Abstract
Acrodermatitis enteropathica (AE) is a rare autosomal recessive pediatric disease characterized by dermatitis, diarrhea, alopecia, and growth failure. The disease results from insufficient uptake of zinc by the intestine and can be fatal unless the diet is supplemented with zinc. To map the gene responsible for AE, a genomewide screen was performed on 17 individuals, including 4 affected individuals, in a consanguineous Jordanian family. Three markers—D8S373, D10S212, and D6S1021—had a pattern consistent with tight linkage to a recessive disease: one allele in the affected sibs and multiple alleles in unaffected sibs and parents. Two-point parametric linkage analysis using FASTLINK identified one region, D8S373, with a maximum LOD score >1.5 (1.94 at D8S373: recombination fraction .001). Twelve additional markers flanking D8S373 were used to genotype the extended family, to fine-map the AE gene. All five affected individuals—including one who was not genotyped in the genomewide screen—were found to be homozygous for a common haplotype, spanning ∼3.5 cM, defined by markers D8S1713 and D8S2334 on chromosomal region 8q24.3. To support these mapping data, seven consanguineous Egyptian families with eight patients with AE were genotyped using these markers, and six patients from five families were found to be homozygous in this region. Multipoint analysis with all consanguineous families, by Mapmaker/Homoz, resulted in a maximum LOD score of 3.89 between D8S1713 and D8S373. Sliding three-point analysis resulted in a maximum LOD score of 5.16 between markers D8S1727 and D8S1744.
Acrodermatitis enteropathica (AE [MIM 201100]) is an inborn error of metabolism resulting in zinc malabsorption and severe zinc deficiency. Symptoms include intermittent simultaneous occurrence of diarrhea and dermatitis, alopecia, and failure to thrive (Dillaha et al. 1953; Van Wouwe 1989). The disorder usually manifests at the time of weaning—or earlier, in infants who are not breast-fed—and can be fatal if untreated. Symptoms other than dermatitis vary with age, and spontaneous remission may occur at adolescence. Barnes and Moynahan (1973) first observed that the disorder was caused by the inability to absorb sufficient zinc, and they achieved a complete cure of all symptoms by zinc supplementation alone. Decreased zinc absorption (Lombeck et al. 1975; Weismann et al. 1979; van den Hamer et al. 1985) and impaired zinc uptake in vitro, evidenced by biopsy of jejunal mucosa (Atherton et al. 1979), were later demonstrated using 65Zn or 69mZn. Zinc supplementation is effective in the treatment of the disease, presumably because the increase in luminal zinc concentration is such that the diffusional component of zinc transport is stimulated (Davies 1980; Steel and Cousins 1985).
The variability of symptoms in patients of different ages makes clinical diagnosis of AE difficult. Typically, plasma- and serum-zinc concentration, as well as urinary excretion of zinc, are half the normal levels (Van Wouwe 1989). However, serum-zinc level is not completely diagnostic, since zinc levels in children are susceptible to a range of conditions (Nishi et al. 1980; Naveh et al. 1982; Rodriguez et al. 1985; Van Wouwe et al. 1988). In cases of doubtful diagnosis, zinc-absorption tests using radioisotopes may be performed in vivo or in vitro. If such tests are unavailable, the clinical response to a daily dose of 3–30 μmol of zinc supplement/kg of body weight, for 5 d, may establish the diagnosis (Krieger 1982, pp. 258–262).
Grider and Young (1996) provided evidence that the AE mutation transiently affects zinc metabolism in human fibroblasts. The activity of the zinc-dependent enzyme, 5′ nucleotidase, and cell-zinc content were both significantly reduced in AE fibroblasts, compared with those in a normal control. AE fibroblasts also exhibited slower-than-normal zinc uptake.
