Abstract
Previous studies have demonstrated that reduced humic substances (HS) can be reoxidized by anaerobic bacteria such as Geobacter, Geothrix, and Wolinella species with a suitable electron acceptor; however, little is known of the importance of this metabolism in the environment. Recently we investigated this metabolism in a diversity of environments including marine and aquatic sediments, forest soils, and drainage ditch soils. Most-probable-number enumeration studies were performed using 2,6-anthrahydroquinone disulfonate (AHDS), an analog for reduced HS, as the electron donor with nitrate as the electron acceptor. Anaerobic organisms capable of utilizing reduced HS as an electron donor were found in all environments tested and ranged from a low of 2.31 × 101 in aquifer sediments to a high of 9.33 × 106 in lake sediments. As part of this study we isolated six novel organisms capable of anaerobic AHDS oxidation. All of the isolates coupled the oxidation of AHDS to the reduction of nitrate with acetate (0.1 mM) as the carbon source. In the absence of cells, no AHDS oxidation was apparent, and in the absence of AHDS, no cell density increase was observed. Generally, nitrate was reduced to N2. Analysis of the AHDS and its oxidized form, 2,6-anthraquinone disulfonate (AQDS), in the medium during growth revealed that the anthraquinone was not being biodegraded as a carbon source and was simply being oxidized as an energy source. Determination of the AHDS oxidized and nitrate reduced accounted for 109% of the theoretical electron transfer. In addition to AHDS, all of these isolates could also couple the oxidation of reduced humic substances to the reduction of nitrate. No HS oxidation occurred in the absence of cells and in the absence of a suitable electron acceptor, demonstrating that these organisms were capable of utilizing natural HS as an energy source and that AHDS serves as a suitable analog for studying this metabolism. Alternative electron donors included simple volatile fatty acids such as propionate, butyrate, and valerate as well as simple organic acids such as lactate and pyruvate. Analysis of the complete sequences of the 16S rRNA genes revealed that the isolates were not closely related to each other and were phylogenetically diverse, with members in the alpha, beta, gamma, and delta subdivisions of the Proteobacteria. Most of the isolates were closely related to known genera not previously recognized for their ability to couple growth to HS oxidation, while one of the isolates represented a new genus in the delta subclass of the Proteobacteria. The results presented here demonstrate that microbial oxidation of HS is a ubiquitous metabolism in the environment. This study represents the first description of HS-oxidizing isolates and demonstrates that microorganisms capable of HS oxidation are phylogenetically diverse.
Humic substances (HS) are ubiquitous in the environment and can be readily isolated from nearly all soils, waters, and sediments. The natural organic matter content of many soils and sediments can account for as much as 10% by weight of the total material (22). In all environments HS are formed from the decomposition of plant, animal, and microbial tissues and tend to be more recalcitrant than their precursors. The functional groups of the HS, which determine the physical and chemical characteristics, vary and depend on the origin and age of the material (22). HS comprise three basic components: humins, humic acids, and fulvic acids. These components are traditionally defined according to their solubilities. Humins are the fraction which is insoluble at all pHs, humic acids are insoluble at pHs below pH 2.0, and fulvic acids are soluble at all pHs.
HS are thought to consist of a skeleton of alkyl or aromatic units cross-linked mainly by oxygen and nitrogen groups, with the major functional groups being carboxylic acid, phenolic and alcoholic hydroxyls, ketone, and quinone groups (27, 42). This structure allows HS to bind both hydrophobic and hydrophilic materials, and thus they play an important role in the fate and transport of heavy metals and contaminating hydrocarbons in the environment (9, 24, 35-37, 38, 46, 54). HS are redox-active compounds and can reduce metals with estimated reduction potentials of 0.5 to 0.7 eV (45). They have been demonstrated to transfer electrons from reduced inorganics (sulfide) and organics (ascorbic acid) to various heavy metals, nitroaromatics, and chlorinated solvents (3, 20, 21, 26, 37, 41, 43, 45, 48). This can have a major effect on the migration and toxicity of many of these compounds (14). It is thought that the redox-reactive components of HS that are involved in these reactions are the quinone moieties (20, 21, 28).
Although HS are ubiquitous, they were always considered inert refractory organic material, especially in anoxic environments. Recent research, however, has demonstrated that HS can in fact play an important role as electron sinks for anaerobic respiratory bacteria and fermentative bacteria, stimulating mineralization of complex organic carbon compounds in the absence of O2 (6, 7, 15, 28, 29). Electron spin resonance studies have confirmed that the quinone moieties are the redox-active components of the HS for these microbial reductive reactions (44), and the HS have been shown to act as soluble electron carriers between microorganisms and insoluble terminal electron acceptors such as Fe(III) oxides (28, 29). When the reduced HS interact with the Fe(III) oxides, they are reoxidized and can thus be recycled (28, 29).
More recently, it has been demonstrated that HS in the reduced form can also serve as suitable electron donors for anaerobic organisms growing on a variety of alternative electron acceptors, such as nitrate and fumarate. In this instance, the organisms obtain carbon from readily degradable limited sources, such as acetate, and simply use the reduced HS as an energy source. Such a metabolism gives these organisms a potential competitive advantage over other heterotrophs in the environment that may require a limited organic compound such as acetate as both a carbon and energy source, thus requiring significantly greater concentrations for growth. Prior studies have demonstrated that pure cultures of Fe(III)-reducing organisms such as Geobacter metallireducens, Geothrix fermentans, and Shewanella alga, which are known to be capable of dissimilatory HS reduction, could alternatively couple reduced HS oxidation to nitrate reduction (30). In addition, other known denitrifiers, such as Paracoccus denitrificans, which are not capable of either dissimilatory HS reduction or Fe(III) reduction could also use reduced HS as an electron donor for denitrification (30).
These studies indicate that reduced HS may play an important role as an electron donor in anaerobic environments, but the organisms primarily responsible for HS oxidation and the environmental relevance of this metabolism are currently unknown. In order to determine the ubiquity and diversity of organisms capable of HS oxidation, we enumerated HS-oxidizing populations in a broad spectrum of environments and isolated six new HS-oxidizing nitrate-reducing bacteria.
