Abstract
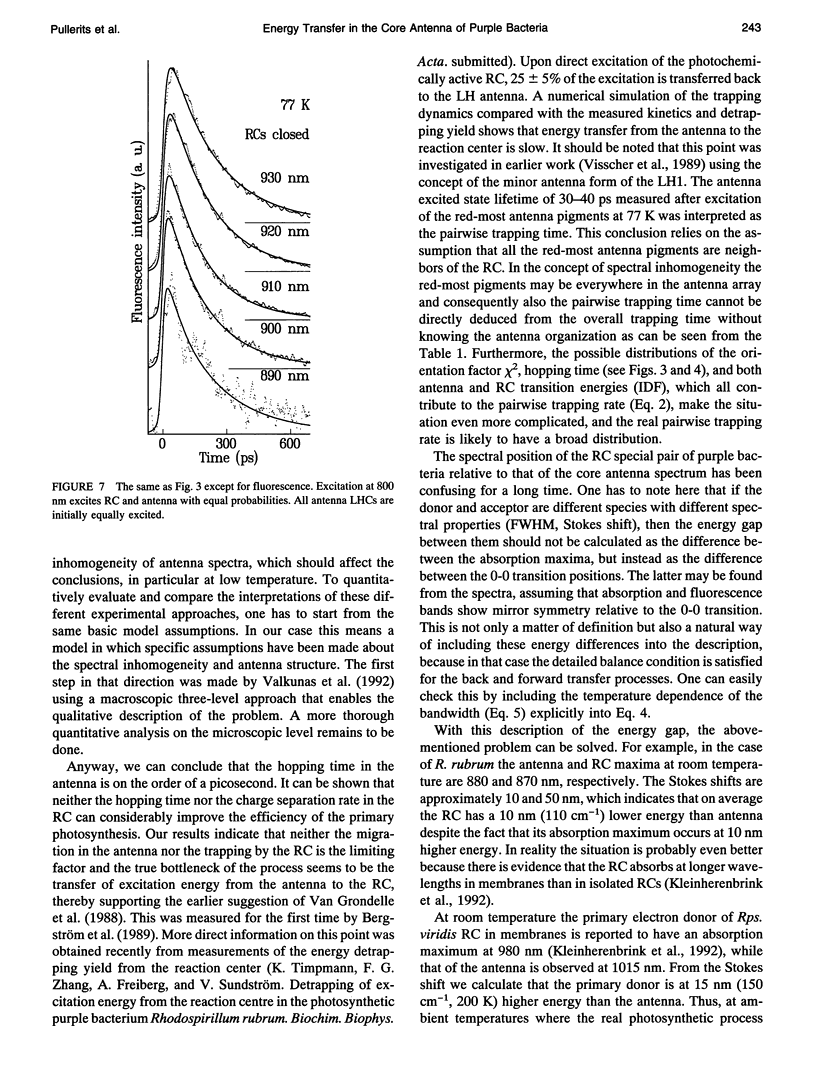
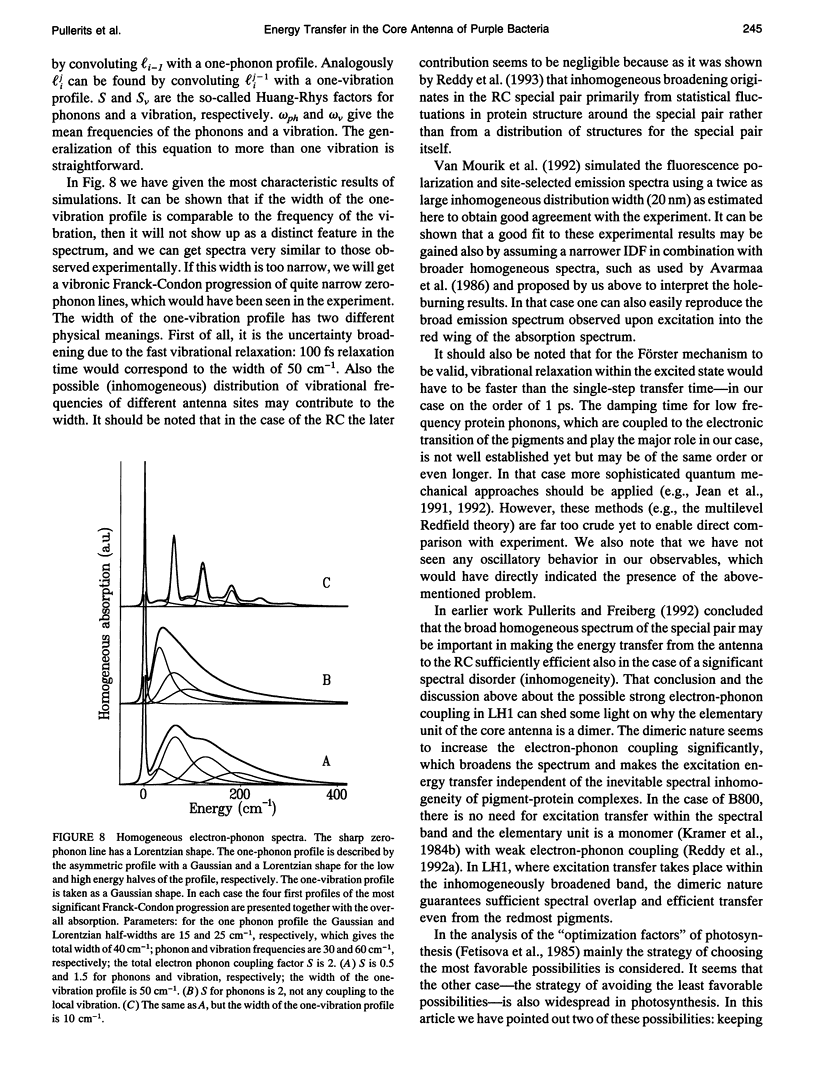
The excited state decay kinetics of chromatophores of the purple photosynthetic bacterium Rhodospirillum rubrum have been recorded at 77 K using picosecond absorption difference spectroscopy under strict annihilation free conditions. The kinetics are shown to be strongly detection wavelength dependent. A simultaneous kinetic modeling of these experiments together with earlier fluorescence kinetics by numerical integration of the appropriate master equation is performed. This model, which accounts for the spectral inhomogeneity of the core light-harvesting antenna of photosynthetic purple bacteria, reveals three qualitatively distinct stages of excitation transfer with different time scales. At first a fast transfer to a local energy minimum takes place (approximately 1 ps). This is followed by a much slower transfer between different energy minima (10-30 ps). The third component corresponds to the excitation transfer to the reaction center, which depends on its state (60 and 200 ps for open and closed, respectively) and seems also to be the bottleneck in the overall trapping time. An acceptable correspondence between theoretical and experimental decay kinetics is achieved at 77 K and at room temperature by assuming that the width of the inhomogeneous broadening is 10-15 nm and the mean residence time of the excitation in the antenna lattice site is 2-3 ps.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang M. C., Callahan P. M., Parkes-Loach P. S., Cotton T. M., Loach P. A. Spectroscopic characterization of the light-harvesting complex of Rhodospirillum rubrum and its structural subunit. Biochemistry. 1990 Jan 16;29(2):421–429. doi: 10.1021/bi00454a017. [DOI] [PubMed] [Google Scholar]
- Deinum G., Kleinherenbrink F. A., Aartsma T. J., Amesz J. The fluorescence yield of Rhodopseudomonas viridis in relation to the redox state of the primary electron donor. Biochim Biophys Acta. 1992 Jan 30;1099(1):81–84. [PubMed] [Google Scholar]
