Abstract
The objective of this study was to compare, by using identical sample types, the Salmonella enterica prevalences and serovar diversities between pigs necropsied on the farm and those necropsied at the abattoir after transport and holding. We necropsied 567 market weight pigs (>70 kg) from six herds. Pigs were alternately assigned to be necropsied on the farm or at the abattoir. One-half of the group was sent in clean, disinfected trailers to slaughter at a commercial abattoir. After transport (mean distance, 169 km) and 2 to 3 h of holding in antemortem pens, these pigs were necropsied. The 50 pigs remaining on the farm were necropsied the following day. The same sample types and amounts were collected for S. enterica culture at both locations. Results show a sevenfold-higher (P < 0.001) S. enterica isolation rate from pigs necropsied at the abattoir (39.9%; 114 of 286) than from those necropsied on the farm (5.3%; 15 of 281). This difference was also observed for each individual herd. All sample types showed a significantly higher prevalence when comparing abattoir to on-farm collection, respectively: lymph nodes, 9.15 versus 3.6%; cecal contents, 13.6 versus 1.8%; 1 g of fecal matter, 25.2 versus 0.7%. Recovery of additional serovars at the abattoir suggests the pigs are receiving S. enterica from extra-farm sources. This study demonstrates that rapid infection during transport, and particularly during holding, is a major reason for increased S. enterica prevalence in swine. This finding identifies the holding pen as an important S. enterica control point in the pork production chain.
It has been reported that pork carcass contamination with Salmonella enterica is primarily related to intestinal S. enterica infections (4, 16, 23). It is assumed that the more S. enterica that is carried into the slaughter process, via the pig's intestines, the greater the risk of equipment and final product contamination. Therefore, reductions in preslaughter infection rates should result in increased pork safety.
A number of studies have reported that S. enterica isolation rates in market swine are 3 to 10 times higher after transport and slaughter compared to rates measured on the farm (1, 11, 20, 24, 25). One possibility for this increase in isolation rates is long-term lairage (greater than 12 h) in contaminated abattoir holding pens (4, 9, 13, 14, 16). In the United States, most abattoirs report that they try to avoid holding pigs for more than 6 to 8 h. However, a 2-h holding period is recommended to improve meat quality (2, 8, 22).
The stress of transport has also been suggested as a reason for increased S. enterica shedding (15; T. J. Stabel and P. J. Fedorka-Cray, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., p. 60, 1999). The physiological changes associated with stress might encourage the recrudescence of latent carriers or it might increase the susceptibility of noncarriers to new infection. Immunological parameters, such as cortisol or beta-endorphins, are increased after transport (7, 15; Stabel and Fedorka-Cray, Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). However, few studies have demonstrated a direct increase in S. enterica shedding or infection due to these physiological changes. Williams and Newell (25) described increased shedding after transport. However, this study used a small number of pigs (n = 20) and the differences in isolation rates were not statistically significant. Isaacson et al. (12) reported increased isolation rates after transport but only if the pigs did not fast before transport. They concluded that transport stress alone did not contribute to the increase in isolation rates. In support of this conclusion, no difference was demonstrated between directly shipped pigs and those stressed by mixing, fasting, and 18 h of holding in a clean, disinfected facility (11).
A weakness in that study, and in others, is the before and after comparison of unmatched sample types and amounts (11, 25). For example, by using 1 g of feces, S. enterica was recovered from 3.4% of pigs tested on the farm. However, after transport and holding and by using colon contents (10 g), cecal contents (10 ml), and ileocecal lymph (ICL) nodes, 71.8% of the same pigs (196 of 273) were positive. Increasing the volume of feces from 1 to 10 g has been shown to double the sensitivity (6). The inclusion of multiple samples from the same pig will increase the likelihood of detecting a positive pig, and the culture of ICL nodes may detect latent nonshedders. Therefore, unmatched comparisons may be invalid.
Additionally, many studies suffer from the possibility of in-plant sample contamination. Samples are often collected from viscera sets after frequent handling along the conveyor belt. It is possible that some isolates were from workers or from the environment and not from the pigs.
The intent of the study reported here was to eliminate some weaknesses by using matching sample types collected from pigs that never entered the slaughter area. The objective was to compare the S. enterica prevalences and serovar diversities between pigs necropsied on the farm and those necropsied at the abattoir.
MATERIALS AND METHODS
Herd selection.