MATERIALS AND METHODS
Sources of soils and sediments.
Soil samples were collected from the top 6 cm of an uncontaminated soil in Clayton Woods, Carbondale, Illinois. In addition, sediment samples were collected from Campus Lake, Southern Illinois University, Carbondale, Illinois; a hydrocarbon-contaminated aquifer at Hanahan, South Carolina; a perchlorate-contaminated sediment at the Naval Surface Warfare Facility, Indian Head, Maryland; a hydrocarbon-contaminated marine harbor at Shelter Island, San Diego Bay, San Diego, California; and a pristine marine site at Key West, Florida.
All samples were freshly collected and transported directly back to the laboratory, where they were immediately assayed for HS-oxidizing, nitrate-reducing bacteria.
Medium and culturing conditions.
Standard anaerobic culturing techniques were used throughout (4, 25, 34). The media were boiled under N2-CO2 (80:20) to remove dissolved O2 and then dispensed into anaerobic pressure tubes and serum bottles under N2-CO2, capped with thick butyl rubber stoppers, and sterilized by autoclaving. The basal media used were either a bicarbonate-buffered freshwater medium (8) or a bicarbonate-buffered marine medium (16). The electron donor, 2,6-anthrahydroquinone disulfonate (AHDS) (5 mM), was prepared by adding an equivalent amount of 2,6-anthraquinone disulfonate (AQDS) to the anoxic medium and further gassing the medium with H2-CO2 (80:20, vol/vol) in the presence of palladium-covered aluminum chips as previously described (14). Sodium salt of acetate (0.1 mM) was added as a suitable carbon source, and nitrate (10 mM) was added as an electron acceptor.
Alternative electron donors were added from sterile anoxic aqueous stocks. Pure aromatic hydrocarbons (benzene, hexadecane, and toluene) were added directly (1 μl to 10 ml of medium). Electron acceptors were also added from anoxic aqueous stocks. Soluble Fe(III) was supplied as Fe(III) chelated with nitrilotriacetic acid [Fe(III)-NTA] (10 mM) (41). Mn(IV) was supplied as synthetic MnO2 that was prepared as described previously (31) to give a final concentration of 10 to 30 mM. Sulfur was supplied as a polysulfide solution prepared as previously outlined (53). All other electron acceptors were prepared as anoxic aqueous stocks of the sodium salts to give final concentrations of 10 mM.
Isolation of HS-oxidizing bacteria.
HS-oxidizing, nitrate-reducing enrichments were established by transferring 1-g subsamples from each of the freshly collected soil and sediment samples into 9 ml of prepared anoxic medium under a gas stream of N2-CO2. AHDS (5 mM) was the electron donor, and nitrate (10 mM) was the electron acceptor. Acetate (0.1 mM) was added as a suitable carbon source. Incubations were done at 30°C in the dark. Positive enrichments were identified by color change of the medium from red to tan as the AHDS was oxidized to AQDS and by microscopic examination. Once a positive enrichment was established, the HS-oxidizing culture was transferred (10% inoculum) into 9 ml of fresh anoxic medium. Isolated colonies were obtained from transfers of positive enrichments by the standard agar shake-tube technique using the medium outlined previously (8, 17). Similarly, some isolates (strains JJ, KC, and HA) were obtained directly from the highest positive dilution tube of the most-probable-number series of the Campus Lake, Indian Head, and Hanahan samples, respectively, using the standard agar shake-tube technique.
Most-probable-number counts.
Numbers of HS-oxidizing, nitrate-reducing bacteria were determined by three-tube most-probable-number counts with 5 mM AHDS as the electron donor in either the marine or freshwater basal medium, depending on the source of the samples. Sodium pyrophosphate (1%, wt/vol) was added to the first dilution tubes in the most-probable-number series to detach the cells from the sediment or soil particles. All most-probable-number tubes were incubated at room temperature in the dark for 60 days prior to analysis. Positives in the most-probable-number series were identified visually by color change of the medium from red to tan as the AHDS was oxidized to AQDS.
16S rRNA gene sequencing and analysis.
The 16S ribosomal DNA (rDNA) sequences were generated as previously described (1, 17). Sequence entry and manipulation was performed with the MacVector 6.1 sequence analysis software program for the Macintosh (Oxford Molecular). Sequences of select 16S rRNAs were downloaded from the Ribosomal Database Project (33) and GenBank (5) into the computer program SeqApp (23). HS-oxidizing bacterial 16S rDNA sequences were added manually to the alignment using secondary-structure information for accurate sequence alignment. Distance, parsimony, and maximum-likelihood analyses of the aligned sequences were performed using PAUP* 4.0d65 (47). Bootstrap analysis was conducted on 100 replications using a heuristic search strategy to assess the confidence level of various clades.
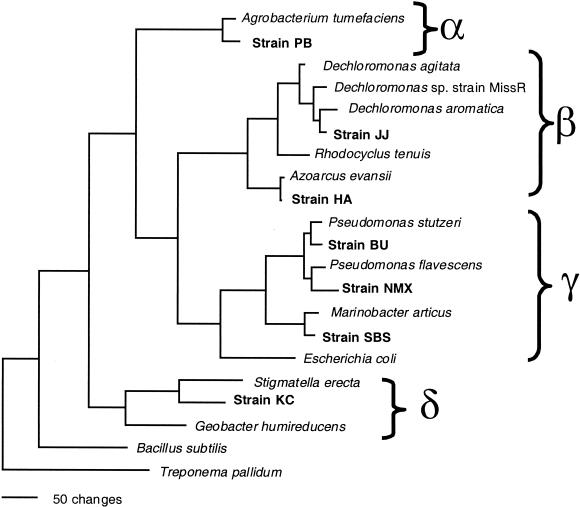
GenBank accession numbers for sequences represented in Fig. 6 are as follows: Agrobacterium tumefaciens, M11223; Dechloromonas agitata, AF047462; Dechloromonas sp. strain MissR, AF170357; Dechloromonas aromatica strain RCB, ΔY032610; Rhodocyclus tenuis, D16208; Azoarcus evansii, X77679; Pseudomonas stutzeri, U26262; Escherichia coli, J01859; Treponema pallidum, M88726; Bacillus subtilis, K00637; Stigmatella erecta, AJ233933; Geobacter humireducens, AF019932; Pseudomonas flavescens, U01916; Marinobacter articus, AF148811; Dechloromonas sp. strain JJ, AY032611; Agrobacterium sp. strain PB, AF482682; Azoarcus sp. strain HA, AF482683; Pseudomonas sp. strain BU, AF482684; Pseudomonas sp. strain NMX, AF482685; Marinobacter sp. strain SBS, AF482686; and strain KC, AF482687.
