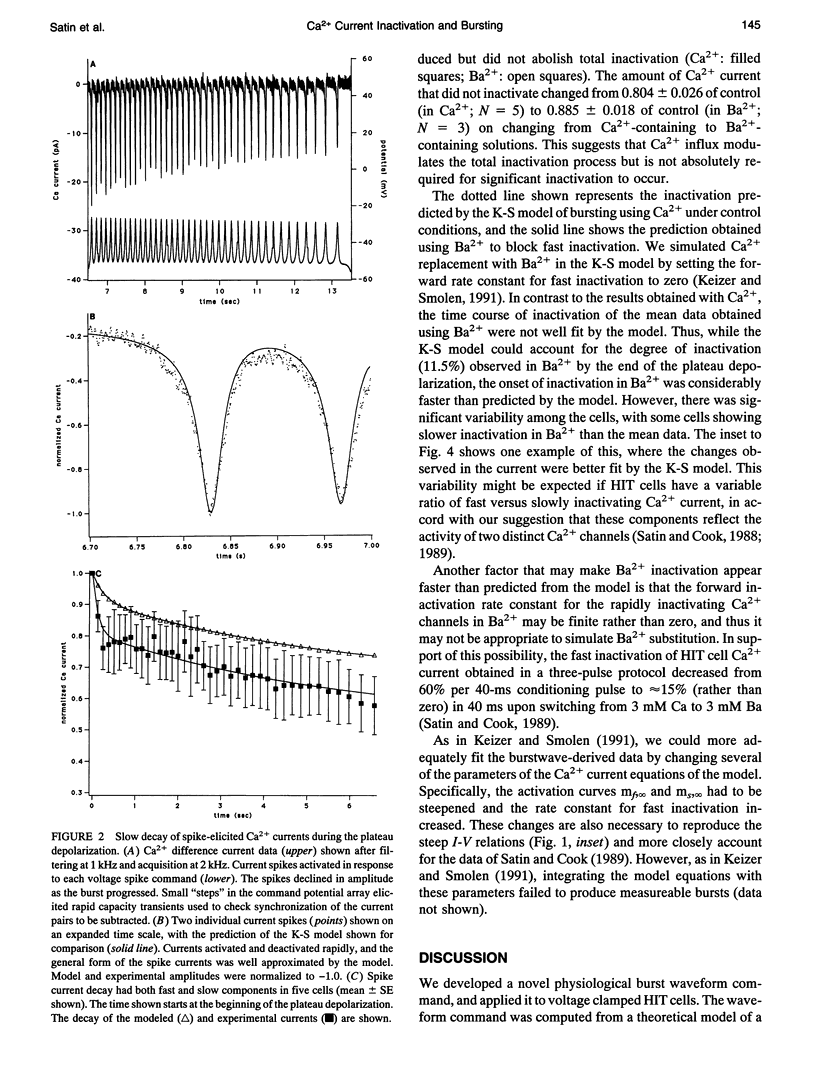
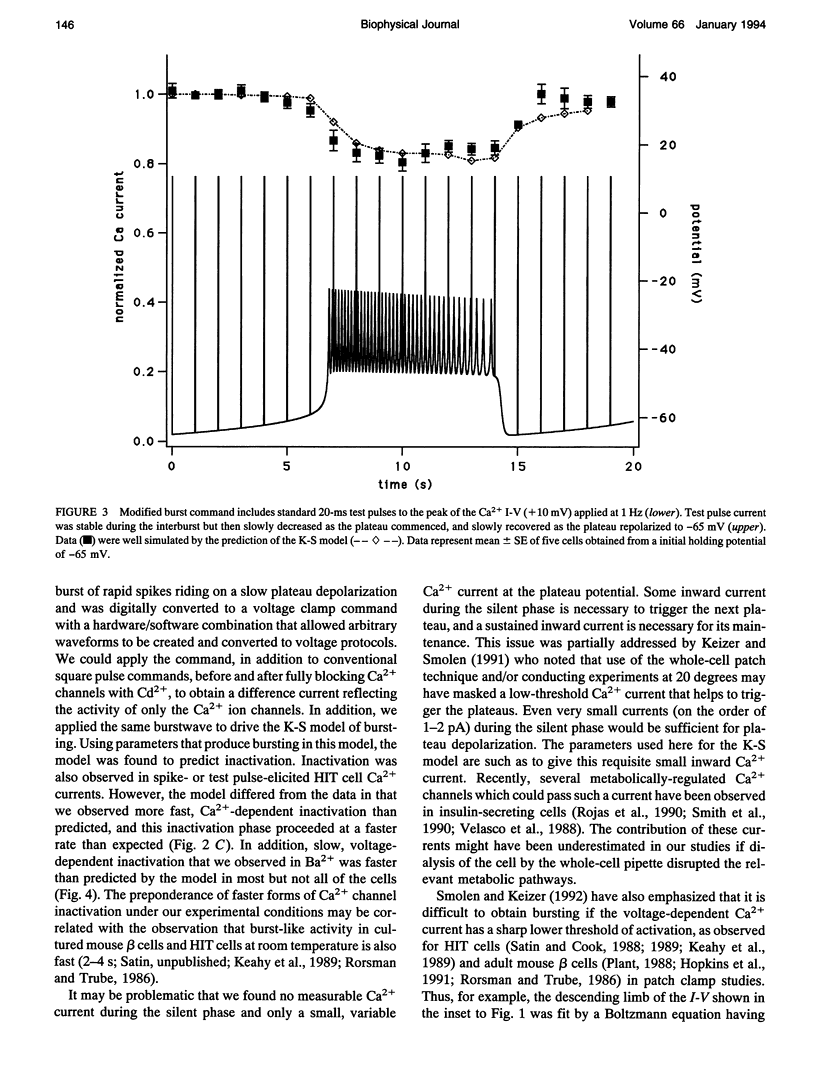
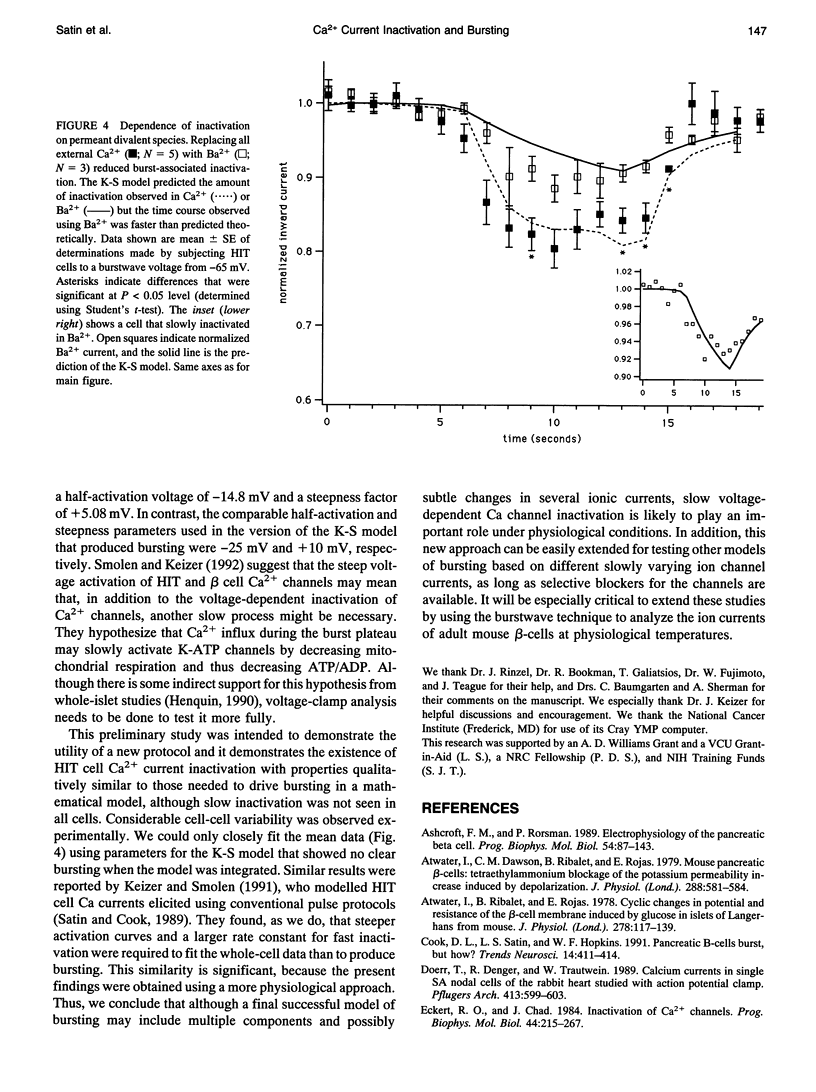
Abstract
A novel voltage-clamp protocol was developed to test whether slow inactivation of Ca2+ current occurs during bursting in insulin-secreting cells. Single insulin-secreting HIT cells were patch-clamped and their Ca2+ currents were isolated pharmacologically. A computed beta-cell burst was used as a voltage-clamp command and the net Ca2+ current elicited was determined as a cadmium difference current. Ca2+ current rapidly activated during the computed plateau and spike depolarizations and then slowly decayed. Integration of this Ca2+ current yielded an estimate of total Ca influx. To further analyze Ca2+ current inactivation during a burst, repetitive test pulses to + 10 mV were added to the voltage command. Current elicited by these pulses was constant during the interburst, but then slowly and reversibly decreased during the depolarizing plateau. This inactivation was reduced by replacing external Ca2+ with Ba2+ as a charge carrier, and in some cells inactivation was slower in Ba2+. Experimental results were compared with the predictions of the Keizer-Smolen mathematical model of bursting, after subjecting model equations to identical voltage commands. In this model, bursting is driven by the slow, voltage-dependent inactivation of Ca current during the plateau active phase. The K-S model could account for the slope of the slow decay of spike-elicited Ca current, the waveform of individual Ca current spikes, and the suppression of test pulse-elicited Ca current during a burst command. However, the extent and rate of fast inactivation were underestimated by the model. Although