- Gingras G., Picorel R. Supramolecular arrangement of Rhodospirillum rubrum B880 holochrome as studied by radiation inactivation and electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1990 May;87(9):3405–3409. doi: 10.1073/pnas.87.9.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean J. M., Chan C. K., Fleming G. R., Owens T. G. Excitation transport and trapping on spectrally disordered lattices. Biophys J. 1989 Dec;56(6):1203–1215. doi: 10.1016/S0006-3495(89)82767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Jean J. M., Werst M. M., Chan C. K., Fleming G. R. Simulations of the temperature dependence of energy transfer in the PSI core antenna. Biophys J. 1992 Jul;63(1):259–273. doi: 10.1016/S0006-3495(92)81589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudzmauskas S., Valkunas L., Borisov A. Y. A theory of excitation transfer in photosynthetic units. J Theor Biol. 1983 Nov 7;105(1):13–23. doi: 10.1016/0022-5193(83)90421-6. [DOI] [PubMed] [Google Scholar]
- Meckenstock R. U., Brunisholz R. A., Zuber H. The light-harvesting core-complex and the B820-subunit from Rhodopseudomonas marina. Part I. Purification and characterisation. FEBS Lett. 1992 Oct 19;311(2):128–134. doi: 10.1016/0014-5793(92)81383-w. [DOI] [PubMed] [Google Scholar]
- Meckenstock R. U., Krusche K., Brunisholz R. A., Zuber H. The light-harvesting core-complex and the B820-subunit from Rhodopseudomonas marina. Part II. Electron microscopic characterisation. FEBS Lett. 1992 Oct 19;311(2):135–138. doi: 10.1016/0014-5793(92)81384-x. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Hinchigeri S. B., Parkes-Loach P. S., Callahan P. M., Sprinkle J. R., Riccobono J. R., Loach P. A. Isolation and characterization of a subunit form of the light-harvesting complex of Rhodospirillum rubrum. Biochemistry. 1987 Aug 11;26(16):5055–5062. doi: 10.1021/bi00390a026. [DOI] [PubMed] [Google Scholar]
- Ormos P., Ansari A., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Sauke T. B., Steinbach P. J., Young R. D. Inhomogeneous broadening in spectral bands of carbonmonoxymyoglobin. The connection between spectral and functional heterogeneity. Biophys J. 1990 Feb;57(2):191–199. doi: 10.1016/S0006-3495(90)82522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picorel R., L'Ecuyer A., Potier M., Gingras G. Structure of the B880 holochrome of Rhodospirillum rubrum as studied by the radiation inactivation method. J Biol Chem. 1986 Mar 5;261(7):3020–3024. [PubMed] [Google Scholar]