FIG. 6.
Phylogenetic tree of the 16S rDNA sequence data set resulting from distance analysis using the Jukes-Cantor correction. The same topology was obtained using either parsimony or maximum likelihood and was supported by bootstrap analysis.
Electron microscopy.
Scanning electron micrographs were prepared using cells grown anaerobically with acetate (10 mM) as the electron donor and nitrate (10 mM) as the electron acceptor as previously described (8) and viewed with a Hitachi S570 scanning electron microscope at 20 kV.
Analytical techniques.
AHDS and AQDS concentrations were determined spectrophotometrically as previously outlined (15, 28, 29). Nitrate concentrations were determined by ion chromatography of aqueous samples using a Dionex DX500 equipped with an AS9-SC column using a sodium carbonate (2 mM)-sodium bicarbonate (7.5 mM) mobile phase at a flow rate of 2 ml/min. Organic acid analysis concentrations were analyzed by high-pressure liquid chromatography (HPLC) with UV detection (Shimadzu SPD-10A) using an HL-75H+ cation exchange column (Hamilton 79476). The eluent was 0.016 N H2SO4 at a flow rate of 0.4 ml per min. N2 gas production was monitored by gas chromatography coupled to thermocouple detection using a Supelco Poapak N 80/100-mesh column and helium as the carrier gas. Growth of cultures on soluble electron acceptors was measured by increase in optical density at 600 nm or by direct microscopic count. Chlorite dismutase enzyme activity was determined by microassay using horseradish peroxidase (Sigma Chemical Corp.) coupled to dianisidine as an electron donor. In the presence of chlorite, a brown color is produced which can be read spectrophotometrically at a wavelength of 450 nm (J. D. Coates, unpublished data).
HS oxidation was determined as previously described (14) by backtitration of HS samples with Fe(III) for 15 min prior to analyzing for Fe(II) by the ferrozine assay (32).
RESULTS
Most-probable-number studies.
Most-probable-number counts with AHDS as the electron donor and nitrate as the electron acceptor indicated that HS-oxidizing bacteria are present in many diverse environments. The HS-oxidizing microbial community represented a significant population in most environments tested (Table 1). The HS-oxidizing population ranged from 2.31 × 101 ± 1.33 × 101 per g in aquifer sediments collected from a petroleum-contaminated aquifer in Hanahan, S.C., to as high as 9.33 × 106 ± 4.17 × 106 per g in aquatic sediments collected from Campus Lake (Table 1).
TABLE 1.
Most-probable-number counts of HS oxidizersa
| Environment sampled | Mean count (cells/g) ± SD |
|---|---|
| Forest soil | 4.27 (± 2.14) × 106 |
| Aquifer sediment (Maryland) | 1.49 (± 6.10) × 104 |
| Aquifer sediment (Sorth Carolina) | 2.31 (± 1.33) × 101 |
| Pristine aquatic sediment (Illinois) | 9.33 (± 4.17) × 106 |
| Marine sediment (Florida) | 2.31 (± 1.33) × 102 |
| Marine sediment (California) | 2.15 (± 8.11) × 102 |
Enumeration studies were performed with AHDS (10 mM) as the electron donor, nitrate (10 mM) as the electron acceptor, and 0.1 mM acetate as the carbon source.
Isolation of HS oxidizers.
After 2 weeks of incubation, good growth was observed in the primary enrichments from all of the environments sampled. Enrichments were transferred into fresh basal medium (10% inoculum). Good growth was observed in the transfer after 1 to 2 weeks as determined by a color change in the medium from red to tan as the AHDS was oxidized to AQDS and by microscopic examination of the culture broths. Highly enriched HS-oxidizing, nitrate-reducing cultures were obtained by sequential transfer over the following weeks prior to serial dilution into agar tubes. Colonies of consistent morphology were apparent in the higher dilution agar tubes from each enrichment after 2 weeks of incubation. Colonies were small, 1 to 4 mm in diameter, and were generally pink in the red agar tube. Several of these colonies were selected from each of the enrichment series, and HS-oxidizing isolates were obtained from all environments sampled.
Phenotypic characteristics.
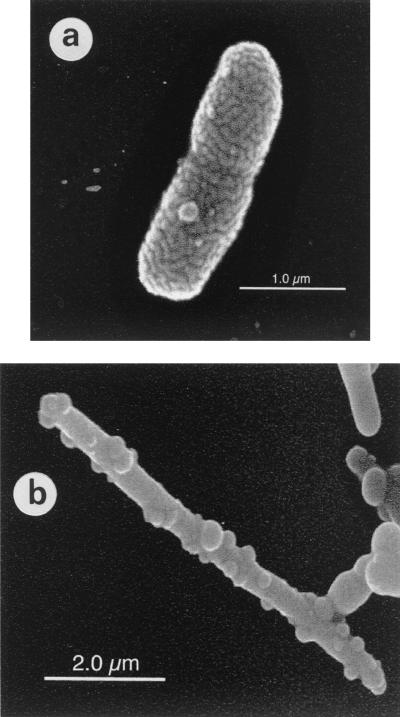
In general, the HS-oxidizing isolates were gram-negative, nonfermenting facultative anaerobes. Morphologically, most of the isolates were motile short rods 0.5 μm in diameter by 2 μm in length (Fig. 1a). One of the isolates, strain KC, was morphologically distinct, being a long thin rod with cells 0.3 μm by 5.3 μm (Fig. 1b). Strain KC was also phenotypically distinct and showed no motility under any of the growth conditions tested, nor did it grow aerobically with any of the alternative media tested. Spores were not evident in wet-mount preparations of any of the isolates when observed by phase contrast microscopy, and no growth was observed in fresh AHDS-nitrate medium after pasteurization at 80°C for 3 min. All of the facultative anaerobic isolates could grow aerobically on L-broth.