Herds eligible for inclusion in this study were those enrolled in the Accelerated Pseudorabies Eradication Program (APEP). This was a voluntary effort of state and federal regulatory agencies (U.S. Department of Agriculture and Animal and Plant Health Inspection Service) to depopulate swine herds that had tested positive for the pseudorabies virus in the previous 12 months. The producer was reimbursed at fair market value for breeding stock and by weight for market animals. To avoid an oversupply of pork, all APEP swine were to be euthanized at a commercial packing plant and sent to rendering. As producers within the state of Iowa were enrolling in the APEP from January 2000 through April 2000, they were invited to participate in this study. The herds needed to have a minimum of 100 animals over 70 kg available for sampling. The final selection of herds included consideration of herd management, statewide location, timing of scheduled depopulation, and our data collection resources. Our goal was to test the first six herds available. Herd selection was not intended to test hypotheses about the effect of management or herd size on S. enterica prevalence. Each herd provided its own control group. Therefore, a convenience sample of herds seemed acceptable and unbiased.
Animal selection.
From each herd, 100 market or finishing swine were to be studied. One-half of the herd was randomly assigned to on-farm necropsy, and the other half was assigned to abattoir necropsy. The selection of 50 pigs for treatment (transport and lairage) and 50 pigs for control (on-farm necropsy) would allow for the detection of an S. enterica prevalence difference of 25% with 95% confidence. Except for breeding stock, all pigs over 70 kg were eligible for inclusion. We selected a predetermined number from each pen so that every eligible pig had an equal chance of being selected.
Sample collection.
Three days before the scheduled depopulation, we collected 1 g antemortem fecal samples (AFEC) by using a fecal loop (Jorgensen Inc., Loveland, Colo.), and we attached alternately colored and uniquely numbered ear tags. The purpose of this fecal collection was to estimate the S. enterica status of the individual farm before the disruptions of depopulation. On the day of depopulation, we determined which ear tag color group would be necropsied on-farm and which group would be necropsied at the abattoir. In all except herd 1, the assignment was random. In herd 1, we selected pigs from the first three pens for farm necropsy and from the next three pens for abattoir necropsy. For all herds, the pigs chosen for abattoir necropsy, with others on the premises, excluding the farm necropsy animals, were transported to a commercial abattoir in disinfected standard commercial livestock trailers. During transport, the pigs were not fed or watered but were provided with water upon arrival from a trough with a constant inflow of fresh water. The maximum time without feed was approximately 5 h.
At the abattoir, the study pigs (n = 50) were held together in a standard holding pen. After holding, they were stunned and exsanguinated. The carcasses were diverted to an open top trailer for necropsy. The ICL node and superficial inguinal lymph (SIL) node were collected by using forceps and scissors scrubbed in 70% ethanol after each pig. The cecal contents (CC), ∼30 ml, were collected through a puncture in the cecum. The necropsy fecal sample (NFEC) was taken, through the rectum, by using the fecal loop (scrubbed in 70% ethanol). A 25-g portion of the gluteal muscle was collected for the detection of S. enterica antibodies.
The pigs (n = 50) to be necropsied on the farm stayed in their original pens on normal feed until the following day. They were then euthanized with a captive bolt gun and immediately moved to a central, on-site location. Samples were collected in the same manner as at the abattoir. Following necropsy, carcasses were removed and rendered.
Sample processing.
The feces (1 g) (AFEC, NFEC) were placed in 10 ml of GN-Hajna (GN) broth and tetrathionate (TET) broth (Becton Dickinson, Sparks, Md.). All remaining samples were placed on ice and transported to the National Animal Disease Center (Ames, Iowa). Samples (farm and abattoir collected) were refrigerated (4.4°C) and processed the following morning. For processing, the SIL nodes (10 g) and the ICL nodes (5 g) were separately macerated in a sterile bag with a rubber mallet. Peptone water (Becton Dickinson) (10 ml) was added, and each sample was homogenized (Stomacher 400 Circulator; Seward Ltd., London, United Kingdom) at 260 rpm for 1 min. One milliliter of supernatant was then added directly to 10 ml (1:10 ratio) of each preenrichment medium (GN and TET). Cecal and fecal samples (10 ml or 10 g) were added directly to 100 ml (1:10 ratio) of preenrichment broth (GN and TET). Culture methods included preenrichment in separate tubes of GN broth (24 h at 37°C) and TET broth (48 h at 37°C) followed by enrichment in Rappaport-Vassiliadis medium (Becton Dickinson) (24 h at 37°C). A loopful of the Rappaport-Vassiliadis medium was then streaked onto brilliant green sulfa agar (Becton Dickinson) (24 h at 37°C) and XLT4 agar (Becton Dickinson) (24 h at 37°C), and after this, a single suspect colony was picked and transferred to triple sugar iron (Becton Dickinson) and lysine iron agar slants (Becton Dickinson) (24 h at 37°C). Biochemically suspect S. enterica isolates were further classified by agglutination with Bacto S. enterica O antiserum groups polyA-I and Vi, B, C1, and E (Becton Dickinson). These isolates were then placed on Trypticase soy agar (Becton Dickinson) slants and shipped to the National Veterinary Services Laboratories (Ames, Iowa) for serotyping.
