Abstract
Frankia strain CcI3 grown in culture produced a hemoglobin which had optical absorption bands typical of a hemoglobin and a molecular mass of 14.1 kDa. Its equilibrium oxygen binding constant was 274 nM, the oxygen dissociation rate constant was 56 s−1, and the oxygen association rate constant was 206 μM−1 s−1.
Frankia, a nitrogen-fixing actinomycete, forms root nodules with plants belonging to eight different plant families. These actinorhizal root nodules are modified lateral roots and contain various amounts of hemoglobin ranging from very low concentrations in Purshia to high concentrations in Casuarina and Myrica (18, 24). In Casuarina, the hemoglobin (or at least most of it) is produced by the plant (6, 12), but the source in other actinorhizal nodules is not known. In nodules from Alnus, which contain intermediate concentrations of hemoglobin, much of the hemoglobin is associated with nodule fractions that contain Frankia (20). Based on this, we hypothesized that Frankia can produce hemoglobin.
Recently a number of truncated hemoglobins that have amino acid sequences that are 20 to 40 residues shorter than classical hemoglobins have been described. They are found in diverse microorganisms, namely, the prokaryotes Synechocystis (8), Mycobacterium (9), and Nostoc (16), and the eukaryotes Paramecium (11), Tetrahymena (14), and Chlamydomonas (7). The amino acid sequences of these hemoglobins indicate that they have a common evolutionary origin and are only distantly related to classical hemoglobins (21). In this paper we report that Frankia produces a hemoglobin that may belong to this group.
Cell culture and hemoglobin extraction.
Cultures of Frankia strain CcI3, originally isolated from root nodules of Casuarina cunninghamiana (27), were grown on a defined nitrogen-free medium (3) with propionate as the carbon source. Growth was in 1-liter flasks without shaking, for a period of 1 to 3 months.
For hemoglobin extraction, the hyphal mat was collected on a polycarbonate membrane filter with 0.4-μm pores, and approximately 1 g of moist hyphae was placed in a 15-ml glass centrifuge tube (Corex no. 8441). Then 1.0 ml of extraction buffer (0.1 M potassium phosphate, 0.13 mM EDTA [pH 7.4]) and 2.0 g of 0.1-mm-diameter glass beads (Biospec Products, Bartlesville, Okla.) were added, and the tube was stoppered and equilibrated with carbon monoxide. After 10 min of incubation at room temperature (to help deplete any remaining O2), the hyphae were disrupted by agitating the tube with a vortex-type tube mixer (23). Agitation was at room temperature for a total of 5 to 10 min, with cycles of 30 to 60 s of agitation followed by 10 s of rest. After centrifugation (10 min at 4,500 × g) the supernatant was collected, and the pellet was reextracted after the addition of 1.0 ml of extraction buffer.
Purification and molecular mass determination.
The crude extract was purified by gel filtration at room temperature on a 1.1- by 40-cm column of Sephacryl S-200, using the same CO-equilibrated buffer that was used for extraction. The same column was used to estimate molecular mass, with cytochrome c and myoglobin from horse skeletal muscle used as standards. The hemoglobin from the gel filtration fractions was studied without further purification.
The molecular mass as determined by gel filtration was 14.1 ± 0.1 kDa (mean ± standard error [SE]; n = 3). This is consistent with the molecular masses of 13.8 and 15 kDa determined by gel filtration for the truncated hemoglobins from Nostoc (16) and Synechocystis (8).
Determination of optical absorption spectra and the O2 equilibrium constant.