FIG. 1.
Scanning electron micrographs of the humic substance-oxidizing isolates (a) strain JJ and (b) strain KC.
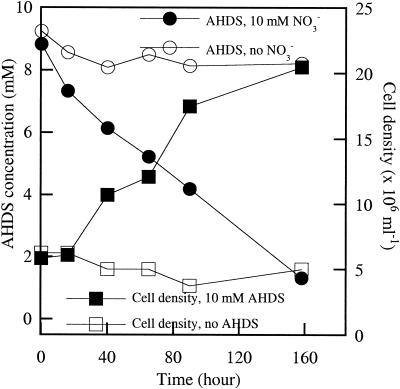
All of the HS-oxidizing isolates were obligate respirers and did not grow on anoxic basal media amended with glucose (10 mM), yeast extract (10 g/liter) and Casamino Acids (10 g/liter) in the absence of a suitable electron acceptor. All isolates tested coupled growth to the oxidation of AHDS with nitrate as the electron acceptor and acetate (0.1 mM) as the carbon source, as shown for strain KC (Fig. 2). In the absence of either nitrate or cells, no AHDS oxidation was apparent, and in the absence of AHDS, no cell density increase was observed (Fig. 2).
FIG. 2.
AHDS oxidation and growth of strain KC with nitrate (10 mM) as the electron acceptor and acetate (0.1 mM) as the carbon source.
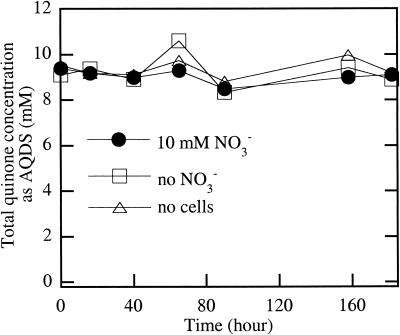
Analysis of the concentration of AQDS and AHDS in the medium during growth revealed that the total anthraquinone concentration remained constant during incubation and it was not being biodegraded as a carbon source by the organisms (Fig. 3). The oxidation of 6.48 mM AHDS resulted in the reduction of 2.38 mM nitrate, giving a stoichiometry of 2.72, which is 109% of the theoretical value according to the formula 5C14H8O8S22− (AHDS) + 2NO3− + 2H+ → 5C14H6O8S22− (AQDS) + N2 + 6H2O.
FIG. 3.
Total quinone concentration in culture broth, measured as AQDS concentration after abiotic air oxidation of samples collected at various time points during the growth of strain KC.
Humic substances as electron donors.
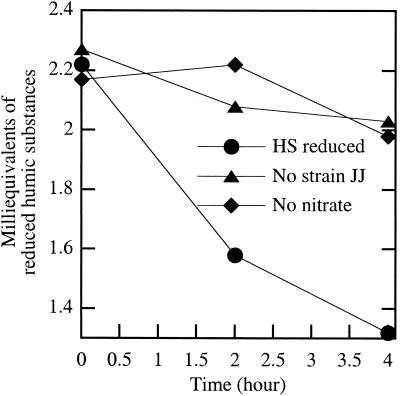
Similarly to AHDS, reduced humic acids were reoxidized by the HS-oxidizing isolates with nitrate as the sole electron acceptor (Fig. 4). No HS oxidation occurred in the absence of cells or if the electron acceptor was omitted (Fig. 4).
FIG. 4.
Oxidation of Aldrich brand humic substances by washed whole-cell suspensions of strain JJ with nitrate as the sole electron acceptor.
Alternative electron donors and acceptors.
In addition to acetate, the HS-oxidizing isolates used short-chain volatile fatty acids and simple dicarboxylic acids as alternative electron donors (Table 2). Three of the HS oxidizers (strains JJ, KC, and NMX) could alternatively use H2 as an electron donor coupled to nitrate reduction. As previously published (13), one of the HS oxidizers, strain JJ, could also use simple aromatic hydrocarbons, including toluene and benzene, as alternative electron donors. As such, this is one of only two organisms described that can oxidize benzene completely to CO2 in the absence of oxygen (13).
TABLE 2.
Compounds used as electron donors by HS-oxidizing isolates with nitrate (10 mM) as the electron acceptor
| Electron donor | Concn | Use by isolate:
|
|||||
|---|---|---|---|---|---|---|---|
| JJ | KC | HA | NMX | PB | SBS | ||
| Hydrogen | 101 kPa | + | + | − | + | − | − |
| Formate | 10 mM | − | − | + | − | + | − |
| Acetate | 10 mM | + | + | + | + | + | + |
| Propionate | 5 mM | + | − | + | + | + | + |
| Butyrate | 5 mM | + | − | + | + | + | + |
| Palmitate | 1 mM | − | + | − | − | + | |
| Ethanol | 10 mM | + | + | + | + | + | + |
| Fumarate | 10 mM | + | + | + | + | + | + |
| Lactate | 10 mM | + | + | + | + | + | + |
| Citrate | 10 mM | + | − | + | − | + | + |
| Pyruvate | 10 mM | + | + | + | + | + | |
| Malate | 10 mM | + | − | + | − | − | − |
| Succinate | 10 mM | + | + | + | − | + | + |
| Glucose | 10 mM | − | + | + | + | + | − |
| Casamino Acids | 1 g/liter | + | + | + | + | + | + |
| Yeast extract | 1 g/liter | − | + | − | − | + | + |
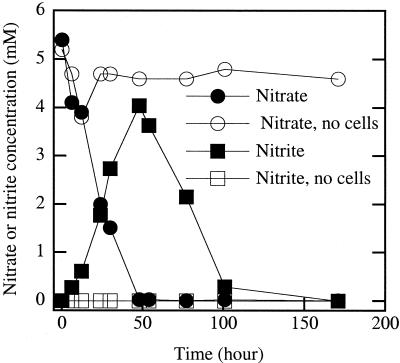
The HS-oxidizing isolates were relatively limited in the range of electron acceptors used. In addition to nitrate, most of the isolates also utilized O2. Strain KC was an anomaly in that it could not grow aerobically or with any electron acceptor tested other than nitrate. Nitrate was generally reduced to N2. In the case of strain JJ, nitrate was transiently reduced to nitrite, which was further reduced to N2 once the nitrate was depleted (Fig. 5). A broad range of alternative electron acceptors tested were not used by the HS-oxidizing isolates.
