Abstract
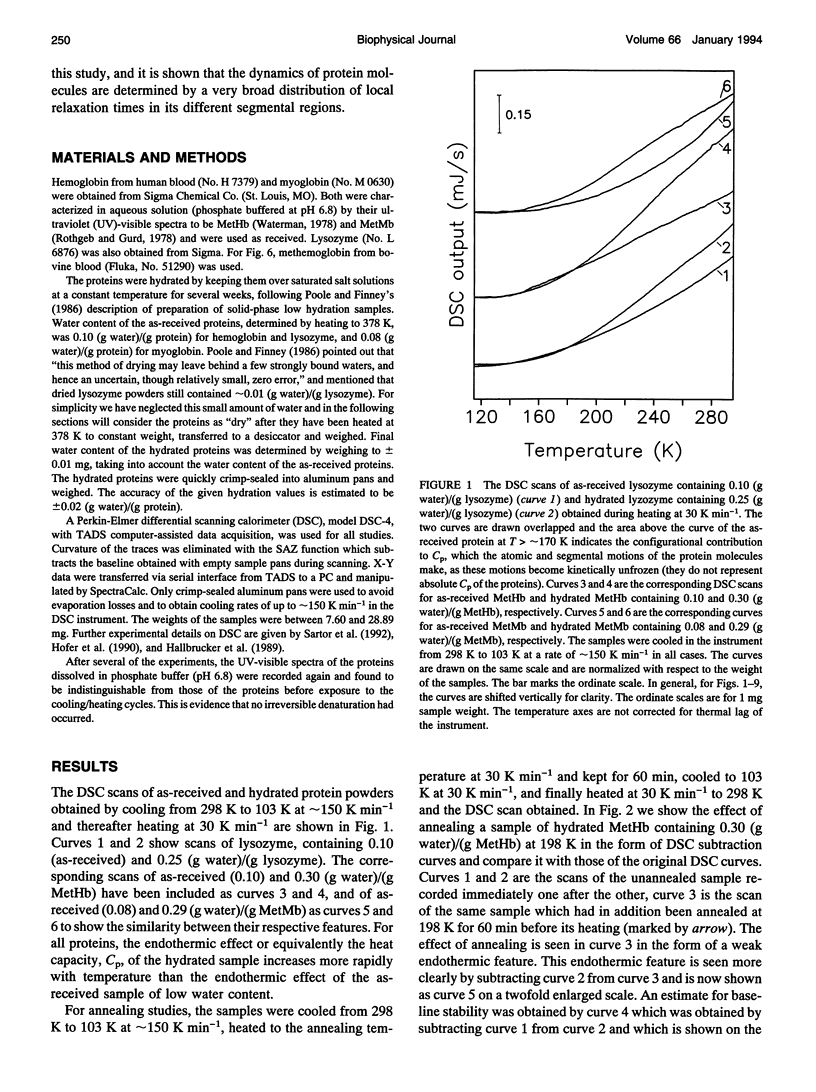
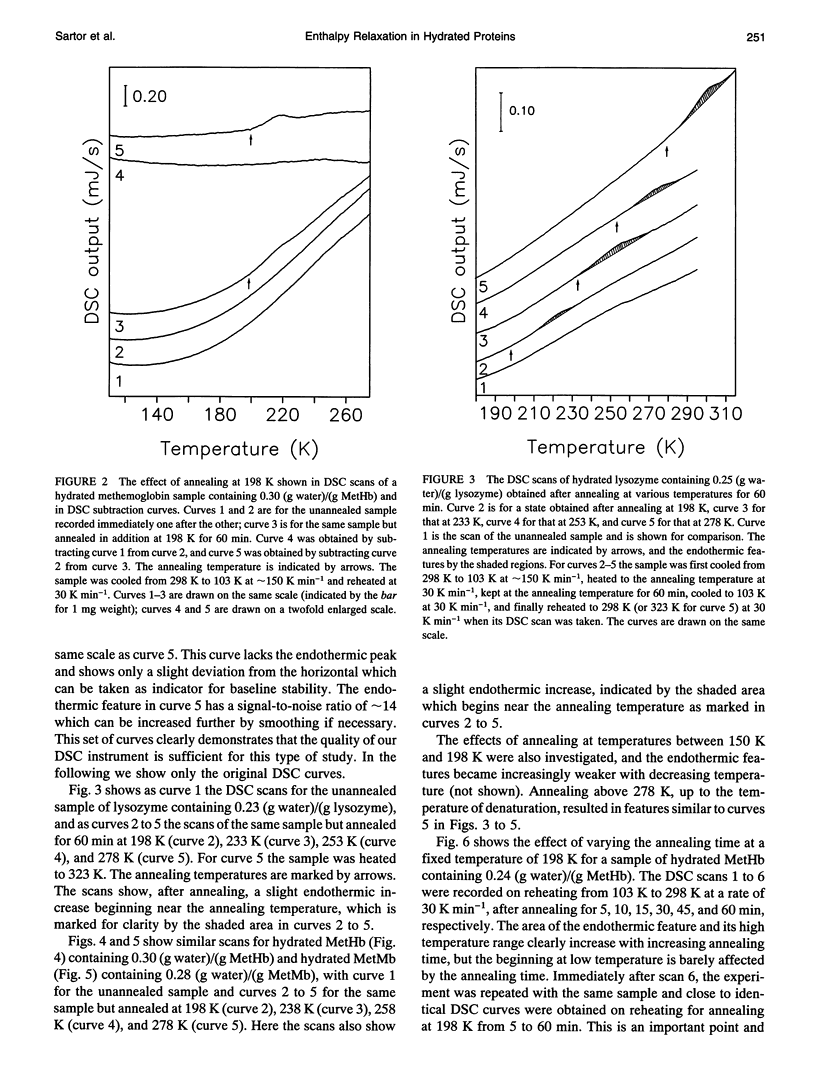
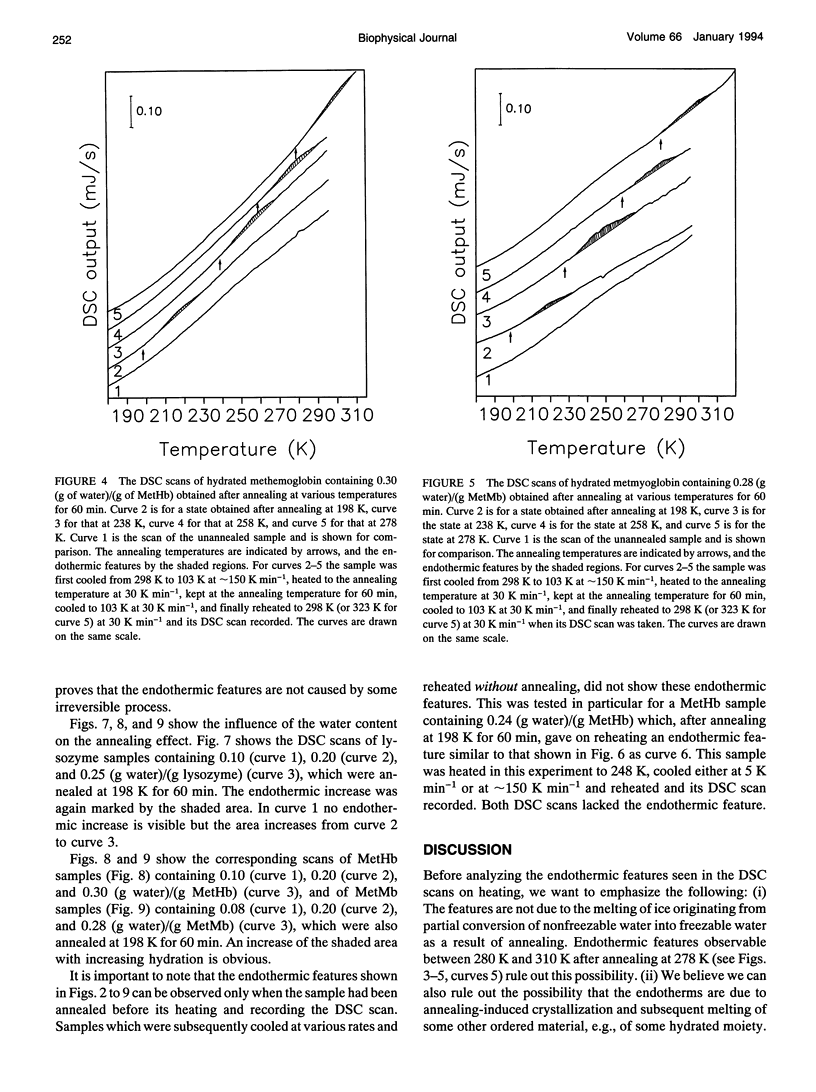
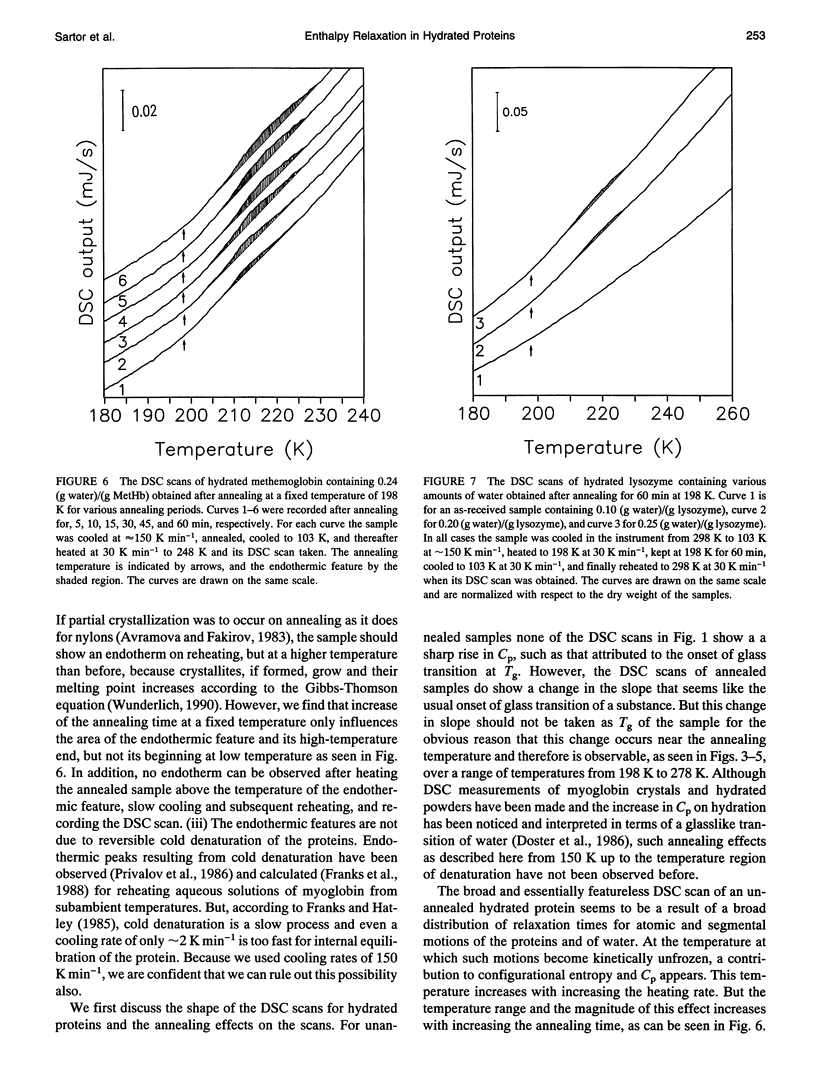
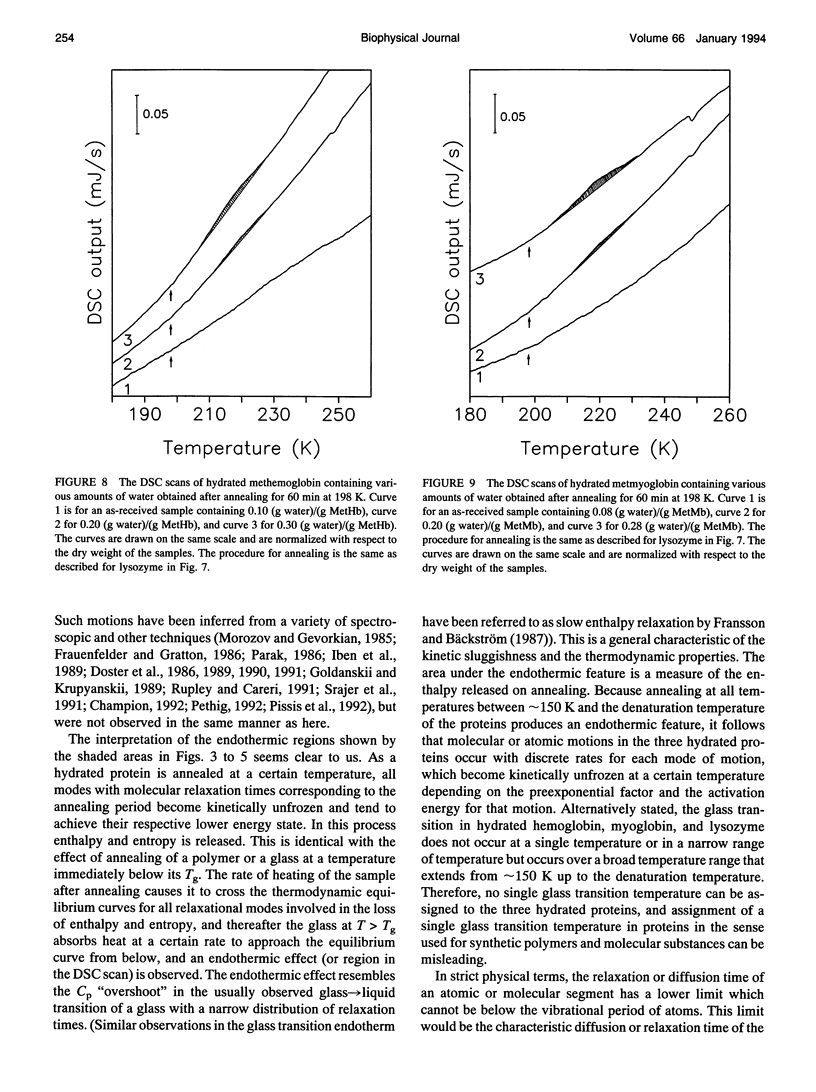
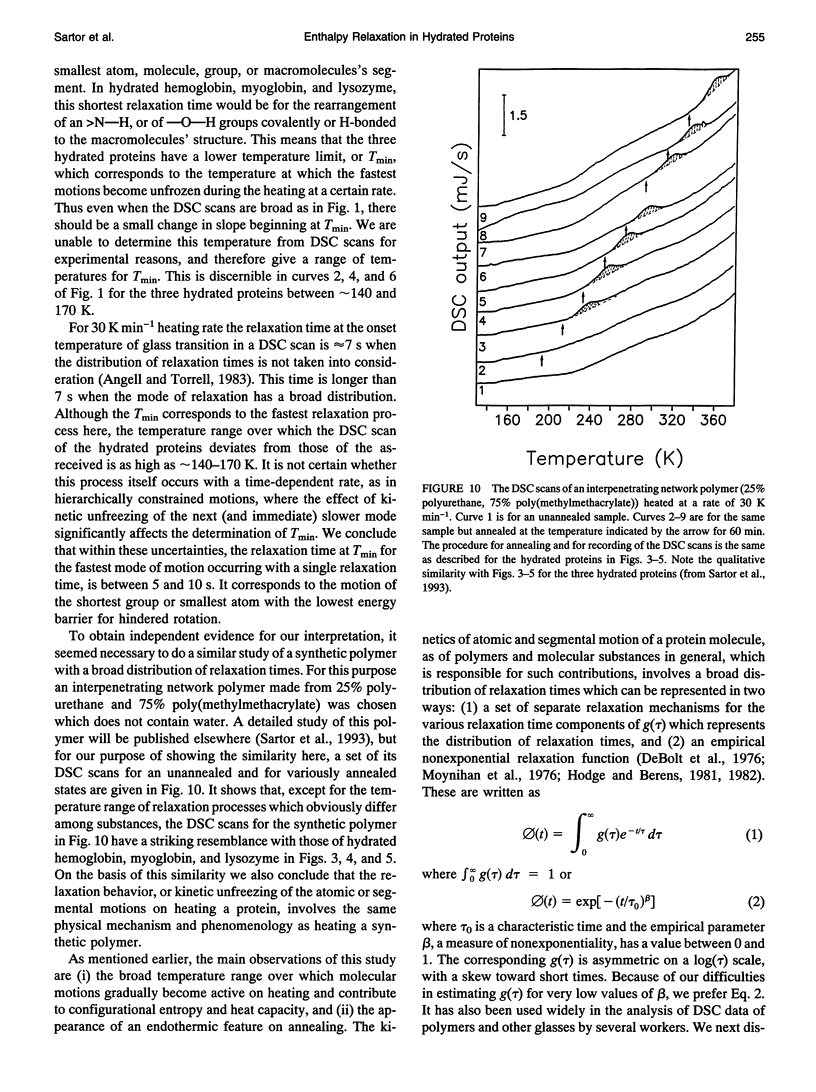
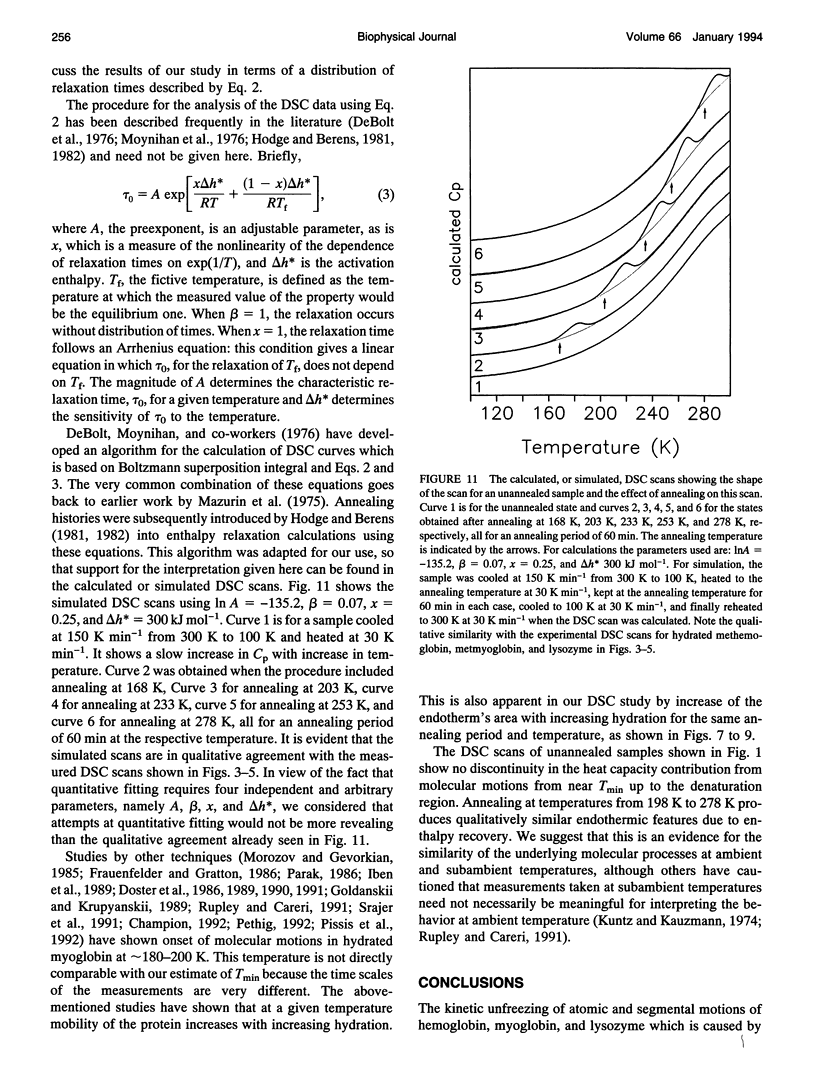
Differential scanning calorimetric (DSC) studies of the glassy states of as-received and hydrated lysozyme, hemoglobin, and myoglobin powders, with water contents of < or = 0.25, < or = 0.30, and < or = 0.29 g/g of protein, show that their heat capacity slowly increases with increasing temperature, without showing an abrupt increase characteristic of glass-->liquid transition. Annealing (also referred to as physical aging) of the hydrated proteins causes their DSC scans to show an endothermic region, similar to an overshoot, immediately above the annealing temperature. This annealing effect appears at