Gel filtration fractions containing carboxyhemoglobin (HbCO) were placed in a tonometer, an enzymatic reduction system (13) was added to maintain the heme iron in the ferrous form, and absorption spectra for HbCO were taken immediately. Carbon monoxide was displaced from the heme iron by competition with oxygen in the presence of light, followed by removal of the displaced CO under vacuum. Three cycles of this treatment converted the ferrous-CO form of the protein to the oxyferrous form as judged by the Soret and visible spectra. Next, the oxygen was removed from the protein by repeated flushing with pure N2, and the spectrum of the resulting deoxyhemoglobin was taken. Following this, the oxygen equilibrium constant was estimated by the tonometric method (17), which involved stepwise addition of measured amounts of oxygen. And lastly, spectra for oxyhemoglobin (HbO2) were taken when addition of more O2 showed no further increase in the HbO2 absorption peaks.
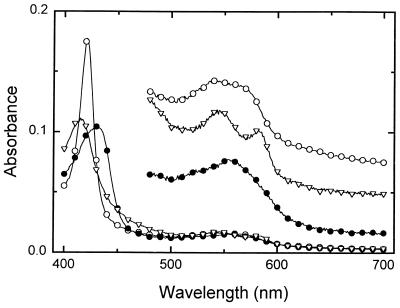
While the carbonmonoxy form of the hemoglobin extracted from Frankia was very stable, the oxy and deoxy forms were not. Measurable loss of absorbance was observed over a time period of minutes at room temperature (approximately 25°C). However, addition of the enzymatic reducing system eliminated this problem, allowing measurements of oxygen equilibrium and dissociation rate. The absorption spectra observed for the carbonmonoxy, oxy, and deoxy forms of the hemoglobin (Fig. 1) are similar to those found for other hemoglobins, including the truncated hemoglobins from Nostoc (22) and Paramecium (11).
FIG. 1.
Optical spectra of the carbonmonoxy (open circles), oxy (triangles), and deoxy (filled circles) forms of Frankia hemoglobin. Data were taken at 1-nm intervals, and symbols were placed at 10-nm intervals. The spectra between 475 and 700 nm are expanded fivefold and vertically displaced from each other to provide clarity.
The oxygen affinity (Kd) of Frankia hemoglobin is in the range reported for hemoglobins believed to function in O2 transport (Table 1). However, its affinity is about 10 times less than those of the hemoglobins from nitrogen-fixing root nodules of legumes and the nonlegumes Parasponia and Casuarina (25). The Hill coefficient for Frankia hemoglobin is not significantly different from 1; thus, oxygen binding is not cooperative. This is consistent with the molecular mass determination by gel filtration, which indicates a mass equal to that of a truncated hemoglobin monomer.
TABLE 1.
Kinetic constants for O2 binding by hemoglobins from Frankia and other organisms
| Organism or protein | k′on (μM−1 s−1) | koff (s−1) | Kd = koff/k′on (nM) | Reference |
|---|---|---|---|---|
| Frankia strain CcI3 | 206a | 56 | 274b | This study |
| Nostoc commune | 390 | 79 | 203 | 22 |
| Paramecium caudatum | 30 | 25 | 838 | 11 |
| Mycobacterium tuberculosis | 25 | 0.2 | 25b | 9 |
| Chlamydomonas eugametos | 0.014 | 7 | ||
| Synechocystis PCC6803 | 0.011 | 8 | ||
| Chironomus HbIII | 300 | 218 | 727 | 1 |
| Sperm whale myoglobin | 14 | 12 | 857 | 19 |
Calculated from the values for koff and Kd.
Calculated from the equilibrium constant for O2 binding.
Oxygen dissociation and association rates.
Oxygen dissociation rate constants (koff) were measured by an Applied Photophysics SF-17 microvolume stopped-flow spectrophotometer as previously described (5). The same Frankia hemoglobin sample used to estimate the oxygen binding constant was used in the kinetic measurements. Previous studies (5) have shown that the presence of the components of the enzymatic reduction system do not affect the determination. The Frankia hemoglobin solutions were deoxygenated by mixing with a 2.0-mg/ml solution of sodium dithionite. Deoxygenation kinetics were followed at 430 nm. Rate constants were estimated by standard least-squares fitting of the data to a first-order mechanism. Each reported value represents the average of four to eight individual determinations. The oxygen association rate constant (k′on) was estimated from the equilibrium binding constant and koff.