FIG. 5.
Nitrate reduction and transient nitrite formation by strain JJ while oxidizing AHDS with acetate as the carbon source.
Optimum growth conditions.
In general, all of the HS-oxidizing isolates demonstrated different optima for pH, temperature, and salinity (Table 3). All of the isolates were mesophilic. Not surprisingly, strain SBS, which was isolated from marine sediments collected from San Diego Bay, showed the highest tolerance to NaCl and was able to grow in concentrations as high as 10% (wt vol−1). Optimum growth was observed at 2% NaCl (wt vol−1). In contrast, strains JJ, KC, and HA were intolerant to NaCl and grew optimally in freshwater medium (i.e., 0% NaCl wt vol−1). Of all the isolates tested, strain KC was the least tolerant to NaCl, and salinities of 0.5% NaCl (wt vol−1) and greater completely inhibited growth.
TABLE 3.
Optimum growth conditions for individual HS-oxidizing isolates with acetate (10 mM) and nitrate (10 mM) as the sole electron donor and acceptor, respectively
| HS-oxidizing isolate | Source of sediment | Optimum temp (°C) | Optimum pH | Optimum salinity (%) |
|---|---|---|---|---|
| JJ | Campus Lake, Ill. | 37 | 6.5 | 0 |
| SBS | San Diego Bay, Calif. | 37 | 7.0 | 2 |
| PB | Potomac River, Md. | 30 | 7.0 | 1 |
| NMX | Shiprock, N. Mex. | 30 | 7.6 | 1 |
| KC | Indian Head, Md. | 37 | 6.5 | 0 |
| HA | Hanahan, S.C. | 37 | 7.5 | 0 |
Phylogeny of the HS-oxidizing isolates.
Analyses of the complete 16S rDNA sequences revealed that all isolates were members of the Proteobacteria (Fig. 6). The HS-oxidizing isolates belonged to four of the five subdivisions (alpha, beta, gamma, and delta) of the Proteobacteria, demonstrating that this metabolism is widespread throughout the phylum (Fig. 6). All of these isolates except for strain KC were closely related (greater than 96% sequence similarity in the 16S rRNA gene) to previously described genera not recognized for the potential to couple growth to the oxidation of reduced humic substances. In contrast, the closest known relative to strain KC was Stigmatella erecta, with less than 90% sequence similarity (88.9%) in the 16S rRNA gene sequence, which, when considered with its unique phenotypic characteristics, indicated that strain KC represents a novel genus in the delta subclass of the Proteobacteria (Fig. 6).
Only one of the isolates, strain JJ, was closely related to organisms previously demonstrated to be capable of HS oxidation. Strain JJ was a member of the Dechloromonas genus in the beta subclass of the Proteobacteria. Its closest relative is “Dechloromonas aromatica” strain RCB (97.7% 16S rDNA sequence similarity), a recently described hydrocarbon-oxidizing perchlorate reducer (13). In contrast to all other members of this genus, strain JJ did not grow by the reduction of either chlorate or perchlorate. In addition, active washed whole-cell suspensions of strain JJ did not dismutate chlorite into chloride and O2, a metabolic capability common to all known perchlorate-reducing bacteria (data not shown).
DISCUSSION
The results of this study demonstrate the hitherto unrecognized ubiquity of microbial humic substance oxidation coupled to nitrate reduction and the broad phylogenetic diversity of the organisms capable of this metabolism. The results of the enumeration studies indicated that nitrate-dependent HS oxidation was present in every environment investigated, including marine samples collected from sites previously demonstrated to be sulfate reducing (10, 11, 18). Whether or not sulfate-reducing bacteria are capable of nitrate-dependent HS oxidation remains to be determined. None of the HS-oxidizing isolates obtained during this study were capable of sulfate reduction.
Previous studies on the nitrate-dependent oxidation of HS demonstrated that many organisms, such as Geobacter species and Shewanella species, known to be capable of the dissimilatory reduction of humic materials could reverse this metabolism and oxidize reduced HS in the presence of a suitable electron acceptor (29). In contrast, some denitrifiers, such as Paracoccus denitrificans (30), and perchlorate reducers, such as Dechloromonas agitata (8), which are incapable of either dissimilatory HS reduction or Fe(III) reduction, did use reduced HS as an electron donor for carbon assimilation and growth, demonstrating that their metabolism is not simply a reversal of the reductive pathway. These previous studies were performed with AHDS as an analog for reduced HS (8, 30).
The fact that all of the isolates obtained in the present study could use reduced humic substances as electron donors indicates that AHDS is a suitable analog of HS for the isolation of nitrate-dependent HS oxidizers. This is similar to observations made in previous studies investigating the ubiquity and diversity of HS-reducing bacteria, in which it was demonstrated that AQDS could be substituted as a reliable analog for oxidized humic substances for the isolation and culturing of HS-reducing bacteria (15).