there are significant differences between the data obtained and the predictions of the K-S model, the overall results show that as predicted by the model, Ca current slowly inactivates during a burst of imposed spikes, and inactivation is dependent on both Ca2+ influx and membrane depolarization. We thus show that clamping cells to their physiological voltage waveform can be readily accomplished and is a powerful approach for understanding the contribution of individual ion currents to bursting.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Atwater I., Ribalet B., Rojas E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J Physiol. 1978 May;278:117–139. doi: 10.1113/jphysiol.1978.sp012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Satin L. S., Hopkins W. F. Pancreatic B cells are bursting, but how? Trends Neurosci. 1991 Sep;14(9):411–414. doi: 10.1016/0166-2236(91)90033-q. [DOI] [PubMed] [Google Scholar]
- Doerr T., Denger R., Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflugers Arch. 1989 Apr;413(6):599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Glucose-induced electrical activity in beta-cells. Feedback control of ATP-sensitive K+ channels by Ca2+? [corrected]. Diabetes. 1990 Nov;39(11):1457–1460. doi: 10.2337/diab.39.11.1457. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Regulation of insulin release by ionic and electrical events in B cells. Horm Res. 1987;27(3):168–178. doi: 10.1159/000180806. [DOI] [PubMed] [Google Scholar]
- Keahey H. H., Rajan A. S., Boyd A. E., 3rd, Kunze D. L. Characterization of voltage-dependent Ca2+ channels in beta-cell line. Diabetes. 1989 Feb;38(2):188–193. doi: 10.2337/diab.38.2.188. [DOI] [PubMed] [Google Scholar]