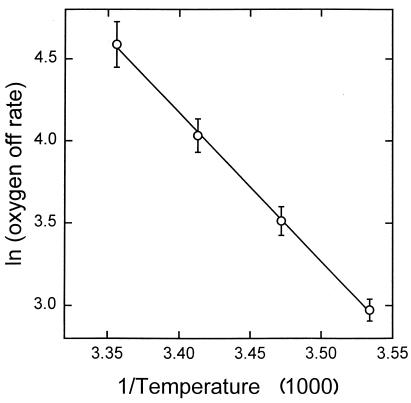
The oxygen dissociation rate (koff) (Table 1) is similar to that observed for Nostoc hemoglobin and is faster than that of many other hemoglobins. The Arrhenius plot of these data is linear between 10 and 25°C and gives an activation energy for dissociation of approximately 18 kcal/mol (Fig. 2), a value consistent with estimates for other monomeric hemoglobins (2).
FIG. 2.
Arrhenius plot of the oxygen dissociation rate (koff) of Frankia hemoglobin. Values are means ± SE for four to eight individual determinations.
The oxygen association rate (k′on), calculated from the koff and the oxygen affinity of Frankia hemoglobin (Table 1), is also similar to that measured for Nostoc hemoglobin. It is notable that the koff for Nostoc hemoglobin is the fastest that has ever been measured.
Effect of combined nitrogen.
Cultures of Frankia strain CcI3 were grown on nitrogen-free medium as before or on the same medium supplemented with NH4Cl (5 mM). The cultures were grown for 28 days, and the hemoglobin concentration in the crude extract was estimated from the HbCO absorption peak at 420 nm by the method of Tjepkema and Asa (24). This method assumes a cell density of 1.0 and converts mass to volume. It underestimates the hemoglobin concentration because not all of the cell mass is cytoplasm. On a fresh mass basis, cultures grown in medium lacking N contained 22.8 ± 1.13 (mean ± SE) μM hemoglobin whereas those grown on medium with N contained only 8.22 ± 1.12 μM hemoglobin.
Our finding that Frankia can produce a hemoglobin in culture strongly supports an earlier suggestion (20) that Frankia produces at least part of the hemoglobin in Alnus nodules. This is further supported by the finding that Frankia strain ArI3, originally isolated from Alnus rubra, also produces a hemoglobin (4). Frankia is also likely to be the source of part of the hemoglobin observed in other actinorhizal nodules. In previous work, evidence was found for two hemoglobins in the actinorhizal nodules of Myrica gale (15). When the hemoglobin from these nodules was purified by gel filtration, much of it eluted in a fraction corresponding to a molecular mass of 38.5 kDa, with an HbCO absorption peak at 416 nm. A smaller amount eluted in a fraction indicating a molecular mass of 16.7 kDa, with an absorption peak at 419 nm (15). Thus, it is possible that the 38.5-kDa hemoglobin was a dimer produced by the host plant and the 16.7-kDa hemoglobin was a monomer produced by Frankia.
The hemoglobins produced by Frankia and Nostoc have high values for both k′on and koff (Table 1). Such values would be well suited for the facilitation of oxygen diffusion over short distances. Whether the concentration of hemoglobin in Frankia is sufficient for simple facilitation of oxygen diffusion is not clear. An overall concentration of 23 μM was found for cultures grown in the absence of combined nitrogen, i.e., under nitrogen-fixing conditions, and such a low concentration may not be sufficient for significant facilitation of O2 diffusion (26). But if much of the hemoglobin is concentrated in specific regions of the cell such as the area near the plasma membrane, then the concentration would be in the range expected for facilitation of oxygen diffusion.