Upon arrival at the laboratory, gluteal muscle samples were frozen (−20°C). Later, these samples were sent to the laboratory of D. L. Harris, Iowa State University, where the Danish-mixed enzyme-linked immunosorbent assay (ELISA) for S. enterica antibodies was performed with the serum exudate (meat juice) (18). The level of antibodies was measured in the ELISA by a colorimetric (wavelength, 490 nm) response expressed as optical density percent (OD%). In this study, a pig was considered ELISA positive if the meat juice OD490% was ≥40.
Statistical analysis.
The S. enterica isolation rates, for farm- and abattoir-collected samples, were compared for each sample type (fecal sample, CC, and lymph nodes) by chi-square analysis. A rate difference for each sample type was calculated by subtracting the farm S. enterica isolation rate (FSIR) from the abattoir S. enterica isolation rate (ASIR). Additionally, the isolation rates were compared between pigs raised in outside lots and those raised in confinement.
The overall S. enterica prevalence in pigs (SPP) was determined, for farm (FSP) and abattoir (ASP) necropsies, by defining a pig positive if any sample collected at necropsy was positive. The prevalence difference (ASP minus FSP), for each herd, was used as the dependant variable in a linear regression model to evaluate the effect of transport distances and abattoir pen holding times. Transport distances were approximated by using zip code data from the farm of origin to the abattoir. Pen holding times were recorded as the elapsed time from unloading until the group was moved out of the pens for euthanasia.
For pigs necropsied at the abattoir, the elapsed time from death to sample collection varied from 15 min to 2 h, as all pigs were euthanized before we began sample collection. In contrast, on-farm samples were collected within 10 to 20 min of euthanasia. To determine if this elapsed time dead affected S. enterica abattoir isolation rates, we ran a linear regression comparing time dead and ASIR for each sample type.
The serology results from the on-farm- and abattoir-necropsied pigs were combined to provide a herd-level estimate of the seroprevalence. This seroprevalence was compared to the ASP.
Disclaimer.
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
RESULTS
Table 1 describes the six herds studied. These farms were small to moderate in size (∼193 sows) by current Midwestern standards. However, based on our observations, most of them could be considered modern, progressive, independent producers. The mean travel distance from farm to abattoir was 169 km. After arrival at the abattoir, some trucks waited up to 2 h before unloading the pigs. In some cases, the pens had been cleaned by high-pressure cold water before the study pigs were placed in them. The mean pen holding time was 2.5 h for all herds. In the study population there was an equal distribution of market pigs raised outside and in confinement (total or partial).
TABLE 1.
Description of herds from which market swine were necropsied for S. enterica isolation
| Herd | Date of depopulation (mo/day/yr) | Herd size (approx no. of sows) | Transport distance (km)a | Holding time (h) | Housing conditions of market swine |
|---|---|---|---|---|---|
| 1 | 1/27/00 | 450 | 153 | 3.25 | Total confinement, fully slatted floors |
| 2 | 3/8/00 | 170 | 182 | 2.5 | Outside lots,b concrete or dirt floors |
| 3 | 3/21/00 | 30 | 132 | 2.5 | Outside lots,b concrete or dirt floors |
| 4 | 3/29/00 | 214 | 77 | 2 | Outside lots,b concrete or dirt floors |
| 5 | 4/4/00 | 125 | 201 | 3 | Total confinement, fully slatted floors |
| 6 | 4/12/00 | 168 | 270 | 1.5 | Partial confinement,c partially slatted floors |
| Mean | 193 | 169 | 2.5 |
Transport distances were estimated from postal codes.
Outside lots included open-faced buildings.