all temperatures between approximately 150 and 300 K. The area under these peaks increases with increasing annealing time at a fixed temperature. The effects are attributed to the presence of a large number of local structures in which macromolecular segments diffuse at different time scales over a broad range. The lowest time scale corresponds to the > N-H and -O-H group motions which become kinetically unfrozen at approximately 150-170 K on heating at a rate of 30 K min-1 and which have a relaxation time of 5-10 s in this temperature range. The annealing effects confirm that the individual glass transition of the relaxing local regions is spread over a temperature range up to the denaturation temperature region of the proteins. The interpretation is supported by simulation of DSC scans in which the distribution of relaxation times is assumed to be exceptionally broad and in which annealing done at several temperatures over a wide range produces endothermic effects (or regions of DSC scans) qualitatively similar to those observed for the hydrated proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooke R., Kuntz I. D. The properties of water in biological systems. Annu Rev Biophys Bioeng. 1974;3(0):95–126. doi: 10.1146/annurev.bb.03.060174.000523. [DOI] [PubMed] [Google Scholar]
- Doster W., Bachleitner A., Dunau R., Hiebl M., Lüscher E. Thermal properties of water in myoglobin crystals and solutions at subzero temperatures. Biophys J. 1986 Aug;50(2):213–219. doi: 10.1016/S0006-3495(86)83455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Doster W, Cusack S, Petry W. Dynamic instability of liquidlike motions in a globular protein observed by inelastic neutron scattering. Phys Rev Lett. 1990 Aug 20;65(8):1080–1083. doi: 10.1103/PhysRevLett.65.1080. [DOI] [PubMed] [Google Scholar]