The oxygen binding properties of the hemoglobins from Frankia and Nostoc contrast sharply with those of most other hemoglobins, including truncated hemoglobins other than those from Nostoc (Table 1). Evidence from Raman spectroscopy suggests an enzymatic, rather than O2 transport, function for the truncated hemoglobins from Synechocystis and Chlamydomonas (10). On the other hand it is interesting that the hemoglobin from the hemolymph of the larvae of Chironomus, an insect, has oxygen association and dissociation rates similar to those of the hemoglobins from Frankia and Nostoc (Table 1). The hemoglobins from certain other invertebrates also have similar properties (25).
In summary, we find that cultures of Frankia produce a hemoglobin with a molecular mass and oxygen binding properties that are similar to those of the hemoglobin produced by Nostoc commune. It is probable that the hemoglobin produced by Frankia in culture is also produced in its nitrogen-fixing symbioses with higher plants.
Acknowledgments
This work was supported by a University of Maine Faculty Research Grant to J.D.T., NRICGP/USDA grant 97-35503-4927 to R.E.C., and CSREES/USDA grant ME08465 to C.R.S.
Footnotes
Maine Agricultural and Forest Experiment Station external publication number 2508.
REFERENCES
- 1.Amiconi, G., E. Antonini, M. Brunori, H. Formaneck, and R. Huber. 1972. Functional properties of native and reconstituted hemoglobins from Chironomus thummi thummi. Eur. J. Biochem. 31:52-58. [DOI] [PubMed] [Google Scholar]
- 2.Antonini, E., and M. Brunori. 1971. Hemoglobin and myoglobin in their interactions with ligands. Elsevier Science Publishing Co., New York, N.Y.
- 3.Baker, D., and D. O'Keefe. 1984. A modified sucrose fractionation procedure for the isolation of frankiae from actinorhizal root nodules and soil samples. Plant Soil 78:23-28. [Google Scholar]
- 4.Beckwith, J. 2002. M.S. thesis. University of Maine, Orono.
- 5.Cashon, R. E., M. E. Vayda, and B. D. Sidell. 1997. Kinetic characterization of myoglobins from vertebrates with vastly different body temperatures. Comp. Biochem. Physiol. B 117:613-620. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, T., E. S. Dennis, J. W. Peacock, J. Landsmann, and K. A. Marcker. 1991. Hemoglobin genes in non-legumes: cloning and characterization of a Casuarina glauca hemoglobin gene. Plant Mol. Biol. 16:339-344. [DOI] [PubMed] [Google Scholar]
- 7.Couture, M., T. K. Das, H. C. Lee, J. Peisach, D. L. Rousseau, B. A. Wittenberg, J. B. Wittenberg, and M. Guertin. 1999. Chlamydomonas chloroplast ferrous hemoglobin. J. Biol. Chem. 274:6898-6910. [DOI] [PubMed] [Google Scholar]
- 8.Couture, M., T. K. Das, P.-Y. Savard, Y. Ouellet, J. B. Wittenberg, B. A. Wittenberg, D. L. Rousseau, and M. Guertin. 2000. Structural investigations of the hemoglobin of the cyanobacterium Synechocystis PCC6803 reveal a unique distal heme pocket. Eur. J. Biochem. 267:4770-4780. [DOI] [PubMed] [Google Scholar]
- 9.Couture, M., S.-R. Yeh, B. A. Wittenberg, J. B. Wittenberg, Y. Ouellet, D. L. Rousseau, and M. Guertin. 1999. A cooperative oxygen-binding hemoglobin from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 96:11223-11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das, T. K., M. Couture, Y. Ouellet, M. Guertin, and D. L. Rousseau. 2001. Simultaneous observation of the O-O and Fe-O2 stretching modes in oxyhemoglobins. Proc. Natl. Acad. Sci. USA 98:479-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das, T. K., R. E. Weber, S. Dewilde, J. B. Wittenberg, B. A. Wittenberg, K. Yamauchi, M.-L. Van Hauwaert, L. Moens, and D. L. Rousseau. 2000. Ligand binding in the ferric and ferrous states of Paramecium hemoglobin. Biochemistry 39:14330-14340. [DOI] [PubMed] [Google Scholar]