Partial confinement. All grow and finish pigs were housed indoors. Gestating sows were in outside lots.
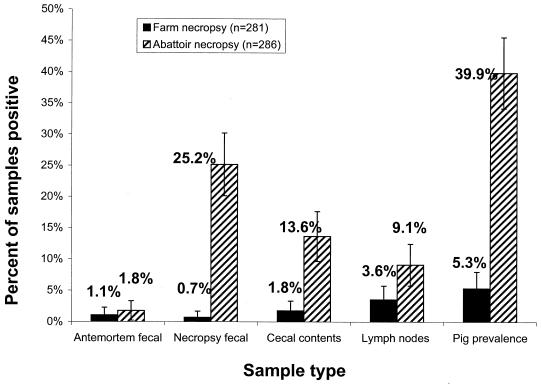
Figure 1 compares the mean S. enterica isolation rates for all herds, separated by samples collected at the farm (FSIR) and at the abattoir (ASIR). There was no significant difference in the antemortem fecal isolation rates between pigs eventually necropsied at the farm (1.1%) and those necropsied at the abattoir (1.8%). In contrast, the fecal samples collected 3 days later at the abattoir necropsy (25.2%) yielded much more S. enterica than those collected at the farm necropsy (0.7%; P < 0.001). CC were also positive more often from abattoir-necropsied pigs (13.6 versus 1.8%; P < 0.05). Results from the ICL nodes were combined with results from the SIL nodes for reasons which will be explained later. These lymph nodes had the smallest rate difference (3.6 versus 9.1%; P < 0.05). The overall SPP was seven times higher (P < 0.001) for pigs that were necropsied after transport and holding (5.3 versus 39.9%).
FIG. 1.
Comparison of S. enterica isolation rates (95% confidence intervals) from market swine (six herds) necropsied at the farm of origin or at the abattoir after 2.5 h of holding in pens. Error bars, 95% confidence interval.
The isolation rates of S. enterica for each herd and sample type and the rate differences (ASIR minus FSIR) are shown in Table 2. Of the 566 AFEC samples collected on the farm 3 days before depopulation, 1.4% were positive. Herd 4 had the highest AFEC rate of 4.4%. After necropsy, S. enterica was isolated more frequently from NFEC, CC, and lymph node data (LNN) collected at the abattoir than those types collected on the farm. These rate differences were significantly different (P < 0.05) for NFEC in herds 2, 3, and 4; for CC in herds 3 and 4; and for LNN in herd 3.
TABLE 2.
S. enterica isolation rates and seroprevalence for pigs necropsied on the farm or at the abattoira
| Herd |
S. enterica isolation rate (%) (no. of samples tested) in:
|
% SPP (no. of samples tested)d
|
% Seroprevalence (mean juice ELISA OD% ≥ 40) (no. of samples tested)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFECb | NFEC
|
CC
|
LNNc
|
|||||||||||||
| Farm | Farm | Abattoir | Rate differencee | Farm | Abattoir | Rate differencee | Farm | Abattoir | Rate differencee | Farm | Abattoir | Rate differencee | Farm | Abattoir | Combined farm and abattoir | |
| 1 | 1.0 (97) | 0 (48) | 2 (50) | 2.0 | 0 (48) | 2 (50) | 2.00 | 6.3 (48) | 6.0 (50) | −0.30 | 6.3 (48) | 10 (50)fg | 3.80 | 0 (48) | 0 (45) | 0 (93)g |
| 2 | 1.1 (94) | 0 (44)f | 30 (50)f | 30.0 | 0 (44) | 4 (50) | 4.00 | 4.5 (44) | 6 (50) | 1.50 | 4.5 (44)f | 38 (50)fg | 33.50 | 77.3 (44) | 58 (50) | 67 (94)g |
| 3 | 0 (89) | 0 (45)f | 36.4 (44)f | 36.4 | 0 (45)f | 13.6 (44)f | 13.64 | 2.2 (45)f | 27.3 (44)f | 25.10 | 2.2 (45)f | 54.5 (44)fg | 52.30 | 0 (44) | 0 (42) | 0 (87)g |
| 4 | 4.4 (90) | 4.4 (45)f | 31.1 (45)f | 26.7 | 4.4 (45)f | 53.3 (45)f | 48.89 | 4.4 (45) | 0 (45) | −4.40 | 8.9 (45)f | 68.9 (45)fg | 60.00 | 13.3 (45) | 11.1 (45) | 12.2 (90)g |
| 5 | 2.0 (98) | 0 (49)f | 51.0 (49)f | 51.0 | 6.1 (49) | 10.2 (49) | 4.08 | 4.1 (49) | 10.2 (49) | 6.10 | 10.2 (49)f | 61.2 (49)fg | 51.00 | 25.0 (48) | 21.3 (47) | 23.5 (95)g |
| 6 | 0.0 (98) | 0 (50) | 2.1 (48) | 2.1 | 0 (50) | 2.1 (48) | 2.08 | 0 (50) | 6.3 (48) | 6.30 | 0 (50)f | 10.4 (48)f | 10.40 | 8.0 (50) | 6.3 (48) | 7.1 (98) |