The biochemical mechanism underlying the zinc-transport defect caused by the AE mutation is still unknown. Analysis of the expression patterns between AE and normal human fibroblasts by two-dimensional gel electrophoresis (Grider and Mouat 1998) and differential display (Muga and Grider 1999) identified several genes that were either down- or up-regulated in AE samples. However, no clear indication of how these changes contributed to the AE phenotype was found; consequently, we have embarked on a genetic approach to discovering the underlying etiology of AE.
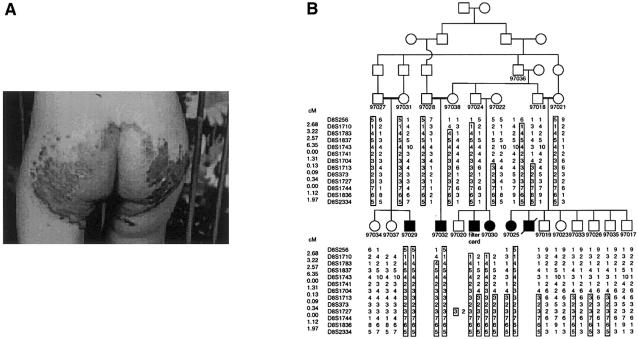
In this study, we sought to localize the genetic defect responsible for AE, by homozygosity mapping (Lander and Botstein 1987) using consanguineous families with affected individuals that were from Jordan and Egypt. We obtained informed consent either from all family members who agreed to participate in the study or from their legal guardians. The proband of the Jordanian family (fig. 1A; filter card in fig. 1B) was first seen at age 5 mo, with a 1-mo history of diaper rash that was resistant to antifungal treatment. The proband's zinc level was 2.2 μmol/liter, whereas the normal reference value for that age group is 9.8–16.8 μmol/liter. There was dramatic improvement with treatment with zinc sulfate (2 mg/kg/d), within a period of 1 wk. Individual 97030 (fig. 1B) is a younger sister of the proband, with a very similar presentation of diaper rash and diarrhea, and, because of the family history, diagnosis was made without testing of her zinc level. She responded excellently to the same treatment and dosage given the proband. Similar symptoms were observed in individuals 97025, 97029, and 97032 (fig. 1B). All were given the same treatment of zinc sulfate, with good response.
Figure 1.
Jordanian pedigree with AE. A, Patient (corresponding to the filter card in the pedigree) showing perianal skin lesions. The perioral region shows an exudative and crusted area on an erythematous base, with irregular outline. The scalp hair is sparse, diarrhea is present, and the elbows and knees show scaly erythematous and thickened skin (psoriasiform). Periungal erythema and slightly swollen folds are present, and the nape of the neck shows slight psoriasiform rash. B, Haplotypes of the Jordanian pedigree, for 13 microsatellite markers at 8q24.3. Haplotypes were constructed by inspection, and those that are homozygous in affected individuals are boxed.
We first performed a genomewide scan of 17 individuals, including 4 affected individuals, from the Jordanian family (pedigree 21; fig. 1B), using a modification of the Weber version 9 marker set (383 microsatellite markers, mean spacing 9 cM, and mean heterozygosity .76). Four blind duplicate samples were placed on each 96-well plate, to assess the amount of genotyping error. For this project and two other concurrent runs, the error rate was 0.15%, on the basis of 3,560 paired genotypes.
We compared the number of alleles present in affected individuals (97025, 97029, 97030, and 97032), unaffected siblings (97019, 97023, 97033, 97034, and 97037), and parents (97018, 97021, 97022, 97024, 97027, 97028, 97031, and 97038), simulating a pooling experiment. Results at three loci—D10S212, D8S373, and D6S1021—were consistent with the pattern expected for an autosomal recessive disease—namely, one allele in the affected individuals and multiple alleles in the unaffected sibs and parents. Moreover, for marker D8S256, adjacent to D8S373, a shift toward homozygosity among the affected sibs was also observed. These data pointed to the chromosomal region 8q24.3 near D8S373 and D8S256 as being a possible candidate location for the AE gene.