- 12.Fleming, A. I., J. B. Wittenberg, B. A. Wittenberg, W. F. Dudman, and C. A. Appleby. 1987. The purification, characterization and ligand-binding kinetics of hemoglobins from root nodules of the non-leguminous Casuarina glauca-Frankia symbiosis. Biochim. Biophys. Acta 911:209-220. [Google Scholar]
- 13.Hayashi, A., T. Suzuki, and M. Shin. 1973. An enzymatic reduction system for metmyoglobin and methemoglobin, and its application to functional studies of oxygen carriers. Biochim. Biophys. Acta 310:309-316. [DOI] [PubMed] [Google Scholar]
- 14.Korenaga, S., J. Igarashi, A. Matsuoka, and K. Shikama. 2000. A primitive myoglobin from Tetrahymena pyriformis: its heme environment, autoxidizability, and genomic DNA structure. Biochim. Biophys. Acta 1543:131-145. [DOI] [PubMed] [Google Scholar]
- 15.Pathirana, S. M., and J. D. Tjepkema. 1995. Purification of hemoglobin from the actinorhizal root nodules of Myrica gale L. Plant Physiol. 107:827-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potts, M., S. V. Angeloni, R. E. Ebel, and D. Bassam. 1992. Myoglobin in a cyanobacterium. Science 256:1690-1692. [DOI] [PubMed] [Google Scholar]
- 17.Riggs, A., and R. A. Wolbach. 1956. Sulphydryl groups and the structure of hemoglobin. J. Gen. Physiol. 39:585-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silvester, W. B., S. L. Harris, and J. D. Tjepkema. 1990. Oxygen regulation and hemoglobin, p. 157-176. In C. R. Schwintzer and J. D. Tjepkema (ed.), The biology of Frankia and actinorhizal plants. Academic Press, San Diego, Calif.
- 19.Springer, B. A., K. D. Egeberg, S. G. Sligar, R. J. Rohlfs, A. J. Mathews, and J. S. Olson. 1989. Discrimination between oxygen and carbon monoxide and inhibition of autooxidation by myoglobin. J. Biol. Chem. 264:3057-3060. [PubMed] [Google Scholar]
- 20.Suharjo, U. K. J., and J. D. Tjepkema. 1995. Occurrence of hemoglobin in the nitrogen-fixing root nodules of Alnus glutinosa. Physiol. Plant. 95:247-252. [Google Scholar]
- 21.Suzuki, T., and K. Imai. 1998. Evolution of myoglobin. Cell. Mol. Life Sci. 54:979-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorsteinsson, M. V., D. R. Bevan, M. Potts, Y. Dou, R. F. Eich, M. S. Hargrove, Q. H. Gibson, and J. S. Olson. 1999. A cyanobacterial hemoglobin with unusual ligand binding kinetics and stability properties. Biochemistry 38:2117-2126. [DOI] [PubMed] [Google Scholar]
- 23.Tisa, L. S., and J. C. Ensign. 1987. Isolation and nitrogenase activity of vesicles from Frankia sp. strain EAN1pec. J. Bacteriol. 169:5054-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tjepkema, J. D., and D. J. Asa. 1987. Total and CO-reactive heme content of actinorhizal nodules and the roots of some non-nodulated plants. Plant Soil 100:225-236. [Google Scholar]
- 25.Weber, R. E., and S. N. Vinogradov. 2001. Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol. Rev. 81:569-628. [DOI] [PubMed] [Google Scholar]
- 26.Wittenberg, J. B. 1992. Functions of cytoplasmic hemoglobins and myohemerythrin. Adv. Comp. Environ. Physiol. 13:59-85. [Google Scholar]
- 27.Zhang, Z., M. F. Lopez, and J. G. Torrey. 1984. A comparison of cultural characteristics and infectivity of Frankia isolates from root nodules of Casuarina species. Plant Soil 78:79-90. [Google Scholar]