Abstract
Kartagener syndrome (KS) is a trilogy of symptoms (nasal polyps, bronchiectasis, and situs inversus totalis) that is associated with ultrastructural anomalies of cilia of epithelial cells covering the upper and lower respiratory tracts and spermatozoa flagellae. The axonemal dynein intermediate-chain gene 1 (DNAI1), which has been demonstrated to be responsible for a case of primary ciliary dyskinesia (PCD) without situs inversus, was screened for mutation in a series of 34 patients with KS. We identified compound heterozygous DNAI1 gene defects in three independent patients and in two of their siblings who presented with PCD and situs solitus (i.e., normal position of inner organs). Strikingly, these five patients share one mutant allele (splice defect), which is identical to one of the mutant DNAI1 alleles found in the patient with PCD, reported elsewhere. Finally, this study demonstrates a link between ciliary function and situs determination, since compound mutation heterozygosity in DNAI1 results in PCD with situs solitus or situs inversus (KS).
Kartagener syndrome (KS [MIM 244400]) (Kartagener 1933) was initially described by Siewert (1904). The respiratory signs (nasal polyposis and bronchiectasis) that are part of the trilogy of symptoms are the long-term results of recurrent upper and lower respiratory tract infections that start early in life. A similar condition, referred to as immotile cilia syndrome (ICS) (ICS1 [MIM 242650]) or later primary ciliary dyskinesia (PCD), is associated with the same respiratory symptoms. In both KS and PCD, respiratory infections are secondary to defective cilia that do not beat efficiently, impairing the drainage of inhaled particles and microbes to the oropharynx. KS and PCD are associated with anomalies of cilia structure—in particular, missing or abnormal dynein arms, abnormal radial spokes, and missing central pair of microtubules (Afzelius 2000). These anomalies may also be observed in the flagella of spermatozoa. The examination of cilia under an electron microscope is difficult, because biopsy specimens are often altered by recurrent infections. Furthermore, it is not always possible to distinguish primary from secondary ciliary alterations unless cells are cultured in a sequential monolayer/suspension system after biopsy (Jorissen and Willems 2000). Because PCD and KS could be observed in the same sibship and even in monozygotic twins (Noone et al. 1999), it was stated that KS was part of PCD and that laterality direction in PCD was randomized, resulting in 50% of patients with situs inversus and hence KS (Afzelius 2000). Nevertheless, there is no large-series study to confirm this hypothesis. Moreover, in two isolated populations of Polynesians, PCD is highly prevalent but not associated with situs inversus (Waite et al. 1981). In addition, a mouse mutant with hydrocephalus and anomalies of cilia and flagella does not exhibit situs inversus (Bryan 1977). This suggests that randomization of situs applies only to a subset of cases of PCD.
The first gene in which mutations were found to be associated with PCD was DNAI1 (MIM 604366) (Pennarun et al. 1999) DNAI1, an axonemal dynein intermediate-chain gene that was isolated from Chlamydomonas reinhardtii, a unicellular alga with two flagella containing an axonemal structure similar to that of human respiratory cilia and sperm tails (Blair and Dutcher 1992; Vallee 1993; Porter 1996). Axonemal dyneins are found in ciliary and flagellar axonemes. The axonemal ultrastructure, which is highly conserved through evolution, is composed of nine outer-doublet microtubules that surround a central pair of microtubules. Two dynein arms—outer and inner—are bound to each peripheral microtubule doublet. Dynein arms are composed of several dynein heavy, intermediate, and light chains. Dynein heavy chains, the main component of dynein arms, are encoded by multiple genes in humans (Milisav et al. 1996; Vaughan et al. 1996; Chapelin et al. 1997; Neesen et al. 1997). An additional dynein heavy chain gene (DNAH10) and a pseudogene (DNAH7p) were recently reported, along with a classification and chromosomal mapping of known human dynein heavy chain genes (Maiti et al. 2000). These dynein arms are essential for ciliary and flagellar beating, which they generate through an ATP-dependent cycle of attachment-detachment to the adjacent microtubule doublet (Witman 1992; Shingyoji 1998). The dynein intermediate gene DNAI1 is localized on 9p13-p21 and is composed of 20 exons encoding a 699–amino acid protein.
