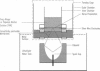
Abstract
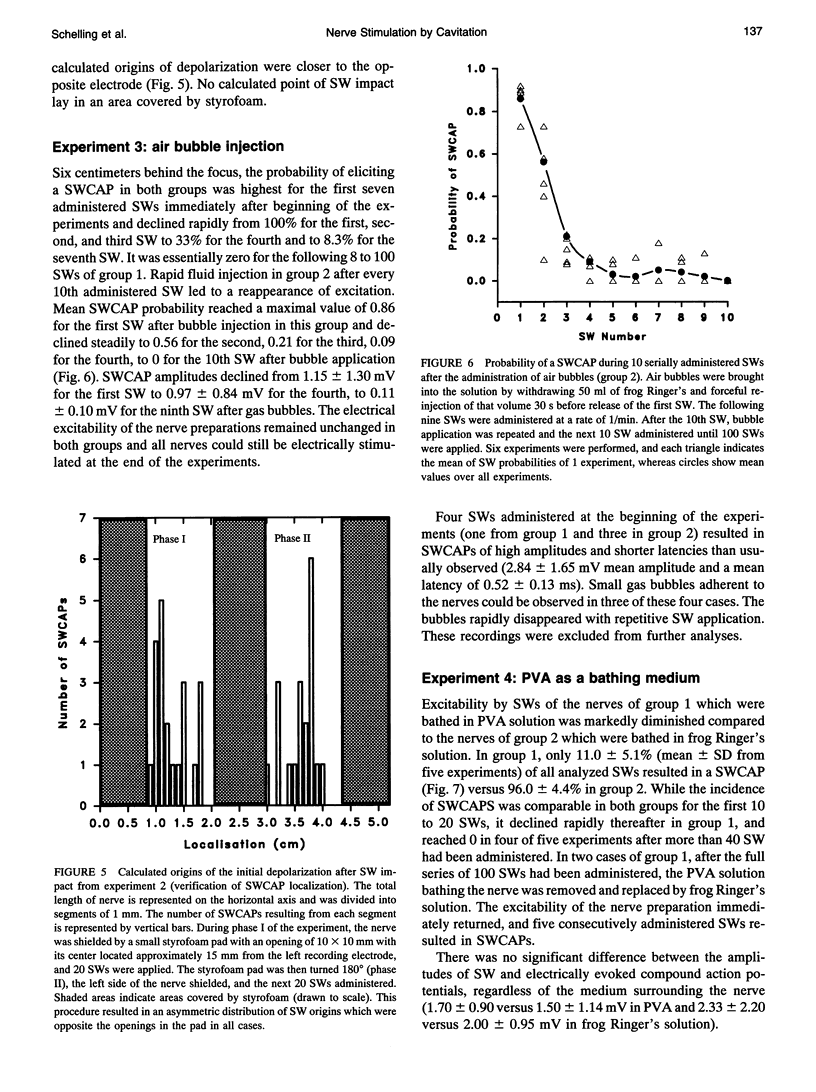
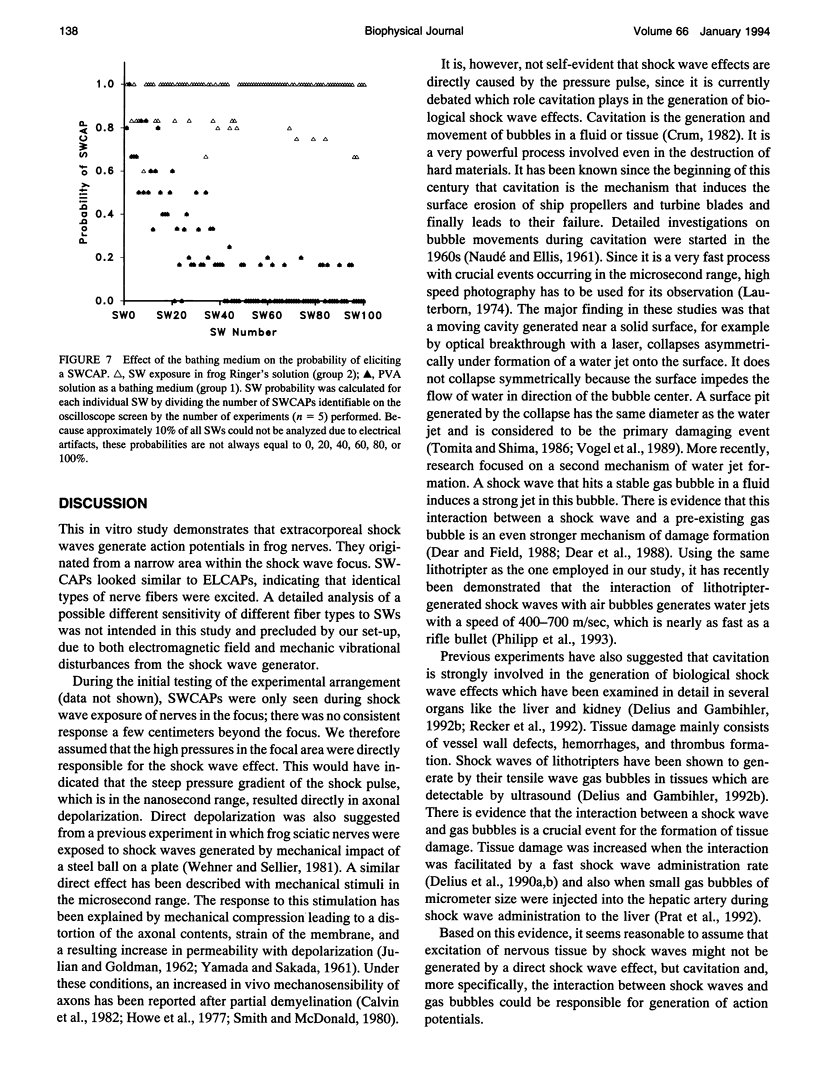
Shock waves (SWs) are single pressure pulses with amplitudes up to over 100 MPa, a rise time of only a few nanoseconds, and a short duration of approximately 2 microseconds. Their clinical application for stone destruction causes pain, indicating nerve stimulation by SWs. To examine this phenomenon, sciatic nerves of frogs were exposed to SWs in an organ bath. The SWs were generated with an experimental Dornier lithotripter model XL1 at an operating voltage of 15 kV. The nerves were mounted in a chamber which allowed electrical nerve stimulation and the registration of electrically and SW-induced compound action potentials (SWCAPs). The chamber was filled with frog Ringer's solution. In a standardized protocol. The first experiment established that 95.0 +/- 4.7% of administered SWs induced action potentials which were lower in amplitude (1.45 +/- 1.14 versus 1.95 +/- 0.95 mV, p = 0.004) but similar in shape to electrically induced compound action potentials. In a second experiment, it was shown that the site of origin of the SWCAPs could be correctly determined by simultaneous recording of action potentials at both ends of the nerve. The mechanism of shock wave stimulation was examined by experiments 3 and 4. In experiment 3, in contrast to the previous experiments, SW exposure of the nerves was performed 6 cm outside the shock wave focus. This resulted in a mean probability of inducing a SWCAP of only 4%. After gas bubble administration, this probability increased to 86% for the first SW released immediately after bubble application and declined to 56% for the second, 21% for the third, to 0 for the 10th SW after fluid injection. This indicates that cavitation, the interaction between shock waves and gas bubbles in fluid or tissues, was involved in SWCAP generation. In experiment 4, nerves were again exposed in the focus, however, the Ringer's solution surrounding the nerve was replaced by polyvinyl alcohol (PVA). PVA is a solution with low cavitation activity.In PVA, the excitability was markedly diminished to 11.0 +/- 5.1% compared with 96.0 +/- 4.4% in control nerves exposed in Ringer's solution. In conclusion, bioeffects of SWs on nervous tissue appear to result from cavitation. It is suggested that cavitation is also the underlying mechanism of SW-related pain during extracorporeal SW lithotripsy in clinical medicine.