Interestingly, of the six genera represented by the isolates obtained in this study, only one, strain JJ, was a member of a genus previously demonstrated to be capable of HS oxidation coupled to nitrate reduction. Strain JJ is a member of the Dechloromonas genus in the beta subclass of the Proteobacteria, a group of organisms known primarily for their ability to grow by the dissimilatory reduction of chlorate and perchlorate [(per)chlorate] (1, 8, 17). This genus was only recently described (1), and together with the Dechlorosoma species, they represent the two dominant groups of perchlorate-reducing bacteria in the environment (1, 8, 12, 17; J. Pollock, L. A. Achenbach, and J. D. Coates, submitted for publication).
Previously we demonstrated that all members of the Dechloromonas genus tested were capable of coupling HS oxidation to either nitrate or (per)chlorate reduction (8, 13, 17). The role of the Dechloromonas species in environments uncontaminated with (per)chlorate is still unknown. However, recent studies have indicated that (per)chlorate-reducing bacteria are metabolically diverse (8, 13, 17) and can survive for extended periods when inoculated into environmental samples in the absence of an additional electron donor and acceptor (12). The ability of these organisms to grow by the oxidation of reduced HS further demonstrates their metabolic versatility.
In contrast to all other members of the Dechloromonas genus (1, 2, 8, 17), strain JJ did not grow by (per)chlorate reduction. In addition, strain JJ did not dismutate chlorite, a transient intermediate formed during the reductive metabolism of (per)chlorate (17, 49) into chloride and oxygen. As such, strain JJ represents the first member of the Dechloromonas genus that is incapable of (per)chlorate reduction. One phylogenetically close relative of the Dechloromonas genus (97.3% 16S sequence similarity to its closest relative, Dechloromonas sp. strain MissR) is the obligate anaerobic Fe(III) reducer Ferribacterium limneticum (1, 2, 19). Similar to strain JJ, previous studies indicated that this organism was also incapable of (per)chlorate reduction (1). Although this organism is phylogenetically closely related to strain JJ (99.5% similarity), extensive phenotypic differences between F. limneticum and strain JJ and phenotypic similarities between strain JJ and Dechloromonas species support the placement of strain JJ in the Dechloromonas genus. Whether or not F. limneticum is capable of growing by nitrate-dependent HS oxidation cannot be determined, as the culture and sole representative of this genus has been lost (D. Cummings, personal communication).
Most of the other isolates obtained in this study were closely related to known genera, such as Pseudomonas in the gamma Proteobacteria and Azoarcus in the beta Proteobacteria, not previously recognized for their ability to grow by humic substance oxidation. In contrast, one of the isolates, strain KC, was phylogenetically distinct and represented a new genus in its own right in the delta subclass of the Proteobacteria. Its closest relative was Stigmatella erecta (88.9% similarity), which is a member of the myxobacteria. Strain KC is morphologically similar to Stigmatella species in that it is a long thin rod (39). However, in contrast to S. erecta and most other myxobacteria (39), strain KC was an obligate anaerobe and did not form fruiting bodies under conditions of starvation.
Myxobacteria are commonly associated with environments of high humus content, such as soils, dung, and the bark of living and dead trees (39). Although their association with these environments has been partially explained by their general ability to decompose complex organics such as cellulose, it is also an environment where reduced HS and nitrate would be prevalent. As demonstrated in this study for strain KC and all the other HS-oxidizing isolates tested, the HS are not biodegraded as a carbon source by these organisms, but rather serve as an energy source for the assimilation of carbon from alternative sources. Whether or not myxobacteria in general can carry out nitrate-dependent HS oxidation in a similar manner is still unknown.
Environmental significance.
Although the geochemistry of HS has been studied for over a century, microbial interactions with HS, especially in the absence of oxygen, are only now being elucidated. HS are considered to be completely recalcitrant in the absence of dissolved oxygen; however, several studies have demonstrated that humic materials are slowly degraded by microbial populations in aerobic environments (references 50 and 52 and references therein). Degradation can be significantly enhanced by exposure of the HS to UV radiation from normal sunlight (52). This is because the absorption of UV by organic macromolecules such as humic and fulvic acids results in the production of biologically labile organic compounds via photolytic decomposition (50, 52). In addition, HS can cause reversible inactivation of microbial extracellular enzymatic activity by the binding of proteins to organic acids, resulting in the aggregation, complexation, and precipitation of the humic substance-protein complex (reference 51 and references therein).
Our and other recent studies of microbial interactions with humic substances in anaerobic environments indicate that HS may play an important role as electron shuttles in the microbial community as a result of their redox properties. The recent discoveries that the quinone moieties of HS can be microbially reduced (44) and that HS can alternatively act as electron acceptors for microbial respiration under strictly anaerobic conditions (15, 28, 29) further emphasize their importance for the mineralization of simple carbon compounds in the natural environment. Similarly, the redox activity of HS may also serve to shuttle electron equivalents formed during microbial fermentation onto terminal inorganic electron acceptors such as Fe(III) and result in the production of a more oxidized end product (6).
The results of the present study demonstrate that HS can also serve as suitable electron donors and energy sources for the assimilation of carbon. Whether or not this can be coupled to an autotrophic metabolism remains to be determined. Similarly, whether or not HS oxidation can compete with alternative metabolisms is still unknown. The environmental effect of the redox cycling of HS is uncertain; however, our previous studies on the effects of HS respiration on the reactivity of humic materials indicate that there is a significant difference in the geochemistry of microbially reduced and oxidized HS (14). Reduction of these compounds resulted in an extensive alteration of the molecular morphology as the colloidal structure of the humic substances collapsed to form small hollow particles, presumably as a result of the increase in the electron density of the molecule (14). In addition, the hydrophobic and hydrophilic interactions of the HS also correlated to the redox state, and as the HS were reduced, the binding of positively charged cations was increased, while the absorption of hydrophobic hydrocarbons was decreased (14).
As humic materials are known to play an important role in the fate and transport of heavy metals and hydrocarbons in contaminated environments (9, 24, 35-37, 38, 46, 54), the microbial cycling of the redox state of the HS could potentially alter the nature and extent of these effects.