Given that the pathophysiology of KS seems close to that of PCD, one could speculate that each gene involved in PCD may be a good candidate to be investigated in KS and that each gene involved in KS may be a good candidate to be investigated in PCD. Alternatively, two distinct genes could underlie KS and PCD. Since the patient with compound mutations in DNAI1 had PCD without situs inversus, we undertook to screen a series of patients with KS for mutations in DNAI1.
In the present report, we studied 34 independent patients with KS. Blood samples were obtained after informed consent. To search for mutations in DNAI1, the 20 exons of the gene were amplified by PCR with intronic primers, according to the method described by Pennarun (1999). Amplified fragments were electrophoresed by SSCP at 7°C and at 20°C, except for exon 13 amplicon, which was digested with AflIII prior to migration, on a 10% acrylamide gel (acrylamide/bisacrylamide: 29/1) with TBE 1× buffer at 16 mA per gel, for 2 h 30 min to 6 h (depending on the size of each fragment). When bandshifts were identified, the corresponding PCR products were cloned in a pGEM-T vector system (Promega) and five or more clones were sequenced in both directions.
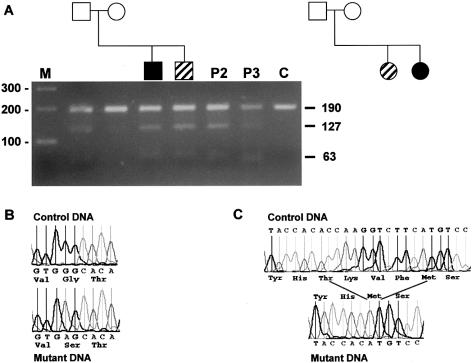
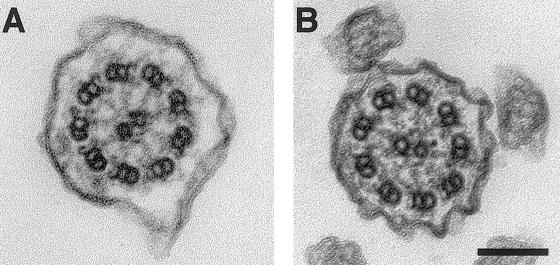
Three independent patients with KS were found to have mutations in the DNAI1 gene. Patient 1 has a brother who has recurrent upper and lower respiratory tract infections and sterility without situs inversus (fig. 1A). Repeated spermograms of his brother demonstrated immotile spermatozoa flagellae. Both brothers also presented ureteral lithiasis, an anomaly that is not usually reported in patients with PCD or KS. The cilia of the brother show absent or truncated outer dynein arms (fig. 2), but no biopsy from patient 1 was available. Both individuals were heterozygous for a common loss-of-function mutation identified in Pennarun's patient: a T insertion at position 3 of the intron 1 donor site that results in absence of splicing and peptide truncation. This mutation was confirmed by digesting the amplicon with HpaI (fig. 1A). In addition to this mutation, patient 1 and his brother had a transition in exon 16 at position 1543 (G→A) that resulted in a glycine to serine transposition at position 515 (fig. 1B). This glycine is highly conserved in both sea urchin IC2 and Chlamydomonas IC78 (Pennarun et al. 1999) and is a potential N-myristoylation site (GTEEGK) (PROSITE, Database of Protein Families and Domains). Moreover, testing parental DNA demonstrated that the splicing error was inherited from the father and the missense mutation from the mother (fig. 1A).
Figure 1.
A, Pedigree tree of the families of patients 1 and 2. Below symbols of the family tree of patient 1 is the agarose gel of exon 1 amplicons after digestion with HpaI. On the left-hand side, the size marker: a 100-bp ladder. Bars on the right-hand side indicate the position of obtained bands (190, 127, and 63 bp). Band 190 bp is the uncut amplicon; bands 127 and 63 bp are the cut bands when there is a T insertion in the splicing site. Blackened symbols indicate patients with KS; oblique stripes indicate patients with PCD. P2 and P3 indicate patients 2 and 3 of this study. B, Electrophoregram of the normal and missense mutations of patient 1. C, Electrophoregram of the normal and the 12-bp deletion observed in patient 3. Both abnormal electrophoregrams were obtained after cloning the amplicons.