| Mean (total) | 1.47 (566) | 0.7 (281) | 25.2 (286) | 24.5 | 1.8 (281) | 13.6 (286) | 11.9 | 3.6 (281) | 9.1 (286) | 5.53 | 5.3 (281) | 39.9 (286) | 34.52 | 20 (280) | 17 (286) | 18.55 (557) |
The 95% confidence limits for S. enterica isolation rates are as follows: AFEC, 0 to 2.3%; NFEC (farm), 0 to 1.7%; NFEC (abattoir), 20.1 to 30.2%; CC (farm), 0.2 to 3.3%; CC (abattoir), 9.7 to 17.6%; LNN (farm), 1.4 to 5.7%; LNN (abattoir), 5.8 to 12.4%. The 95% confidence limits for pig prevalence were 2.7 to 8.0% (farm) and 34.2 to 45.5% (abattoir). The 95% confidence limits for seroprevalence were 15.3 to 24.7% (farm), 12.5 to 21.4% (abattoir), and 15 to 22% (combined farm and abattoir).
1-g fecal samples taken 3 days before depopulation.
Results from the ICL and SIL nodes are combined.
SPP is defined as S. enterica isolation from any of the samples collected at necropsy (1 g of feces, CC, or lymph nodes).
Rate difference is the abattoir percentage minus the farm percentage.
Percentages are significantly different between farm and abattoir for that sample type and herd (P < 0.05).
Percentages are significantly different between pig prevalence (abattoir) and seroprevalence (combined farm and abattoir) for that herd (P < 0.05).
For each herd, the SPP determined by combining results from all sample types was higher at the abattoir than at the farm. With the exception of herd 1, the prevalence was significantly higher (P < 0.05). Herd 4 had the largest prevalence difference between the farm (8.9%) and the abattoir (68.9%). Herds 3 and 4 also had large rate differences, over 50 percentage points.
The LNN reported in Table 2 combine SIL and ICL node results. S. enterica was only isolated from SIL nodes in herds 3 and 5. For herd 3, seven SIL nodes were reported as S. enterica positive but none of the ICL nodes were positive. For herd 5, five SIL nodes were positive and all ICL nodes were negative. We expected that pigs reflecting systemic salmonellosis in the SIL nodes might also be positive in some of the ICL nodes. Therefore, the possibility of mislabeled lymph nodes during sample collection was considered for these two herds. Subsequently, the ICL and SIL node results were pooled for all analyses.
Table 2 also shows seroprevalence estimates for pigs necropsied on the farm and at the abattoir and combined data. Seroprevalence estimates varied greatly by herd, from 0 to 67%. With the exception of herd 2, the ASP was higher than the seroprevalence estimate (combined farm and abattoir) for herds (3 and 4) with a low seroprevalence (<15% seropositive) as well as for herds (2 and 5) with a high seroprevalence (>15% seropositive). The ASP was higher than the FSP.
Overall, a minority of the culture-positive pigs showed evidence of historical S. enterica infection as measured by the Danish-mixed ELISA. Of the 127 culture-positive pigs (farm and abattoir) with ELISA data, only 23.6% were seropositive (OD490% ≥ 40). Of the 34 pigs with positive lymph nodes (SIL, ICL), 23% were seropositive.
Linear regression analysis showed no effect of herd size, transport distance, or holding time on the rate differences (ASIR minus FSIR) for all sample types. However, as these were herd-level variables (n = 6 observations), the statistical power to detect an effect was predictably low.
The regression analysis of elapsed time dead and ASIR showed no difference for any of the sample types. Samples collected at the end of the 2-h period were no more likely to be S. enterica positive than samples collected at the beginning (P = 0.99).