- Keizer J., Smolen P. Bursting electrical activity in pancreatic beta cells caused by Ca(2+)- and voltage-inactivated Ca2+ channels. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3897–3901. doi: 10.1073/pnas.88.9.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. P., Sutton R., Ashcroft F. M. Voltage-activated calcium and potassium currents in human pancreatic beta-cells. J Physiol. 1991 Nov;443:175–192. doi: 10.1113/jphysiol.1991.sp018829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCobb D. P., Beam K. G. Action potential waveform voltage-clamp commands reveal striking differences in calcium entry via low and high voltage-activated calcium channels. Neuron. 1991 Jul;7(1):119–127. doi: 10.1016/0896-6273(91)90080-j. [DOI] [PubMed] [Google Scholar]
- Plant T. D. Properties and calcium-dependent inactivation of calcium currents in cultured mouse pancreatic B-cells. J Physiol. 1988 Oct;404:731–747. doi: 10.1113/jphysiol.1988.sp017316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B., Beigelman P. M. Cyclic variation of K+ conductance in pancreatic beta-cells: Ca2+ and voltage dependence. Am J Physiol. 1979 Sep;237(3):C137–C146. doi: 10.1152/ajpcell.1979.237.3.C137. [DOI] [PubMed] [Google Scholar]
- Rojas E., Hidalgo J., Carroll P. B., Li M. X., Atwater I. A new class of calcium channels activated by glucose in human pancreatic beta-cells. FEBS Lett. 1990 Feb 26;261(2):265–270. doi: 10.1016/0014-5793(90)80568-4. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santerre R. F., Cook R. A., Crisel R. M., Sharp J. D., Schmidt R. J., Williams D. C., Wilson C. P. Insulin synthesis in a clonal cell line of simian virus 40-transformed hamster pancreatic beta cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4339–4343. doi: 10.1073/pnas.78.7.