Figure 2.
Electron-microscopic specimen of a normal case (A) and of the brother of patient 1 (B). The outer dynein arms are shortened or missing. Bar: 0.1 μm. Tracheal biopsies were fixed with 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4, postfixed 1 h at 4°C in 1% osmium tetroxyde in 0.1 M cacodylate, and, after 1 h impregnation in 2% aqueous uranyl acetate, embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and were examined at 80 KV with a JEOL 2000EX electron microscope.
Both patient 2 and her sister (fig. 1A) (who has PCD without situs inversus) have the same compound mutations as patient 1; both also had children. Patient 3, who has no other affected sibling, has situs inversus totalis, chronic sinusitis, bronchitis, recurrent otitis, and aplasia of frontal sinus. She refused to have a nasal or bronchial biopsy. She has compound DNAI1 gene defects. One gene defect is the same splice defect as observed in patients 1 and 2. The other mutation is a 12-bp deletion (fig. 1C), which results in truncation of DNAI1 by four amino acids in the fourth WD domain. This deletion removes a phenylalanine at position 556, which is conserved between sea urchin IC2, Chlamydomonas IC78, and human DNAI1.
To confirm that the missense and deletion mutations were causative, we tested 200 normal chromosomes by SSCP analysis and StyI digestion, respectively (the 12-bp deletion removes a StyI site). None showed the same pattern of migration as the DNA of patients.
The T insertion in intron 1 is a highly prevalent DNAI1 mutation, since it represents four of eight mutant alleles so far reported in patients with PCD and KS. This observation suggests that the patients with DNAI1 splice defect were related. Nevertheless, we were unable to demonstrate a familial relationship between these patients who originated from different geographic areas. To test for a founder effect, we searched for polymorphisms in the vicinity of this splice defect. We found no variation in the 5′ untranslated sequence or in the intronic sequence of the amplicon; however, we detected a variant in the coding sequence of exon 1: a G→T transversion at position +22 that changes the encoded amino acid from alanine to serine and suppresses a NlaIV restriction site. This variation was considered to be a polymorphism, since this alanine residue is not conserved between human, sea urchin, and Chlamydomonas. Moreover, this transversion was found in several control DNAs. Unfortunately, this polymorphism was uninformative in this study, since patients 1, 2, and 3 were all homozygous G/G.
In this series, 31 (91%) of 34 patients with KS had no mutations, which strongly suggests that KS is, like PCD (Pennarun et al. 1999), a heterogeneous condition. This hypothesis was confirmed by showing, by means of both SSCP and DNA sequencing, that two of the nine patients with consanguineous parents were heterozygous at one or both of the polymorphic sites, described elsewhere, in exon 11 of DNAI1 (Pennarun et al. 1999). Furthermore, in three pairs of affected siblings with nonconsanguineous parents, two had discordant genotypes at this locus. These data demonstrate KS heterogeneity, because it is very unlikely that four recombination events would have occurred in this small sample within a gene of 62 kb (Centre de Ressources INFOBIOGEN).
In conclusion, we have demonstrated that mutation in DNAI1 may result in KS, thus establishing the first molecular basis for KS. Moreover, this work confirms the attractive hypothesis that, in some patients with PCD, the proper body asymmetry is the result of random determination of left-right asymmetry, since in two sibships compound heterozygous mutations resulted in PCD with or without left-right inversion. This is also the first molecular demonstration of autosomal recessive inheritance of lateralization defects. Before DNAI1, other genes have been shown to be associated with laterality disturbances in humans: ZIC3 (Gebbia et al. 1997), LEFTY A (Kosaki et al. 1999a), ACVR2B (Kosaki et al. 1999b), and CFC1 (Bamford et al. 2000). However, in the autosomal genes (LEFTY A, ACVR2B, and CFC1), no patients with compound mutations were found, which suggests that the inheritance mode is not recessive, as in the case of DNAI1, but is di- or trigenic.