Acknowledgments
Support for this research was from grant N00014-99-10371 from the Office of Naval Research to J.D.C.
REFERENCES
- 1.Achenbach, L. A., R. A. Bruce, U. Michaelidou, and J. D. Coates. 2001. Dechloromonas agitata N.N. gen., sp. nov. and Dechlorosoma suillum N.N. gen., sp. nov., two novel environmentally dominant (per)chlorate-reducing bacteria and their phylogenetic position. Int. J. Syst. E vol. Microbiol. 51:527-533. [DOI] [PubMed] [Google Scholar]
- 2.Achenbach, L. A., and J. D. Coates. 2000. Disparity between bacterial phylogeny and physiology. ASM News 66:714-716. [Google Scholar]
- 3.Alberts, J. J., J. E. Schindler, and R. W. Miller. 1974. Elemental mercury evolution mediated by humic acid. Science 184:895-896. [DOI] [PubMed] [Google Scholar]
- 4.Balch, W. E., G. E. Fox, L. J. Magrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43:260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson, D. A., M. S. Boguski, D. J. Lipman, J. Ostell, and B. F. Ouellette. 1998. GenBank. Nucleic Acids Res. 26:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benz, M., B. Schink, and A. Brune. 1998. Humic acid reduction by Propionibacterium freudenreichii and other fermenting bacteria. Appl. Environ. Microbiol. 64:4507-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley, P. M., F. H. Chapelle, and D. R. Lovley. 1998. Humic acids as electron acceptors for anaerobic microbial oxidation of vinyl chloride and dichloroethene. Appl. Environ. Microbiol. 64:3102-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce, R. A., L. A. Achenbach, and J. D. Coates. 1999. Reduction of (per)chlorate by a novel organism isolated from a paper mill waste. Environ. Microbiol. 1:319-331. [DOI] [PubMed] [Google Scholar]
- 9.Buffle, J. 1984. Natural organic matter and metal-organic interactions in aquatic systems, p. 165-199. In H. Sigel (ed.), Metal ions in biological systems, vol. 18. Marcel Dekker, Inc., New York, N.Y. [Google Scholar]
- 10.Coates, J. D., R. T. Anderson, and D. R. Lovley. 1996. Anaerobic hydrocarbon degradation in petroleum-contaminated harbor sediments under sulfate-reducing and artificially imposed iron-reducing conditions. Environ. Sci. Technol. 30:2784-2789. [Google Scholar]
- 11.Coates, J. D., R. T. Anderson, and D. R. Lovley. 1996. Anaerobic oxidation of polycyclic aromatic hydrocarbons under sulfate-reducing conditions. Appl. Environ. Microbiol. 62:1099-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coates, J. D., R. A. Bruce, J. A. Patrick, and L. A. Achenbach. 1999. Hydrocarbon bioremediative potential of (per)chlorate-reducing bacteria. Bioremediation J. 3:323-334. [Google Scholar]
- 13.Coates, J. D., R. Chakraborty, J. G. Lack, S. M. O'Connor, K. A. Cole, K. S. Bender, and L. A. Achenbach. 2001. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature 411:1039-1043. [DOI] [PubMed] [Google Scholar]
- 14.Coates, J. D., R. Chakraborty, S. M. O'Connor, C. Schmidt, and J. Thieme. 2001. The geochemical effects of microbial humic substances reduction. Acta Hydrochim. Hydrobiol. 28:420-427. [Google Scholar]
- 15.Coates, J. D., D. J. Ellis, E. L. Blunt-Harris, C. V. Gaw, E. Roden, and D. R. Lovley. 1998. Recovery of humic-reducing bacteria from a diversity of environments. Appl. Environ. Microbiol. 64:1504-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coates, J. D., D. J. Lonergan, and D. R. Lovley. 1995. Desulfuromonas palmitatis sp. nov., a long-chain fatty acid oxidizing Fe(III) reducer from marine sediments. Arch. Microbiol. 164:406-413. [PubMed] [Google Scholar]
- 17.Coates, J. D., U. Michaelidou, R. A. Bruce, S. M. O'Connor, J. N. Crespi, and L. A. Achenbach. 1999. The ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 65:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coates, J. D., J. Woodward, J. Allen, P. Philp, and D. R. Lovley. 1997. Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Appl. Environ. Microbiol. 63:3589-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings, D. E., F. Caccavo, Jr., S. Spring, and R. F. Rosenzweig. 1999. Ferribacterium limneticum gen. nov., sp. nov., an Fe(III)-reducing microorganism isolated from mining-impacted freshwater lake sediments. Arch. Microbiol. 171:183-188. [Google Scholar]
- 20.Curtis, G. P., and M. Reinhard. 1994. Reductive dehalogenation of hexachloroethane, carbon tetrachloride, and bromoform by anthrahydroquinone disulfonate and humic acid. Environ. Sci. Technol. 28:2393-2401. [DOI] [PubMed] [Google Scholar]
- 21.Dunnivant, F. M., R. P. Schwarzenbach, and D. L. Macalady. 1992. Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environ. Sci. Technol. 26:2133-2142. [Google Scholar]
- 22.Gaffney, J. S., N. A. Marley, and S. B. Clark. 1996. Humic and fulvic acids and organic colloidal materials in the environment, p. 2-16. In J. S. Gaffney, N. A. Marley, and S. B. Clark (ed.), Humic and fulvic acids: isolation, structure, and environmental role, vol. 651. American Chemical Society, Washington, D.C. [Google Scholar]
- 23.Gilbert, D. G. 1993. SeqApp, 1.9a157 ed. Biocomputing Office, Biology Dept., Indiana University, Bloomington, Ind.