- Pullerits T., Freiberg A. Kinetic model of primary energy transfer and trapping in photosynthetic membranes. Biophys J. 1992 Oct;63(4):879–896. doi: 10.1016/S0006-3495(92)81688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy N. R., Kolaczkowski S. V., Small G. J. A photoinduced persistent structural transformation of the special pair of a bacterial reaction center. Science. 1993 Apr 2;260(5104):68–71. doi: 10.1126/science.260.5104.68. [DOI] [PubMed] [Google Scholar]
- Ries B, Bässler H, Grünewald M, Movaghar B. Monte Carlo study of relaxation and diffusion in glassy systems. Phys Rev B Condens Matter. 1988 Apr 1;37(10):5508–5517. doi: 10.1103/physrevb.37.5508. [DOI] [PubMed] [Google Scholar]
- Seely G. R. Effects of spectral variety and molecular orientation on energy trapping in the photosynthetic unit: a model calculation. J Theor Biol. 1973 Jul;40(1):173–187. doi: 10.1016/0022-5193(73)90170-7. [DOI] [PubMed] [Google Scholar]
- Stark W., Kühlbrandt W., Wildhaber I., Wehrli E., Mühlethaler K. The structure of the photoreceptor unit of Rhodopseudomonas viridis. EMBO J. 1984 Apr;3(4):777–783. doi: 10.1002/j.1460-2075.1984.tb01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visschers R. W., Chang M. C., van Mourik F., Parkes-Loach P. S., Heller B. A., Loach P. A., van Grondelle R. Fluorescence polarization and low-temperature absorption spectroscopy of a subunit form of light-harvesting complex I from purple photosynthetic bacteria. Biochemistry. 1991 Jun 11;30(23):5734–5742. doi: 10.1021/bi00237a015. [DOI] [PubMed] [Google Scholar]
- Zhang F. G., Gillbro T., van Grondelle R., Sundström V. Dynamics of energy transfer and trapping in the light-harvesting antenna of Rhodopseudomonas viridis. Biophys J. 1992 Mar;61(3):694–703. doi: 10.1016/S0006-3495(92)81874-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