This work, although refuting the hypothesis that two closely linked loci may be involved in KS and in PCD, confirms the link between abnormal ciliary function and situs determination (Afzelius 1999). A link between cilia motility and lateralization determination was already provided by mouse mutants defective in genes required for cilia growth (Hfh4) (Chen et al. 1998; Brody et al. 2000) or beating (Kif3B and Kif3A) (Nonaka et al. 1998; Takeda et al. 1999). Nodal cells of the mouse embryo have a unique cilium at their apical pole. This cilium does not actually beat but displays an anticlockwise vortex movement. Nonaka et al. (1998) showed elegantly, by adding a dye to the extraembryonic fluid, that cilia rotation induces a leftward directional flow to this extraembryonic fluid, potentially concentrating on the left side and depleting on the right side, which are critical factors that would initiate the cascade of molecular events leading to normal lateralization (Nonaka et al. 1998). If this vortexing movement is absent, as it is in Kif3A- and Kif3B-deficient mice (Nonaka et al. 1998; Takeda et al. 1999), there is presumably no left-right gradient of signaling factors, and hence laterality is randomized. In support of this hypothesis, Okada et al. (1999) showed that the mouse mutants iv (Supp et al. 1997, 1999) and inv (Mochizuki et al. 1998; Morgan et al. 1998), which present laterality defects, do not display a normal nodal cilia motility. Curiously, the iv mutation is located in the lrd (left right dynein) gene, which encodes a dynein-heavy–chain gene, although homozygous mice do not express any respiratory symptoms.
In contrast with Kif3A and Kif3B mouse mutants, which present severe developmental anomalies, the Hfh4 mutant mouse has randomized laterality, hydrocephalus, and growth retardation but survives up to 12 wk after birth. Interestingly, Hfh4 -/- mice have nodal cilia; it is not known, however, whether these cilia are functional (Brody et al. 2000). Because Kif3A- and Kif3B-deficient mice die before ciliogenesis of airway epithelial cells, it is also not known whether these mutants have normal ciliary growth and motility. These data suggest that Hfh4 is a candidate gene to be considered in cases of human PCD. Indeed, the human ortholog gene HFH4, recently renamed FOXJ1 (MIM 602291), was screened in a series of human patients with PCD, and no mutations were identified; however, it was not specified whether any patients had ciliary aplasia (Maiti et al. 2000). Aplasia of cilia accounts for ∼4% of PCD (Jorissen et al. 2000), and it would be worthwhile repeating FOXJ1 screening in patients with bona fide ciliary aplasia, a condition that is best evidenced after in vitro culture of respiratory epithelial cells (Jorissen et al. 2000).
DNAI1 maps to 9p13-p21, even though this chromosomal region was not detected as potentially harboring a gene implicated, by linkage analysis, in PCD (Blouin et al. 2000). It confirms that it is difficult to detect linkage in such a highly heterogeneous autosomal recessive condition. In the two cases in which electron microscopy was available, DNAI1 mutations were associated with abnormal outer dynein arms. Nevertheless, this anomaly is not specific to this gene defect, since Pennarun et al. (1999) excluded DNAI1 mutations in two patients with PCD from consanguineous families who had abnormal outer dynein arms.
In this report, we have provided evidence for the high prevalence of a splicing defect in the four independent cases of compound heterozygous DNAI1 gene defects so far reported. Further studies are required to confirm this high prevalence and to conclude whether the underlying molecular mechanism is the result of a founder effect or a recurrent mutation event.