Full text
PDF
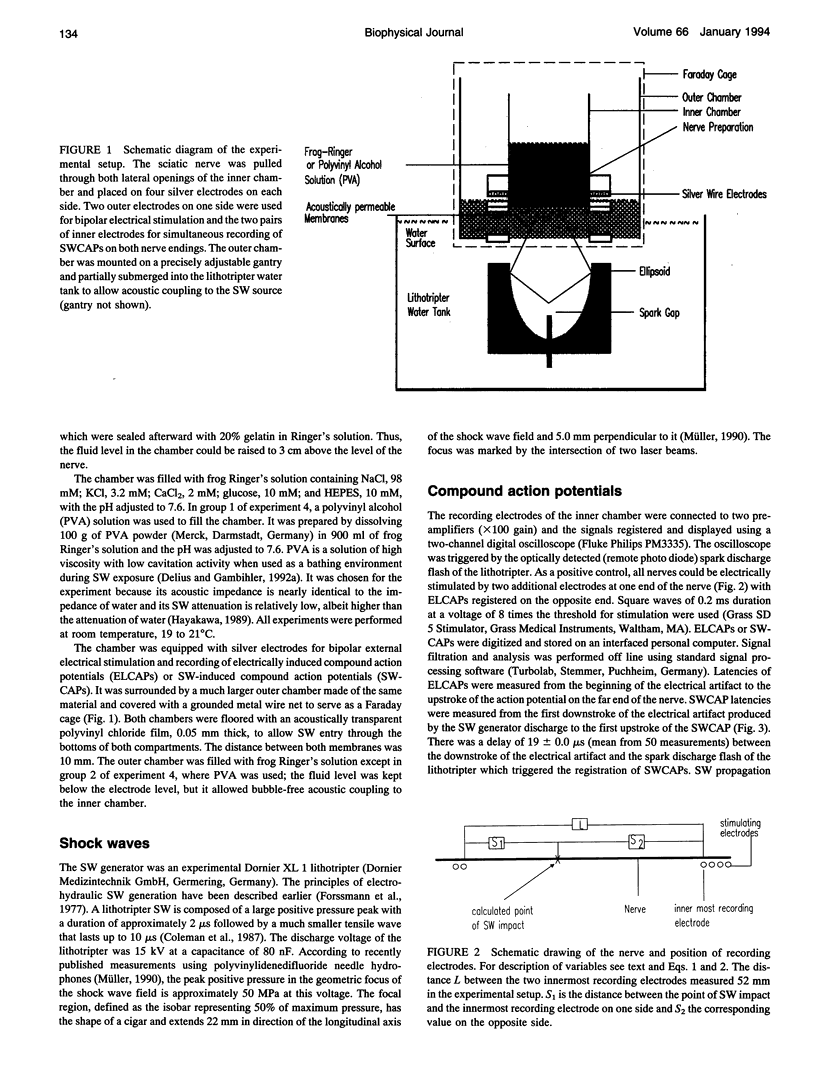
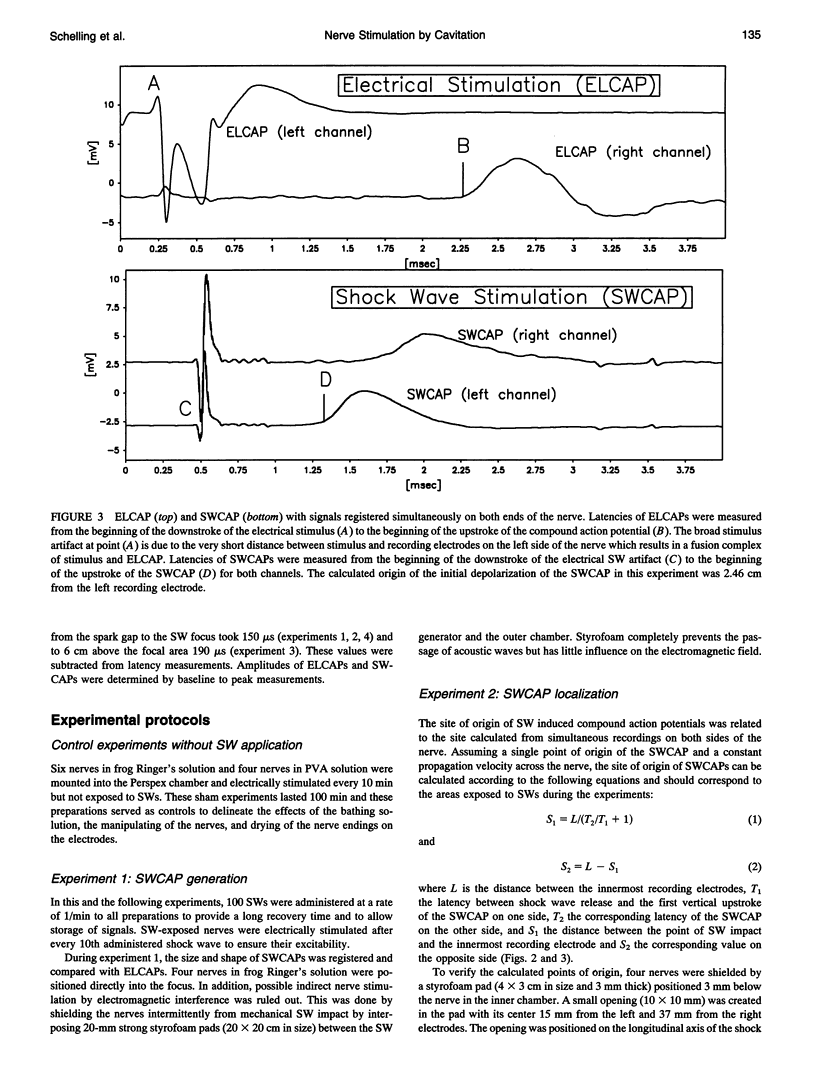
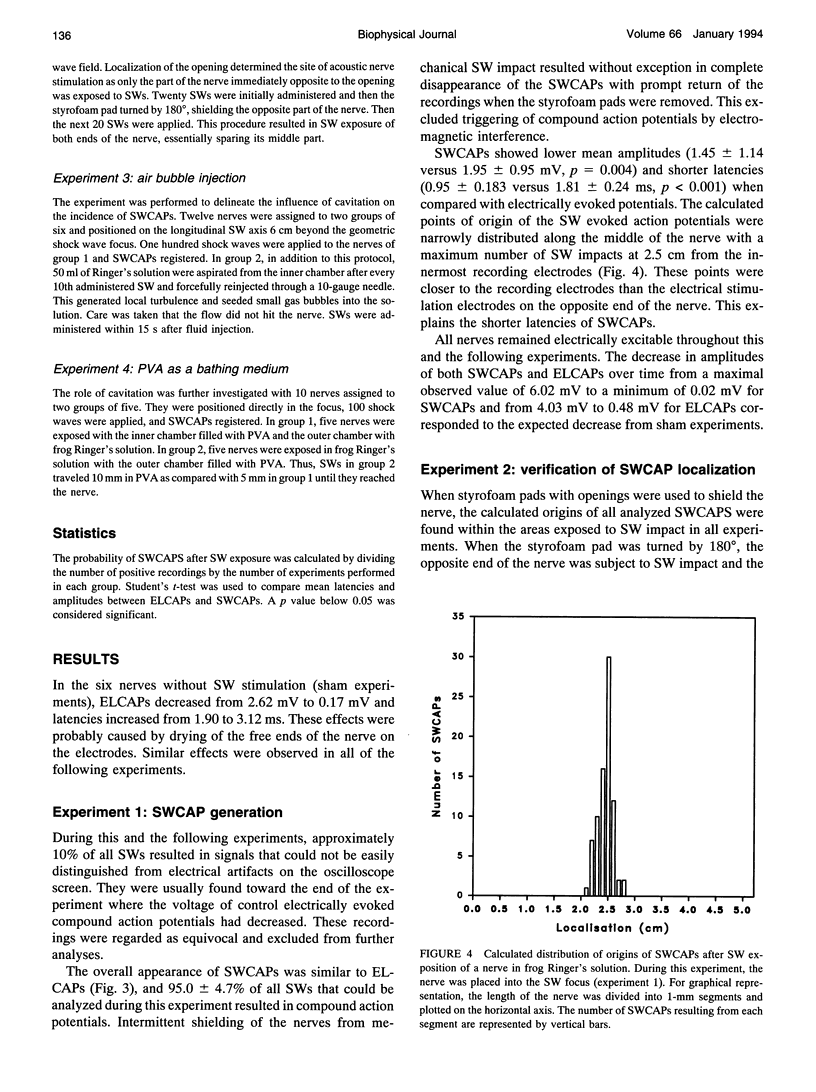


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brümmer F., Brenner J., Bräuner T., Hülser D. F. Effect of shock waves on suspended and immobilized L1210 cells. Ultrasound Med Biol. 1989;15(3):229–239. doi: 10.1016/0301-5629(89)90067-7. [DOI] [PubMed] [Google Scholar]
- Calvin W. H., Devor M., Howe J. F. Can neuralgias arise from minor demyelination? Spontaneous firing, mechanosensitivity, and afterdischarge from conducting axons. Exp Neurol. 1982 Mar;75(3):755–763. doi: 10.1016/0014-4886(82)90040-1. [DOI] [PubMed] [Google Scholar]
- Coleman A. J., Saunders J. E. A survey of the acoustic output of commercial extracorporeal shock wave lithotripters. Ultrasound Med Biol. 1989;15(3):213–227. doi: 10.1016/0301-5629(89)90066-5. [DOI] [PubMed] [Google Scholar]
- Coleman A. J., Saunders J. E., Preston R. C., Bacon D. R. Pressure waveforms generated by a Dornier extra-corporeal shock-wave lithotripter. Ultrasound Med Biol. 1987 Oct;13(10):651–657. doi: 10.1016/0301-5629(87)90063-9. [DOI] [PubMed] [Google Scholar]
- Delius M., Gambihler S. Sonographic imaging of extracorporeal shock wave effects in the liver and gallbladder of dogs. Digestion. 1992;52(1):55–60. doi: 10.1159/000200939. [DOI] [PubMed] [Google Scholar]