The possibility that chromosomal region 8q24.3 is the location of the AE gene was supported by two types of linkage analysis. We used FASTLINK (Lathrop et al. 1984; Cottingham 1993) for two-point parametric linkage analysis of the genotyping data, to find the maximum LOD score for each marker. An autosomal recessive–inheritance pattern with gene frequency .001 and 100% penetrance was assumed, and marker-allele frequencies were estimated from the data. This analysis identified one region defined by two markers with maximum LOD scores ⩾0.75: 1.94 (recombination fraction [θ]=.001) at D8S373 and 0.75 (θ=.100) at D8S256 (table 1). Nonparametric linkage analysis using the Haseman-Elston (Haseman and Elston 1972) method as implemented in Sib-pair (Duffy 1997) identified the same region, with P<.05: P=.0098 at D8S256, and P=.0361 at D8S373. Three analyses suggested the 8q24.3 region near markers D8S373 and D8S256 as being the possible AE candidate region.
Table 1.
Markers with a LOD Score ⩾0.75, from Genome Scan of a Jordanian Family with AE
| Marker | Maximum TotalTwo-Point LOD Score | θ |
| D1S1589 | 1.48 | .001 |
| D2S1356 | 1.48 | .001 |
| D6S1021 | .78 | .001 |
| D7S3051 | .92 | .001 |
| D8S256 | .75 | .100 |
| D8S373 | 1.94 | .001 |
| D10S212 | .77 | .001 |
| D16S3253 | .93 | .001 |
| D19S245 | 1.03 | .001 |
On the basis of these results, we further genotyped the extended Jordanian family (fig. 1B), for 11 additional markers from D8S256 to the telomere. Two individuals (97022 and the spouse of 97036) who were distant relatives of the family share with other family members a haplotype that segregates with the disease. The shared haplotype spans a 3.5-cM interval from marker D8S1713 to the telomeric marker D8S2334 (fig. 1B). Several recombination events were observed in this pedigree, but none narrowed the candidate region further.
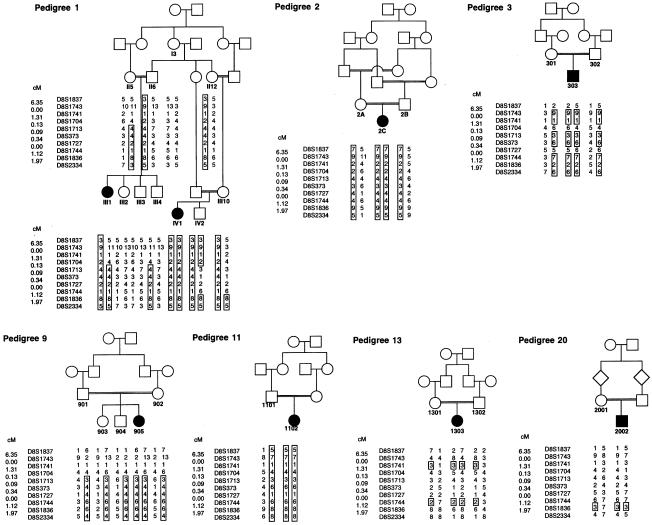
The 10 most distal markers of 8q24.3 were then used to genotype seven consanguineous families from Egypt, as shown in figure 2. Of the eight affected individuals, six (from pedigrees 1, 2, 3, 9, and 11; fig. 2) were shown to be homozygous for most of the loci, whereas none of the unaffected relatives were similarly homozygous. A closer examination of the genotyping data revealed that unaffected individual IV2 in pedigree 1 is homozygous for the two most distal markers, D8S1836 and D8S2334. If this region is homozygous by descent, it would be excluded from the candidate interval, narrowing it to 1.5 cM. However, genotyping of additional markers would be needed to draw this conclusion. Patient 303 from pedigree 3 presented a paradox, being heterozygous (with alleles 5 and 6) for marker D8S1727 but homozygous for neighboring markers. An unlikely possible explanation is that marker locations are not accurate. Other possible explanations include a calling error or a prior change in size of the microsatellite.