Acknowledgments
We are indebted to the patients for their participation. Robert Kelly is warmly thanked for reviewing the manuscript. This work was supported by the Fondation de la Recherche Médicale, the SESERAC, and the Région Rhône-Alpes. Cécile Guichard is a student of the Ecole Pratique des Hautes Etudes, Paris.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Centre de Ressources INFOBIOGEN, http://www.infobiogen.fr/srs/ (accession number AL359088)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for KS [MIM 244400], ICS1 [MIM 242650], and DNAI1 [MIM 604366])https://omim.org/entry/
- PROSITE, Database of Protein Families and Domains, http://www.expasy.ch/prosite
References
- Afzelius BA (1999) Asymmetry of cilia and of mice and men. Int J Dev Biol 43:283–286 [PubMed] [Google Scholar]
- ——— (2000) Ciliary structure in health and disease. Acta Otorhinolaryngol Belg 54:287–291 [PubMed] [Google Scholar]
- Bamford RN, Roessler E, Burdine RD, Saplakoglu U, de la Cruz J, Splitt M, Towbin J, Bowers P, Marino B, Schier AF, Shen MM, Muenke M, Casey B (2000) Loss-of-function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat Genet 26:365–369 [DOI] [PubMed] [Google Scholar]
- Blair DF, Dutcher SK (1992) Flagella in prokaryotes and lower eukaryotes. Curr Opin Genet Dev 2:756–767 [DOI] [PubMed] [Google Scholar]
- Blouin JL, Meeks M, Radhakrishna U, Sainsbury A, Gehring C, Duriaux Saïl G, Bartoloni L, Dombi V, O'Rawe A, Walne A, Chung E, Afzelius BA, Armengot M, Jorissen M, Schidlow DV, van Maldergem L, Walt H, Gardiner RM, Probst D, Guerne PA, Delozier-Blanchet CD, Antonarakis SE (2000) Primary ciliary dyskinesia: a genome-wide linkage analysis reveals extensive locus heterogeneity. Eur J Hum Genet 8:109–118 [DOI] [PubMed] [Google Scholar]
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD (2000) Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol 23:45–51 [DOI] [PubMed] [Google Scholar]
- Bryan J (1977) Spermatogenesis revisited. IV. Abnormal spermiogenesis in mice homozygous for another male-sterility-inducing mutation, hpy (hydrocephalic-polydactyly). Cell Tissue Res 180:187–201 [DOI] [PubMed] [Google Scholar]
- Chapelin C, Duriez B, Magnino F, Goossens M, Escudier E, Amselem S (1997) Isolation of several human axonemal dynein heavy chain genes: genomic structure of the catalytic site, phylogenetic analysis and chromosomal assignment. FEBS Lett 412:325–330 [DOI] [PubMed] [Google Scholar]
- Chen J, Knowles HJ, Hebert JL, Hackett BP (1998) Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest 102:1077–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbia M, Ferrero GB, Pilia G, Bassi MT, Aylsworth A, Penman-Splitt M, Bird LM, Bamforth JS, Burn J, Schlessinger D, Nelson DL, Casey B (1997) X-linked situs abnormalities result from mutations in ZIC3. Nat Genet 17:305–308 [DOI] [PubMed] [Google Scholar]
- Jorissen M, Willems T (2000) Success rates of respiratory epithelial cell culture techniques with ciliogenesis for diagnosing primary ciliary dyskinesia. Acta Otorhinolaryngol Belg 54:357–365 [PubMed] [Google Scholar]
- Jorissen M, Willems T, van der Schueren B, Verbeken E, de Boeck K (2000) Ultrastructural expression of primary ciliary dyskinesia after ciliogenesis in culture. Acta Otorhinolaryngol Belg 54:343–356 [PubMed] [Google Scholar]
- Kartagener M (1933) Zur Pathologie der Bronchiektasien: Bronchiektasien bei Situs viscerum invertus. Beitr Klin Tuberk 83:489–501 [Google Scholar]
- Kosaki K, Bassi MT, Kosaki R, Lewin M, Belmont J, Schauer G, Casey B (1999a) Characterization and mutation analysis of human LEFTY A and LEFTY B, homologues of murine genes implicated in left-right axis development. Am J Hum Genet 64:712–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaki R, Gebbia M, Kosaki K, Lewin M, Bowers P, Towbin JA, Casey B (1999b) Left-right axis malformations associated with mutations in ACVR2B, the gene for human activin receptor type IIB. Am J Med Genet 82:70–76 [DOI] [PubMed] [Google Scholar]