- Delius M., Jordan M., Liebich H. G., Brendel W. Biological effects of shock waves: effect of shock waves on the liver and gallbladder wall of dogs--administration rate dependence. Ultrasound Med Biol. 1990;16(5):459–466. doi: 10.1016/0301-5629(90)90168-c. [DOI] [PubMed] [Google Scholar]
- Ellisman M. H., Palmer D. E., André M. P. Diagnostic levels of ultrasound may disrupt myelination. Exp Neurol. 1987 Oct;98(1):78–92. doi: 10.1016/0014-4886(87)90073-2. [DOI] [PubMed] [Google Scholar]
- Filipczyński L., Piechocki M. Estimation of the temperature increase in the focus of a lithotripter for the case of high rate administration. Ultrasound Med Biol. 1990;16(2):149–156. doi: 10.1016/0301-5629(90)90143-z. [DOI] [PubMed] [Google Scholar]
- Forssmann B., Hepp W., Chaussy C., Eisenberger F., Wanner K. Eine Methode zur berührungsfreien Zertrümmerung von Nierensteinen durch Stosswellen. Biomed Tech (Berl) 1977 Jul-Aug;22(7-8):164–168. doi: 10.1515/bmte.1977.22.7-8.164. [DOI] [PubMed] [Google Scholar]
- Fry F. J., Kossoff G., Eggleton R. C., Dunn F. Threshold ultrasonic dosages for structural changes in the mammalian brain. J Acoust Soc Am. 1970 Dec;48(6 Suppl):1413+–1413+. doi: 10.1121/1.1912301. [DOI] [PubMed] [Google Scholar]
- GRAY J. A., RITCHIE J. M. Effects of stretch on single myelinated nerve fibres. J Physiol. 1954 Apr 28;124(1):84–99. doi: 10.1113/jphysiol.1954.sp005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUG O., PAPE R. Nachweis der Ultraschallkavitation im Gewebe. Strahlentherapie. 1954;94(1):79–99. [PubMed] [Google Scholar]