Approximately twice as many different serovars were isolated from abattoir-necropsied pigs as from the farm-necropsied pigs (Table 3). For most of the herds, many additional serovars were recovered at the abattoir compared to the farm. Only herd 1 seemed to have the same type and number of serovars at the farm and the abattoir. As noted, herd 1 was the only herd that did not show a significant difference between FSIR and ASIR. Additionally, it was the only herd where the pigs were not randomly assigned to necropsy location. At the abattoir, herd 3 had a large number of S. enterica serovar Typhimurium subsp. copenhagen isolations (n = 25). Nine of those 25 isolates were from LNN. Notably, this was the only herd with a significant difference in the isolation rates for LNN. This serovar was the one most frequently isolated from all lymph nodes in all herds.
TABLE 3.
S. enterica serovars from tissues collected at farm and abattoir necropsy for six swine herds
| Herd |
S. enterica serovar(s)a (no. of recoveries) found in:
|
||||||
|---|---|---|---|---|---|---|---|
| AFEC | NFEC
|
CC
|
LNN
|
||||
| Farm | Abattoir | Farm | Abattoir | Farm | Abattoir | ||
| 1 | None typeable | TYC (1) | TYC (1) | TYC (2), AGN (1) | TYC (2), AGN (1) | ||
| 2 | None typeable | DER (5), INF (2), RDG (4), TYC (1) | DER (1) | CHK (2) | CHK (3) | ||
| 3 | No isolates | BOV (1), AGN (1), DER (1), MUS (1), TYC (12) | TYC (4) | MAN (1) | TYC (9), AGN (1), BOV (1) | ||
| 4 | DER (3) | DER (2) | DER (3), HEI (2), LIL (1), SPA (1), MAN (1) | DER (2) | DER (19), OHI (1), TYC (2) | DER (2) | |
| 5 | HEI (1), LON (1) | DER (2), MUS (1), MUC (16), LON (1), MVD (3) | LON (2) | MUC (2), MVD (2), OHI (2) | MUC (1), TYC (1) | DER (2), MUC (3), HEI (1) | |
| 6 | No isolates | MUC (1) | None typeable | AGN (2), CHK (1) | |||
AGN, Agona; ANA, Anatum; BOV, Bovis-morbificans; BRD, Braenderup; CHK, Choleraesuis subsp. kunzendorf; DER, Derby; HEI, Heidelberg; INF, Infantis; LIL, Lille; LON, London; MAN, Manhattan; MBA, Mbandaka; MUS, Muenster; MUC, Muenchen; MVD, Montevideo; OHI, Ohio; RDG, Reading; SEN, Senftenberg; SPA, Saint Paul; TYC, Typhimurium subsp. copenhagen.
DISCUSSION
The APEP provided a unique research opportunity to necropsy a large number of market swine (n = 567). We were able to estimate the ASP without the possibility of in-plant contamination. We demonstrated increased serovar diversity in pigs after transport and holding. Finally, we were able to compare the FSP and ASP by using matched sample types (1 g of feces, CC, colon contents, lymph nodes).
The only remaining differences between the two groups were access to feed, method of euthanasia, and the elapsed time from death to sample collection. Decreased feed intake (∼5 h) in the abattoir group does not explain the increased isolation rate, as Isaacson et al. reported decreased rates from fasted pigs, and it would not account for the increase in serovar diversity we observed (12). It is not expected that the method of euthanasia would affect isolation rates. The regression analysis of elapsed time dead on ASIR showed that higher levels at the abattoir were not due to elapsed time dead.
The study pigs in herd 1 were assigned differently than the pigs in the subsequent herds. The first three pens tested were assigned to on-farm necropsy, and the remaining pens were assigned to the abattoir. This change was made to accommodate the producer's loading requirements. Detailed analysis of pig-specific results (data not shown) suggests that we encountered a cluster of infection in two of the pens necropsied on the farm. Therefore, the resulting farm prevalence was higher than would have occurred if the pigs had been alternately assigned. It is likely, with random assignment, that herd 1 would have also demonstrated a significantly (P < 0.05) higher ASP.
The data suggest that most infections discovered at the abattoir were not due to the recrudescence of latently infected pigs. Many of the latently infected pigs should have been detected by lymph node culture on the farm (26). Additionally, latently infected pigs should have shown serological evidence of infection as measured by the Danish-mixed ELISA (17). If recrudescent infection caused the increase in ASP, then the culture rate should be similar to the seroprevalence rate (Table 2). However, since the culture prevalence is higher than the seroprevalence (39.9 versus 17%), we posit that the pigs were infected after leaving the farm.