Figure 2.
Pedigrees of seven consanguineous Egyptian families with genotypes of 10 microsatellite markers at 8q24.3. For compactness, only the relevant portions of the pedigrees are shown. Haplotypes/markers that are homozygous in affected individuals are boxed.
Two other findings are notable. First, different haplotypes are found in all homozygous patients, arguing against the hypothesis of a common origin for AE in the Egyptian population. Second, the two patients from pedigrees 13 and 20 showed no significant homozygosity in chromosomal region 8q24.3. Although this finding may suggest the existence of another locus that could cause AE, we also note that patient 2002 has no records of zinc-level measurement and response to zinc-supplement therapy. Given the difficulty of AE diagnosis, we cannot exclude the possibility of a misdiagnosis.
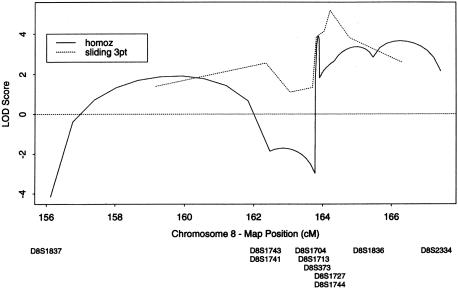
By parametric analysis using FASTLINK, a maximum two-point LOD score of 4.54 was found at marker D8S1744 (table 2). Mapmaker/Homoz was used to calculate multipoint LOD scores for all eight consanguineous families, by homozygosity mapping (Kruglyak et al. 1995). The maximum LOD score obtained in this region was 3.89 (fig. 3). Sliding three-point analysis of the same families was also performed using FASTLINK (Terwilliger and Ott 1994). The maximum LOD score obtained was 5.16 between markers D8S1727 and D8S1744.
Table 2.
Two-Point LOD Scores for Markers at 8q24.3
|
LOD Score at θ = |
||||||||
| Marker | Location(cM) | .0 | .01 | .05 | .1 | .2 | .3 | .4 |
| D8S264 | .73 | −∞ | −7.22 | −3.57 | −2.06 | −.81 | −.29 | −.07 |
| D8S1130 | 22.41 | −∞ | −4.47 | −1.89 | −.93 | −.22 | .01 | .06 |
| D8S1106 | 26.43 | −5.24 | −3.20 | −1.47 | −.75 | −.21 | −.04 | .00 |
| D8S1145 | 37.04 | −1.75 | −1.09 | −.63 | −.47 | −.24 | −.10 | −.03 |
| D8S136 | 43.96 | −.31 | .51 | .99 | 1.05 | .87 | .59 | .29 |
| D8S1771 | 50.05 | −1.80 | −1.00 | −.50 | −.30 | −.10 | −.10 | .00 |
| D8S1477 | 60.34 | −1.59 | −1.20 | −.28 | .07 | .23 | .18 | .07 |
| D8S1110 | 67.27 | −4.57 | −2.84 | −1.23 | −.67 | −.33 | −.21 | −.12 |
| D8S1113 | 77.89 | −1.34 | −.50 | .02 | .16 | .18 | .12 | .05 |
| D8S1136 | 82.26 | −∞ | −1.91 | −.32 | .24 | .46 | .31 | .08 |
| D8S2324 | 94.08 | −∞ | −4.05 | −1.83 | −.88 | −.17 | .01 | .02 |
| D8S1119 | 101.01 | −4.46 | −2.87 | −1.27 | −.65 | −.22 | −.09 | −.05 |
| D8S1132 | … | −7.21 | −5.23 | −3.01 | −1.92 | −.91 | −.42 | −.15 |
| D8S592 | 125.27 | −3.75 | −2.19 | −1.06 | −.64 | −.32 | −.18 | −.07 |
| D8S1128 | 139.53 | −∞ | −2.86 | −1.68 | −1.02 | −.44 | −.17 | −.04 |
| D8S256 | 148.12 | −.75 | 1.08 | 1.45 | 1.35 | .89 | .44 | .14 |
| D8S1710 | 150.80 | −∞ | −.56 | .37 | .79 | .88 | .67 | .36 |
| D8S1783 | 154.02 | 1.07 | 1.12 | 1.68 | 1.82 | 1.54 | 1.02 | .46 |
| D8S1837 | 156.59 | −∞ | −.17 | .33 | .32 | .11 | .08 | .07 |
| D8S1743 | 162.94 | .63 | .52 | .52 | .40 | −.01 | −.06 | −.03 |
| D8S1741 | 162.94 | −.25 | −.13 | .62 | .92 | .87 | .62 | .32 |
| D8S1704 | 164.25 | −.02 | .10 | .78 | .91 | .73 | .58 | .34 |