- Howe J. F., Loeser J. D., Calvin W. H. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain. 1977 Feb;3(1):25–41. doi: 10.1016/0304-3959(77)90033-1. [DOI] [PubMed] [Google Scholar]
- Müller M. Dornier-Lithotripter im Vergleich. Vermessung der Stosswellenfelder und Fragmentationswirkungen. Biomed Tech (Berl) 1990 Nov;35(11):250–262. [PubMed] [Google Scholar]
- Prat F., Ponchon T., Berger F., Chapelon J. Y., Gagnon P., Cathignol D. Hepatic lesions in the rabbit induced by acoustic cavitation. Gastroenterology. 1991 May;100(5 Pt 1):1345–1350. [PubMed] [Google Scholar]
- Sackmann M., Pauletzki J., Sauerbruch T., Holl J., Schelling G., Paumgartner G. The Munich Gallbladder Lithotripsy Study. Results of the first 5 years with 711 patients. Ann Intern Med. 1991 Feb 15;114(4):290–296. doi: 10.7326/0003-4819-114-4-290. [DOI] [PubMed] [Google Scholar]
- Schelling G., Mendl G., Weber W., Pauletzki J., Sackmann M., Pöppel E., Peter K. Patient controlled analgesia for extracorporeal shock wave lithotripsy of gallstones. Pain. 1992 Mar;48(3):355–359. doi: 10.1016/0304-3959(92)90084-O. [DOI] [PubMed] [Google Scholar]