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin L. S., Cook D. L. Calcium current inactivation in insulin-secreting cells is mediated by calcium influx and membrane depolarization. Pflugers Arch. 1989 May;414(1):1–10. doi: 10.1007/BF00585619. [DOI] [PubMed] [Google Scholar]
- Satin L. S., Cook D. L. Evidence for two calcium currents in insulin-secreting cells. Pflugers Arch. 1988 Apr;411(4):401–409. doi: 10.1007/BF00587719. [DOI] [PubMed] [Google Scholar]
- Satin L. S., Hopkins W. F., Fatherazi S., Cook D. L. Expression of a rapid, low-voltage threshold K current in insulin-secreting cells is dependent on intracellular calcium buffering. J Membr Biol. 1989 Dec;112(3):213–222. doi: 10.1007/BF01870952. [DOI] [PubMed] [Google Scholar]
- Sherman A., Rinzel J., Keizer J. Emergence of organized bursting in clusters of pancreatic beta-cells by channel sharing. Biophys J. 1988 Sep;54(3):411–425. doi: 10.1016/S0006-3495(88)82975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A., Rorsman P., Ashcroft F. M. Modulation of dihydropyridine-sensitive Ca2+ channels by glucose metabolism in mouse pancreatic beta-cells. Nature. 1989 Nov 30;342(6249):550–553. doi: 10.1038/342550a0. [DOI] [PubMed] [Google Scholar]
- Smolen P., Keizer J. Slow voltage inactivation of Ca2+ currents and bursting mechanisms for the mouse pancreatic beta-cell. J Membr Biol. 1992 Apr;127(1):9–19. doi: 10.1007/BF00232754. [DOI] [PubMed] [Google Scholar]
- Velasco J. M., Petersen J. U., Petersen O. H. Single-channel Ba2+ currents in insulin-secreting cells are activated by glyceraldehyde stimulation. FEBS Lett. 1988 Apr 25;231(2):366–370. doi: 10.1016/0014-5793(88)80851-2. [DOI] [PubMed] [Google Scholar]