| D8S1713 | 164.38 | 2.34 | 2.21 | 2.15 | 1.98 | 1.32 | .75 | .33 |
| D8S373 | 164.47 | 2.39 | 2.26 | 2.18 | 1.97 | 1.18 | .64 | .30 |
| D8S1727 | 164.81 | 2.80 | 2.67 | 2.18 | 1.60 | .76 | .42 | .22 |
| D8S1744 | 164.81 | 4.54 | 4.42 | 3.90 | 3.26 | 2.12 | 1.25 | .59 |
| D8S1836 | 165.93 | 1.76 | 1.62 | 1.50 | 1.30 | .80 | .53 | .28 |
| D8S2334 | 167.90 | 3.35 | 3.24 | 2.79 | 2.20 | 1.08 | .28 | .00 |
Figure 3.
Graph of three-point and multipoint LOD scores, against distance (in cM), at 8q24.3. For sliding three-point analysis, the maximum LOD score is 5.16. Homozygosity mapping gives a maximum LOD score of 3.89. Mapmaker/Homoz was used for homozygosity mapping.
We conclude that AE maps within a 3.5-cM interval near the telomere on chromosomal region 8q24.3. A search of GeneMap'99 in this region revealed >70 known genes and UniGene clusters in this interval. Research is underway to test these candidates for expression in the intestine and for mutations that cause AE.
Acknowledgments
We are indebted to the families that participated in this study. K.W. is grateful to Bing Zhou for stimulating discussions and to Anjali Shah for help with genotyping. Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through federal contract N01-HG-65403 from the National Institutes of Health to the Johns Hopkins University. J.G. is an investigator for, and K.W. is an associate of, the Howard Hughes Medical Institute.
Electronic-Database Information
The accession number and URLs for data in this article are as follows:
- Center for Inherited Disease Research, http://www.cidr.jhmi.edu/ (for the Weber version 9 marker set)
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics/ (for order and genetic distances of markers on 8q)
- GeneMap’99, http://www.ncbi.nlm.nih.gov/genemap99/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for AE [MIM 201100]) [PubMed]
- UniGene, http://www.ncbi.nlm.nih.gov/UniGene/
References
- Atherton DJ, Muller DP, Aggett PJ, Harries JT (1979) A defect in zinc uptake by jejunal biopsies in acrodermatitis enteropathica. Clin Sci 56:505–507 [DOI] [PubMed] [Google Scholar]
- Barnes PM, Moynahan EJ (1973) Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med 66:327–329 [PMC free article] [PubMed] [Google Scholar]
- Cottingham RW Jr, Idury RM, Schäffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- Davies NT (1980) Studies on the absorption of zinc by rat intestine. Br J Nutr 43:189–203 [DOI] [PubMed] [Google Scholar]
- Dillaha CJ, Lorincz AL, Aavik OR (1953) Acrodermatitis enteropathica: review of the literature and report of a case successfully treated with diodoquin. JAMA 152:509–512 [DOI] [PubMed] [Google Scholar]
- Duffy D (1997) Sib-pair: a program for non-parametric linkage/association analysis. Am J Hum Genet Suppl 61:A197 [Google Scholar]
- Grider A, Mouat MF (1998) The acrodermatitis enteropathica mutation affects protein expression in human fibroblasts: analysis by two-dimensional gel electrophoresis. J Nutr 128:1311–1314 [DOI] [PubMed] [Google Scholar]