Rapid infection during holding is the most likely explanation for the large increase in S. enterica isolation rates and the increased serodiversity. Rapid infection among herd-mates during transport cannot be ruled out. This possibility is particularly evident in herds 4 and 5, where there was an increase of farm resident serovars. However, in these two herds and the others, additional nonfarm serovars were also recovered at the abattoir. It is not clear if our culture methods are unbiased in detecting all serovars of S. enterica. However, the same methods were used on samples from both locations, so any possible bias would occur in both sets of samples.
In another study, it was experimentally demonstrated that it is feasible for market-weight swine to acquire infection from the floors of contaminated pens in as little as 2 h (10). We have observed pigs ingesting and/or inhaling the organism from the on-floor slurry. Rapid transport throughout the gastrointestinal tract seems possible. It has been shown that a fluid marker can reach the cecum in 2 h (3). Inhaled organisms may travel through the lymphatic system, as 10-week-old esophagotomized pigs were positive in the cecum 3 h after intranasal inoculation (5). Additionally, two recent publications have reported that holding pens in commercial abattoirs are usually contaminated with S. enterica (19, 21).
The weight of evidence suggests that rapid infection, particularly in holding pens, is the major cause of increased S. enterica isolation from market swine. This finding identifies abattoir holding pens as an important S. enterica control point in the pork production chain. Future research should focus on mechanisms of infection and interventions to reduce the risk from holding pens.
Acknowledgments
We thank the critical cooperation of the USDA-APHIS, Ronnie Blair, the producers, and abattoir involved in this study as well as D. L. Harris (serum ELISA), USDA-APHIS-National Veterinary Service Laboratories, and the technical assistance of Deborah Buffington, Brad Chriswell, Sharon Franklin, Jared Gailey, and Carol Wiltsey.
This study was supported in part by a grant from the Tri-State Food Safety Consortium.
REFERENCES
- 1.Berends, B. R., H. A. P. Urlings, and J. M. A. Snijders. 1996. Identification and quantification of risk factors in animal management and transport regarding Salmonella spp. in pigs. Int. J. Food Microbiol. 30:37-53. [DOI] [PubMed] [Google Scholar]
- 2.Berg, E. 1998. Critical points affecting fresh pork quality within the packing plant, p. 269-286. In Proceedings of the Pork Quality and Safety Summit, July 14-15, 1998. National Pork Producers Council, Des Moines, Iowa.
- 3.Clemens, E. T., C. E. Stevens, and M. Southworth. 1975. Sites of organic acid production and pattern of digesta movement in the gastrointestinal tract of swine. J. Nutr. 105:759-768. [DOI] [PubMed] [Google Scholar]
- 4.Craven, J. A., and D. B. Hurst. 1982. The effect of time in lairage on the frequency of salmonella infection in slaughtered pigs. J. Hyg. 88:107-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedorka-Cray, P. J., L. C. Kelley, T. J. Stabel, J. T. Gray, and J. A. Laufer. 1995. Alternate routes of invasion may affect pathogenesis of Salmonella typhimurium in swine. Infect. Immun. 63:2658-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funk, J., P. R. Davies, and M. A. Nichols. 2000. The effect of sample weight on detection of Salmonella enterica in swine feces. J. Vet. Diagn. Investig. 12:412-418. [DOI] [PubMed] [Google Scholar]
- 7.Geverink, N. A., R. H. Bradshaw, E. Lambooij, V. M. Wiegant, and D. M. Broom. 1998. Effects of simulated lairage conditions on the physiology and behaviour of pigs. Vet. Rec. 143:241-244. [DOI] [PubMed] [Google Scholar]
- 8.Grandin, T. 1994. Methods to prevent PSE and blood splash, p. 206-209. In Proceedings for the Lehman Conference. University of Minnesota, St. Paul, Minn.
