Microbiol. 2:251-265. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann, R., and R. Michel. 2001. Distribution of free-living amoebae (FLA) during preparation and supply of drinking water. Int. J. Hyg. Environ. Health 203:215-219. [DOI] [PubMed] [Google Scholar]
- 16.Hollis, T., Y. Honda, T. Fukamizo, E. Marcotte, P. J. Day, and J. D. Robertus. 1997. Kinetic analysis of barley chitinase. Arch. Biochem. Biophys. 344:335-342. [DOI] [PubMed] [Google Scholar]
- 17.Hunsley, D., and D. Kay. 1976. Wall structure of the Neurospora hyphal apex: immunofluorescent localization of wall surface antigens. J. Gen. Microbiol. 96:233-248. [DOI] [PubMed] [Google Scholar]
- 18.John, D. T. 1993. Opportunistically pathogenic free-living amoebae, p. 143-246. In J. P. Kreier and J. R. Baker (ed.), Parasitic protozoa, 2nd ed., vol. 3. Academic Press, Inc., San Diego, Calif. [Google Scholar]
- 19.Kasprzak, W., and A. C. Majewska. 1985. Improvement in isolation and axenic growth of Giardia intestinalis strains. Trans. R. Soc. Trop. Med. Hyg. 79:551-557. [DOI] [PubMed] [Google Scholar]
- 20.Kneipp, L. F., A. F. B. Andrade, W. de Souza, J. Angluster, C. S. Alviano, and L. R. Travassos. 1998. Trichomonas vaginalis and Tritrichomonas foetus: expression of chitin at the cell surface. Exp. Parasitol. 89:195-204. [DOI] [PubMed] [Google Scholar]
- 21.Linder, E., L. Lundin, and H. Vorma. 1987. Detection of Pneumocystis carinii in lung-derived samples using monoclonal antibodies to an 82 kDa parasite component. J. Immunol. Methods 98:57-62. [DOI] [PubMed] [Google Scholar]
- 22.Linder, M., T. Nevanen, L. Söderholm, O. Bengs, and T. T. Teeri. 1998. Improved immobilization of fusion proteins via cellulose-binding domains. Biotechnol. Bioeng. 60:642-647. [DOI] [PubMed] [Google Scholar]
- 23.Linder, M., I. Salovuori, L. Ruohonen, and T. T. Teeri. 1996. Characterization of a double cellulose-binding domain: synergistic high affinity binding to cellulose. J. Biol. Chem. 271:21268-21272. [DOI] [PubMed] [Google Scholar]
- 24.Linder, M., K. Selber, T. Nakari-Setälä, M. Qiao, M.-R. Kula, and M. Penttilä. 2001. The hydrophobins HFBI and HFBII from Trichoderma reesei showing efficient interactions with nonionic surfactants in aqueous two-phase systems. Biomacromolecules 2:511-517. [DOI] [PubMed] [Google Scholar]
- 25.Linder, M., and T. T. Teeri. 1996. The cellulose-binding domain of the major cellobiohydrolase of Trichoderma reesei exhibits true reversibility and a high exchange rate on crystalline cellulose. Proc. Natl. Acad. Sci. USA 93:12251-12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linder, M., and T. T. Teeri. 1997. The roles and function of cellulose-binding domains. J. Biotechnol. 57:15-28. [DOI] [PubMed] [Google Scholar]
- 27.Linder, M., J. Winiecka-Krusnell, and E. Linder. 2001. Identification of Acanthamoebae in clinical and environmental samples using cellulose-binding domains of cellulase as immunocytochemical marker for cyst wall cellulose, p. 198-201. In Proceedings of the IXth International Meeting on the Biology and Pathogenicity of Free-living Amoebae, Paris, July 2001. John Libbey Eurotext, Paris, France.
- 28.Martines, H. M., M. S. Osato, and R. L. Font. 1987. The value of calcofluor white in the diagnosis of mycotic and Acanthamoeba infections of the eye and ocular adnexa. Ophthalmology 94:23-26. [DOI] [PubMed] [Google Scholar]
- 29.Neff, R. J., Benton, W. F., and R. H. Neff. 1964. The composition of the mature cyst wall of soil ameba Acanthamoeba sp. J. Cell Biol. 23:66A. [Google Scholar]
- 30.Page, F. C. 1988. A new key to freshwater and soil Gymnamoeba with instructions for culture. Freshwater Association, Natural Environment Research Council, London, United Kingdom.
- 31.Rohr, U., S. Weber, R. Michel, F. Selenka, and M. Wilhelm. 1998. Comparison of free-living amoebae in hot water systems of hospitals with isolates from moist sanitary areas by identifying genera and determining temperature tolerance. Appl. Environ. Microbiol. 64:1822-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanden, G. N., W. E. Morrill, B. S. Fields, R. F. Breiman, and J. M. Barbaree. 1992. Incubation of water samples containing amoebae improves detection of legionellae by the culture method. Appl. Environ. Microbiol. 58:2001-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svedhem, V., M. Lebbad, J. Struve, B. Veress, E. Andstrom, A. Aust-Kettis, and E. Linder. 1998. Microsporidia in duodenal biopsies from 72 HIV-infected patients with abdominal complaints. APMIS 106:535-538. [DOI] [PubMed] [Google Scholar]
- 34.Taylor, J. T., C. H. Haigler, D. G. Kilburn, and R. L. Blanton. 1996. Detection of cellulose with improved specificity using laser-based instruments. Biotech. Histochem. 71:215-223. [DOI] [PubMed] [Google Scholar]
- 35.Tomlinson, G., and E. A. Jones. 1962. Isolation of cellulose from the cyst wall of a soil amoeba. Biochim. Biophys. Acta 63:194-200. [DOI] [PubMed] [Google Scholar]
- 36.Visvesvara, G. S. 1987. Laboratory diagnosis, p. 193-215. In E. G. Rondanelli (ed.), Amphizoic amoebae. Human pathology. Piccin Nuova Libraria, Padua, Italy.
- 37.Walker, A. N., R. E. Garner, and M. N. Horst. 1990. Immunocytochemical detection of chitin in Pneumocystis carinii. Infect. Immun. 58:412-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward, H. D., J. Alroy, B. I. Lev, G. T. Keusch, and M. E. A. Pereira. 1985. Identification of chitin as a structural component of Giardia cysts. Infect. Immun. 49:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeltins, A., and H. Schrempf. 1995. Visualization of alpha-chitin with a specific chitin-binding protein (CHB1) from Streptomyces olivaceoviridis. Anal. Biochem. 231:287-294. [DOI] [PubMed] [Google Scholar]