- Grider A, Young EM (1996) The acrodermatitis enteropathica mutation transiently affects zinc metabolism in human fibroblasts. J Nutr 126:219–224 [DOI] [PubMed] [Google Scholar]
- Haseman JK, Elston RC (1972) The investigation of linkage between a quantitative trait and a marker locus. Behav Genet 2:3–19 [DOI] [PubMed] [Google Scholar]
- Krieger I (1982) Mineral deficiencies in pediatric disorders of feeding, nutrition and metabolism. John Wiley & Sons, New York [Google Scholar]
- Kruglyak L, Daly MJ, Lander ES (1995) Rapid multipoint linkage analysis of recessive traits in nuclear families, including homozygosity mapping. Am J Hum Genet 56:519–527 [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Botstein D (1987) Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236:1567–1570 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombeck T, Schnippering HG, Ritzl F, Feinendegen LE, Bremer HJ (1975) Absorption of zinc in acrodermatitis enteropathica. Lancet 1:855 [DOI] [PubMed] [Google Scholar]
- Muga SJ, Grider A (1999) Partial characterization of a human zinc-deficiency syndrome by differential display. Biol Trace Elem Res 68:1–12 [DOI] [PubMed] [Google Scholar]
- Naveh Y, Lightman A, Zinder O (1982) Effect of diarrhea on serum zinc concentrations in infants and children. J Pediatr 101:730–732 [DOI] [PubMed] [Google Scholar]
- Nishi Y, Lifshitz F, Bayne MA, Daum F, Silverberg M, Aiges H (1980) Zinc status and its relation to growth retardation in children with chronic inflammatory bowel disease. Am J Clin Nutr 33:2613–2621 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Soto G, Torres S, Venegas G, Castillo-Duran C (1985) Zinc and copper in hair and plasma of children with chronic diarrhea. Acta Paediatr Scand 74:770–774 [DOI] [PubMed] [Google Scholar]
- Steel L, Cousins RJ (1985) Kinetics of zinc absorption by luminally and vascularly perfused rat intestine. Am J Physiol 248:G46–G53 [DOI] [PubMed] [Google Scholar]
- Terwilliger JD, Ott J (1994) Handbook of genetic linkage. Johns Hopkins University Press, Baltimore [Google Scholar]
- van den Hamer CJA, Cornelisse C, Hoogenraad TU, Van Wouwe JP (1985) Use of 69mZn loading for monitoring of zinc malabsorption. In: Mills CF, Brenner I, Chesters JK (eds) Trace elements in man and animals—TEMA 5. Commonwealth Agricultural Bureaux, Slough, United Kingdom, pp 689–691 [Google Scholar]
- Van Wouwe JP (1989) Clinical and laboratory diagnosis of acrodermatitis enteropathica. Eur J Pediatr 149:2–8 [DOI] [PubMed] [Google Scholar]
- Van Wouwe JP, Van Gelderen HH, Enschede FAJ, Van de Velde EA (1988) Acute diarrhea, non-responsive to dietary restriction, zinc deficiency and subclinical growth retardation in pre-school children. Trace Elem Med 5:90–92 [Google Scholar]
- Weismann K, Hoe S, Knudsen L, Sorensen SS (1979) 65Zinc absorption in patients suffering from acrodermatitis enteropathica and in normal adults assessed by whole-body counting technique. Br J Dermatol 101:573–579 [DOI] [PubMed] [Google Scholar]