- 24.Hesketh, N., M. N. Jones, and E. Tipping. 1996. The interaction of some pesticides and herbicides with humic substances. Anal. Chim. Acta 327:191-201. [Google Scholar]
- 25.Hungate, R. E. 1969. A roll tube method for cultivation of strict anaerobes. Methods Microbiol. 3B:117-132. [Google Scholar]
- 26.Kahn, T. R., C. H. Langford, and G. B. Skippen. 1984. Complexation and reduction as factors in the link between metal ion concentrations and organic matter in the Indian River. Org. Geochem. 7:261-266. [Google Scholar]
- 27.Livens, F. R. 1991. Chemical reactions of metals with humic material. Environ. Pollut. 70:183-208. [DOI] [PubMed] [Google Scholar]
- 28.Lovley, D. R., J. D. Coates, E. L. Blunt-Harris, E. J. P. Phillips, and J. C. Woodward. 1996. Humic substances as electron acceptors for microbial respiration. Nature 382:445-448. [Google Scholar]
- 29.Lovley, D. R., J. L. Fraga, E. L. Blunt-Harris, L. A. Hayes, E. J. P. Phillips, and J. D. Coates. 1998. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim. Hydrobiol. 26:152-157. [Google Scholar]
- 30.Lovley, D. R., J. L. Fraga, J. D. Coates, and E. L. Blunt-Harris. 1999. Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1:89-98. [DOI] [PubMed] [Google Scholar]
- 31.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron and manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lovley, D. R., and E. J. P. Phillips. 1987. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol 53:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, J. M. Stredwick, G. M. Garrity, B. Li, G. J. Olsen, S. Pramanik, T. M. Schmidt, and J. M. Tiedje. 2000. The RDP (Ribosomal Database Project) continues. Nucleic Acids Res. 28:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, T. L., and M. J. Wolin. 1974. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl. Microbiol. 27:985-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moulin, V., and C. Moulin. 1995. Fate of actinides in the presence of humic substances under conditions relevant to nuclear waste disposal. Appl. Geochem. 10:573-580. [Google Scholar]
- 36.Nelson, D. M., W. R. Penrose, J. O. Karttunen, and P. Mehlhaff. 1985. Effects of dissolved organic carbon on the adsorption properties of plutonium in natural waters. Environ. Sci. Technol. 19:127-131. [Google Scholar]
- 37.Perdue, E. M. 1989. Effects of humic substances on metal speciation, p. 281-295. In I. H. Suffet and P. MacCarthy (ed.), Aquatic humic substances: influence on fate and treatment of pollutants, vol. 219. American Chemical Society, Washington, D.C. [Google Scholar]
- 38.Rebhun, M., F. De Smedt, and J. Rwetabula. 1996. Dissolved humic substances for remediation of sites contaminated by organic pollutants. Binding-desorption model predictions. Water Res. 30:2027-2038. [Google Scholar]
- 39.Reichenbach, H., and M. Dworkin. 1991. The myobacteria, p. 3416-3487. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. IV. Springer-Verlag, New York, N.Y. [Google Scholar]
- 40.Roden, E. E., and D. R. Lovley. 1993. Dissimilatory Fe(III) reduction by the marine microorganism Desulfuromonas acetoxidans. Appl. Environ. Microbiol. 59:734-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindler, J. E., D. J. Williams, and A. P. Zimmerman. 1976. Investigation of extracellular electron transport by humic acids, p. 109-115. In J. O. Nriagu (ed.), Environmental biogeochemistry, vol. 1. Ann Arbor Science, Ann Arbor, Mich. [Google Scholar]
- 42.Schulten, H. R., B. Plage, and M. Schnitzer. 1991. A chemical structure for humic substances. Naturwissenschaften 78:311-312. [DOI] [PubMed] [Google Scholar]
- 43.Schwarzenbach, R. P., R. Stierli, K. Lanz, and J. Zeyer. 1990. Quinone and iron porphyrin mediated reduction of nitroaromatic compounds in homogeneous aqueous solution. Environ. Sci. Technol. 24:1566-1574. [Google Scholar]
- 44.Scott, D. T., D. M. McKnight, E. L. Blunt-Harris, S. E. Kolesar, and D. R. Lovley. 1998. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 32:2984-2989. [Google Scholar]
- 45.Skogerboe, R. K., and S. A. Wilson. 1981. Reduction of ionic species by fulvic acid. Anal. Chem. 53:228-232. [Google Scholar]
- 46.Stevenson, F. J. 1982. Humus chemistry: genesis, composition, reactions, p. 1-25. John Wiley & Sons, New York, N.Y.
- 47.Swofford, D. L. 1999. PAUP*: Phylogenetic Analysis Using Parsimony (and other methods), version 4.0. Sinauer Associates, Sunderland, Mass.
- 48.Szilagyi, M. 1971. Reduction of Fe3+ ion by humic acid preparations. Soil Sci. 111:233-235. [Google Scholar]
- 49.van Ginkel, C., G. Rikken, A. Kroon, and S. Kengen. 1996. Purification and characterization of chlorite dismutase: a novel oxygen-generating enzyme. Arch. Microbiol. 166:321-326. [DOI] [PubMed] [Google Scholar]
- 50.Wetzel, R. G. 1992. Gradient-dominated ecosystems: sources and regulatory functions of dissolved organic matter in freshwater ecosystems. Hydrobiologia 229:181-198. [Google Scholar]
- 51.Wetzel, R. G. 1993. Humic compounds from wetlands: complexation, inactivation, and reactivation of surface-bound and extracellular enzymes. Int. Ver. Theor. Angew. Limnol. Verh. 25:122-128. [Google Scholar]
- 52.Wetzel, R. G., P. G. Hatcher, and T. S. Bianchi. 1995. Natural photolysis by ultraviolet irradiance of recalcitrant dissolved organic matter to simple substrates for rapid bacterial metabolism. Limnol. Oceangr. 40:1369-1380. [Google Scholar]
- 53.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria. Environ. Sci. Technol. 26:725-733. [Google Scholar]
- 54.Zhang, Y.-J., N. D. Bryan, F. R. Livens, and M. N. Jones. 1996. Complexing of metal ions by humic substances, p. 194-206. In J. S. Gaffney, N. A. Marley, and S. B. Clark (ed.), Humic and fulvic acids: Isolation, structure, and environmental role, vol. 651. American Chemical Society, Washington, D.C. [Google Scholar]