- Maiti AK, Bartoloni L, Mitchison HM, Meeks M, Chung E, Spiden S, Gehrig C, Rossier C, DeLozier-Blanchet CD, Blouin J, Gardiner RM, Antonarakis SE (2000) No deleterious mutations in the FOXJ1 (alias HFH-4) gene in patients with primary ciliary dyskinesia. Cytogenet Cell Genet 90:119–122 [DOI] [PubMed] [Google Scholar]
- Maiti AK, Mattéi MG, Jorissen M, Volz A, Ziegler A, Bouvagnet P (2000) Identification, tissue specific expression, and chromosomal localisation of several human dynein heavy chain gene. Eur J Hum Genet 8:923–932 [DOI] [PubMed] [Google Scholar]
- Milisav I, Jones MH, Affara NA (1996) Characterization of a novel human dynein-related gene that is specifically expressed in testis. Mamm Genome 7:667–672 [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Sajoh Y, Tsuchiya K, Shirayoshi Y, Takai S, Taya C, Yonekawa H, Yamada K, Nihei H, Nakatsuji N, Overbeek PA, Hamada H, Yokoyama T (1998) Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature 395:177–181 [DOI] [PubMed] [Google Scholar]
- Morgan D, Turnpenny L, Goodship J, Dai W, Majumder K, Matthews L, Gardner A, Schuster G, Vien L, Harrison W, Elder FF, Penman-Splitt M, Overbeek P, Strachan T (1998) Inversin, a novel gene in vertebrate left-right axis pathway, is partially deleted in the inv mouse. Nat Genet 20:149–155 [DOI] [PubMed] [Google Scholar]
- Neesen J, Koehler MR, Kirschner R, Steinlein C, Kreutzberger J, Engel W, Schmid M (1997) Identification of dynein heavy chain genes expressed in human and mouse testis: chromosomal localization of an axonemal dynein gene. Gene 200:193–202 [DOI] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N (1998) Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95:829–837 [DOI] [PubMed] [Google Scholar]
- Noone PG, Bali D, Carson JL, Sannuti A, Gipson CL, Ostrowski LE, Bromberg PA, Boucher RC, Knowles MR (1999) Discordant organ laterality in monozygotic twins with primary ciliary dyskinesia. Am J Med Genet 82:155–160 [DOI] [PubMed] [Google Scholar]
- Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N (1999) Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell 4:459–468 [DOI] [PubMed] [Google Scholar]
- Pennarun G, Escudier E, Chapelin C, Bridoux AM, Cacheux V, Roger G, Clément A, Goossens M, Amselem S, Duriez B (1999) Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am J Hum Genet 65:1508–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME (1996) Axonemal dyneins: assembly, organization, and regulation. Curr Opin Cell Biol 8:10–17 [DOI] [PubMed] [Google Scholar]
- Shingyoji C, Higuchi H, Yoshimura M, Katayama E, Yanagida T (1998) Dynein arms are oscillating force generators. Nature 393:711–714 [DOI] [PubMed] [Google Scholar]
- Siewert A (1904) Über einen Fall von Bronchiectasie bei einem Patienten mit Situs inversus viscerum. Berl Klin Wochenschr 41:139–141 [Google Scholar]
- Supp DM, Brueckner M, Kuehn MR, Witte DP, Lowe LA, McGrath J, Corrales J, Potter SS (1999) Targeted deletion of the ATP binding domain of left-right dynein confirms its role in specifying development of left-right asymmetries. Development 126:5495–5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supp DM, Witte DP, Potter SS, Brueckner M (1997) Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature 389:963–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N (1999) Left-right asymmetry and kinesin superfamily protein KIF3A new insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J Cell Biol 145:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R (1993) Molecular analysis of the microtubule motor dynein. Proc Natl Acad Sci USA 90:8769–8772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan KT, Mikami A, Paschal BM, Holzbaur ELF, Hughes SM, Echeverri CJ, Moore KJ, Gilbert DJ, Copeland NG, Jenkins NA, Vallee RB (1996) Multiple mouse chromosomal loci for dynein-based motility. Genomics 36:29–38 [DOI] [PubMed] [Google Scholar]
- Waite DA, Wakefield SJ, Mackay JB, and Ross IT (1981) Mucociliary transport and ultrastructural abnormalities in Polynesian bronchiectasis. Chest 80:896–898 [DOI] [PubMed] [Google Scholar]
- Witman GB (1992) Axonemal dyneins. Curr Opin Cell Biol 4:74–79 [DOI] [PubMed] [Google Scholar]