- 9.Hansen, R., R. Rogers, S. Emge, and N. J. Jacobs. 1964. Incidence of Salmonella in the hog colon as affected by handling practices prior to slaughter. J. Am. Vet. Med. Assoc. 145:139-140. [PubMed] [Google Scholar]
- 10.Hurd, H. S., J. K. Gailey, J. D. McKean, and M. H. Rostagno. 2001. Rapid infection in market-weight swine following exposure to a Salmonella Typhimurium-contaminated environment. Am. J. Vet. Res. 62:1194-1197. [DOI] [PubMed] [Google Scholar]
- 11.Hurd, H. S., J. D. McKean, I. V. Wesley, and L. A. Karriker. 2001. The effect of lairage on Salmonella isolation from market swine. J. Food Prot. 64:939-944. [DOI] [PubMed] [Google Scholar]
- 12.Isaacson, R. E., L. D. Firkins, and R. M. Weigel. 1999. Effect of transportation and feed withdrawal on shedding of Salmonella Typhimurium among experimentally infected pigs. Am. J. Vet. Res. 60:1155-1158. [PubMed] [Google Scholar]
- 13.Kampelmacher, E. H., P. A. M. Guinee, K. Hoestra, and A. Van Keulen. 1963. Further studies on Salmonella in slaughterhouses and in normal slaughter pigs. Zentbl. Vetmed. Reihe B 10:1-27. [Google Scholar]
- 14.McDounagh, V. P., and H. G. Smith. 1958. The significance of the abattoir in salmonella infection in Bradford. J. Hyg. 56:271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGlone, J. J., J. L. Salak, E. A. Lumpkin, R. I. Nicholoson, M. Gibson, and R. Norman. 1993. Shipping stress and social status effects on pig performance, plasma cortisol, natural killer cell activity, and leukocyte numbers. J. Anim. Sci. 71:888-896. [DOI] [PubMed] [Google Scholar]
- 16.Morgan, I. R., F. L. Krautil, and J. A. Craven. 1987. Effect of time in lairage on caecal and carcass salmonella contamination of slaughter pigs. Epidemiol. Infect. 98:323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen, B., D. Baggesen, F. Bager, J. Haugegaard, and P. Lind. 1995. The serological response to Salmonella serovars typhimurium and infantis in experimentally infected pigs. The time course followed with an indirect anti-LPS ELISA and bacteriological examinations. Vet. Microbiol. 47:205-218. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen, B., L. Ekeroth, F. Bager, and P. Lind. 1998. Use of muscle fluid as a source of antibodies for serologic detection of Salmonella infection in slaughter pig herds. J. Vet. Diagn. Investig. 10:158-163. [DOI] [PubMed] [Google Scholar]
- 19.Rostagno, M. H., H. S. Hurd, J. D. McKean, C. Ziemer, and R. C. Leite. 2001. Abattoir holding pens as a source of Salmonella for swine, p. 298-300. In Proceedings of the 4th International Symposium on the Epidemiology and Control of Salmonella and other Food Borne Pathogens in Pork, Leipzig, Germany. University of Leipzig, Leipzig, Germany.
- 20.Shotts, E. B., Jr., W. T. Martin, and M. M. Galton. 1962. Further studies on Salmonella in human and animal foods and in the environment of processing plants, p. 309-318. In Proceedings of the 65th Annual Meeting of the U.S. Livestock Sanitary Association. MacCrellish and Quigley Co., Trenton, NJ.
- 21.Swanenburg, M., H. A. P. Urlings, D. A. Keuzenkamp, and J. M. A. Snijders. 2001. Salmonella in the lairage of pig slaughterhouses. J. Food Prot. 64:12-16. [DOI] [PubMed] [Google Scholar]
- 22.Warriss, P. D., S. N. Brown, J. E. Edwards, M. H. Anil, and D. P. Fordham. 1992. Time in lairage needed by pigs to recover from the stress of transport. Vet. Rec. 131:194-196. [DOI] [PubMed] [Google Scholar]
- 23.Widders, P. R., K. J. Coates, I. R. Morgan, and A. Pointon. 1996. Investigation of Salmonella contamination of pigs in Australia, p. 28. In Proceedings of the 1st International Symposium on the Ecology of Salmonella in Pork Production. USDA Agricultural Research Service, Ames, Iowa.
- 24.Williams, L. P., Jr., and K. W. Newell. 1967. Patterns of Salmonella excretion in market swine. Am. J. Public Health 57:466-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams, L. P., Jr., and K. W. Newell. 1970. Salmonella excretion in joy-riding pigs. Am. J. Public Health 60:926-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood, R. L., R. Rose, N. E. Coe, and K. E. Ferris. 1991. Experimental establishment of persistent infection in swine with a zoonotic strain of Salmonella newport. Am. J. Vet. Res. 52:813-819. [PubMed] [Google Scholar]