- Franks F., Hatley R. H., Friedman H. L. The thermodynamics of protein stability. Cold destabilization as a general phenomenon. Biophys Chem. 1988 Sep;31(3):307–315. doi: 10.1016/0301-4622(88)80037-1. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Gratton E. Protein dynamics and hydration. Methods Enzymol. 1986;127:207–216. doi: 10.1016/0076-6879(86)27017-2. [DOI] [PubMed] [Google Scholar]
- Goldanskii V. I., Krupyanskii Y. F. Protein and protein-bound water dynamics studied by Rayleigh scattering of Mössbauer radiation (RSMR). Q Rev Biophys. 1989 Feb;22(1):39–92. doi: 10.1017/s003358350000336x. [DOI] [PubMed] [Google Scholar]
- Iben IE, Braunstein D, Doster W, Frauenfelder H, Hong MK, Johnson JB, Luck S, Ormos P, Schulte A, Steinbach PJ. Glassy behavior of a protein. Phys Rev Lett. 1989 Apr 17;62(16):1916–1919. doi: 10.1103/PhysRevLett.62.1916. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Parak F. Correlation of protein dynamics with water mobility: Mössbauer spectroscopy and microwave absorption methods. Methods Enzymol. 1986;127:196–206. doi: 10.1016/0076-6879(86)27016-0. [DOI] [PubMed] [Google Scholar]
- Pethig R. Protein-water interactions determined by dielectric methods. Annu Rev Phys Chem. 1992;43:177–205. doi: 10.1146/annurev.pc.43.100192.001141. [DOI] [PubMed] [Google Scholar]
- Poole P. L., Finney J. L. Solid-phase protein hydration studies. Methods Enzymol. 1986;127:284–293. doi: 10.1016/0076-6879(86)27023-8. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Griko YuV, Venyaminov SYu, Kutyshenko V. P. Cold denaturation of myoglobin. J Mol Biol. 1986 Aug 5;190(3):487–498. doi: 10.1016/0022-2836(86)90017-3. [DOI] [PubMed] [Google Scholar]
- Rothgeb T. M., Gurd F. R. Physical methods for the study of myoglobin. Methods Enzymol. 1978;52:473–486. doi: 10.1016/s0076-6879(78)52052-1. [DOI] [PubMed] [Google Scholar]
- Rupley J. A., Careri G. Protein hydration and function. Adv Protein Chem. 1991;41:37–172. doi: 10.1016/s0065-3233(08)60197-7. [DOI] [PubMed] [Google Scholar]
- Srajer V., Reinisch L., Champion P. M. Investigation of laser-induced long-lived states of photolyzed MbCO. Biochemistry. 1991 May 21;30(20):4886–4895. doi: 10.1021/bi00234a008. [DOI] [PubMed] [Google Scholar]
- VAN DEN BERG L., ROSE D. Effect of freezing on the pH and composition of sodium and potassium phosphate solutions; the reciprocal system KH2PO4-Na2-HPO4-H2O. Arch Biochem Biophys. 1959 Apr;81(2):319–329. doi: 10.1016/0003-9861(59)90209-7. [DOI] [PubMed] [Google Scholar]
- Waterman M. R. Spectral characterization of human hemoglobin and its derivatives. Methods Enzymol. 1978;52:456–463. doi: 10.1016/s0076-6879(78)52050-8. [DOI] [PubMed] [Google Scholar]