- Schelling G., Weber W., Sackmann M., Peter K. Pain control during extracorporeal shock wave lithotripsy of gallstones by titrated alfentanil infusion. Anesthesiology. 1989 Jun;70(6):1022–1023. doi: 10.1097/00000542-198906000-00027. [DOI] [PubMed] [Google Scholar]
- Smith K. J., McDonald W. I. Spontaneous and mechanically evoked activity due to central demyelinating lesion. Nature. 1980 Jul 10;286(5769):154–155. doi: 10.1038/286154a0. [DOI] [PubMed] [Google Scholar]
- Wehner H. D., Sellier K. Shockwave-induced compound action potentials in the peripheral nerve. Z Rechtsmed. 1981;86(4):239–243. doi: 10.1007/BF00200664. [DOI] [PubMed] [Google Scholar]
- YAMADA M., SAKADA S. Effects of mechanical stimulation on the nerve fiber. Jpn J Physiol. 1961 Aug 15;11:378–384. doi: 10.2170/jjphysiol.11.378. [DOI] [PubMed] [Google Scholar]
- YOUNG R. R., HENNEMAN E. Functional effects of focused ultrasound on mammalian nerves. Science. 1961 Nov 10;134(3489):1521–1522. doi: 10.1126/science.134.3489.1521. [DOI] [PubMed] [Google Scholar]
- YOUNG R. R., HENNEMAN E. Reversible block of nerve conduction by ultrasound. Arch Neurol. 1961 Jan;4:83–89. doi: 10.1001/archneur.1961.00450070085009. [DOI] [PubMed] [Google Scholar]
- Zakharov S. I., Bogdanov KYu, Rosenshtraukh L. V., Gavrilov L. R., Yushin V. P. The effect of acoustic cavitation on the contraction force and membrane potential of rat papillary muscle. Ultrasound Med Biol. 1989;15(6):561–565. doi: 10.1016/0301-5629(89)90189-0. [DOI] [PubMed] [Google Scholar]