Abstract
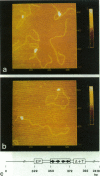
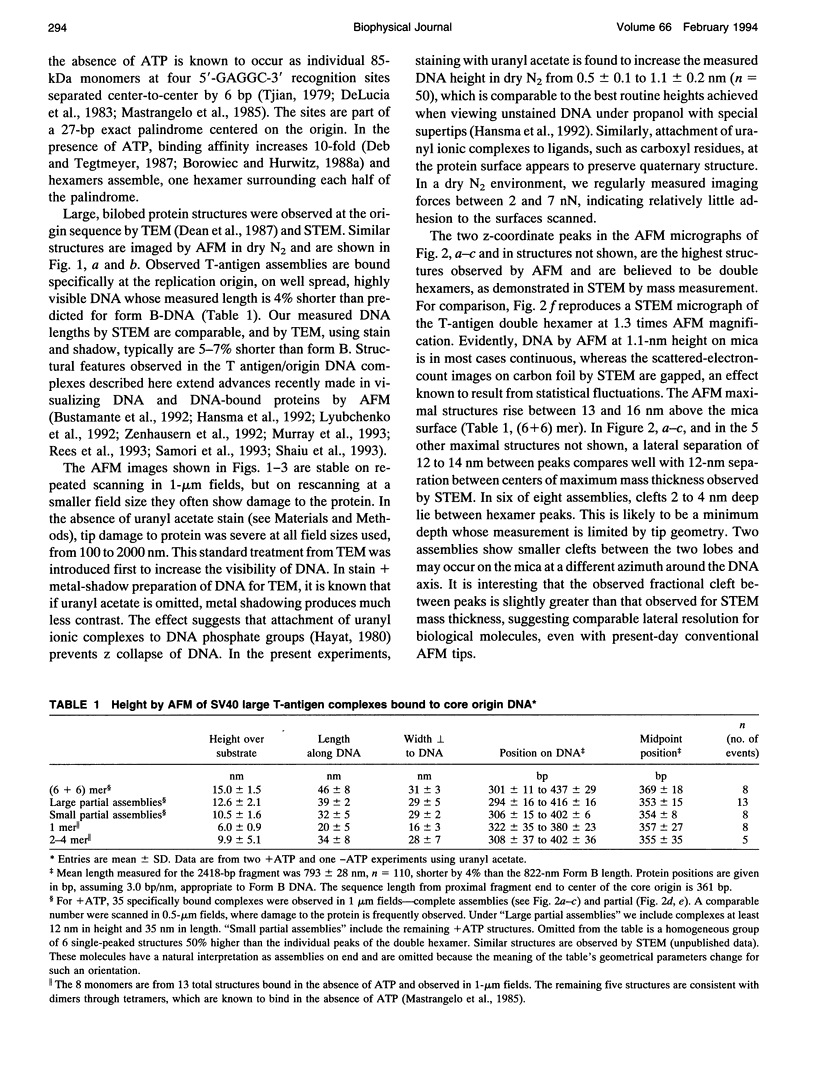
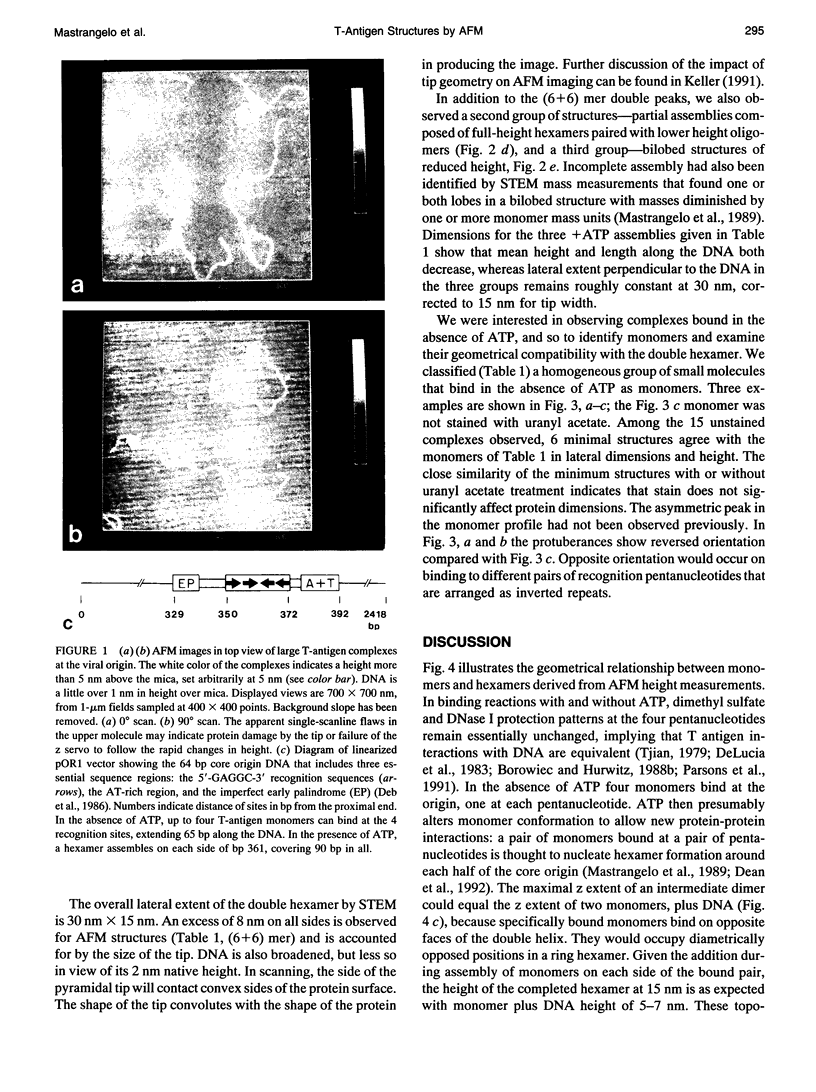
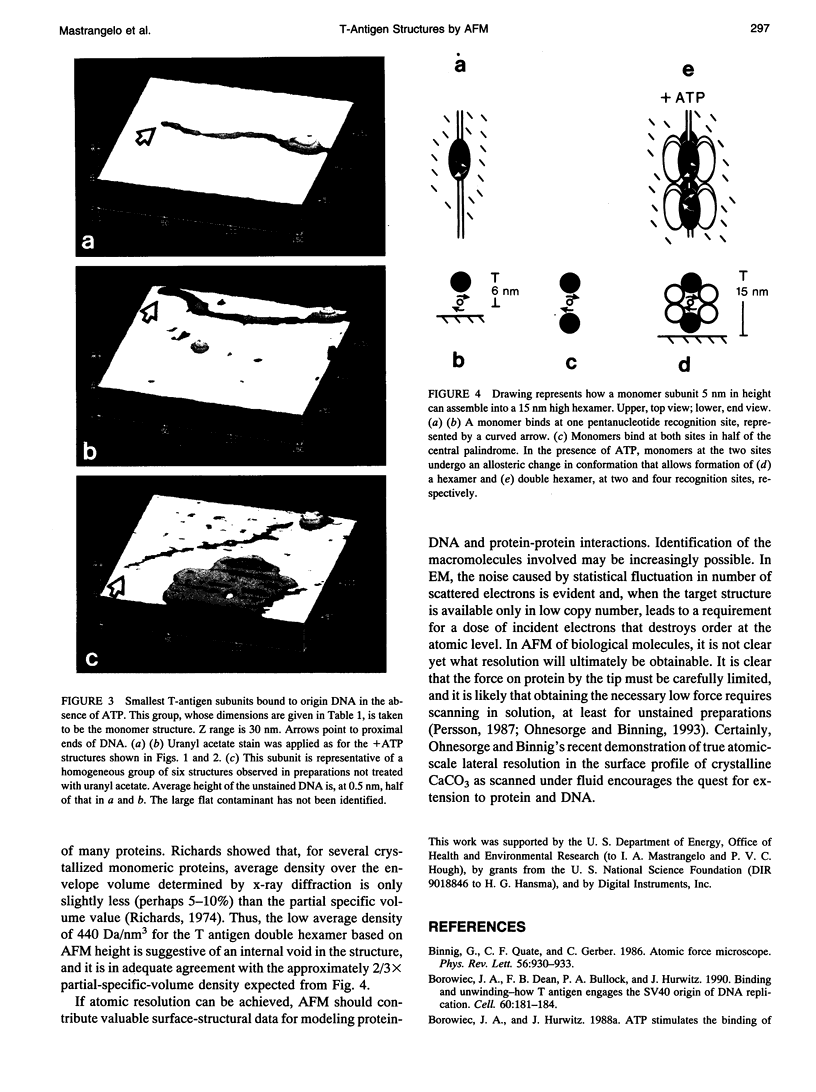
For inorganic crystals such as calcite (CaCO3), Atomic Force Microscopy (AFM) has provided surface structure at atomic resolution (Ohnesorge and Binnig, 1993). As part of a broad effort to obtain high resolution for an individual protein or protein assembly (Binnig et al., 1986; Rugar and Hansma, 1990; Radmacher et al., 1992), we applied AFM to study the ATP-dependent double hexamer of SV40 large T antigen, which assembles around the viral origin of DNA replication. Multimeric mass has been determined in two-dimensional projected images by Scanning Transmission Electron Microscopy (STEM) (Mastrangelo et al., 1989). By AFM, if the DNA-protein preparation has been stained positively by uranyl acetate, the contour at the junction between hexamers is visible as a cleft, 2-4 nm deep. The cleft, whether determined as a fraction of height by AFM or as a fraction of mass thickness by STEM, is of comparable magnitude. On either side of the cleft, hexamers attain a maximum height of 13-16 nm. Monomers found in the absence of ATP show heights of 5-7 nm. Taken together, the z coordinates provide a surface profile of complete and partial replication assemblies consistent with the spatial distribution of recognition pentanucleotides on the DNA, and they contribute direct geometrical evidence for a ring-like hexamer structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Dean F. B., Bullock P. A., Hurwitz J. Binding and unwinding--how T antigen engages the SV40 origin of DNA replication. Cell. 1990 Jan 26;60(2):181–184. doi: 10.1016/0092-8674(90)90730-3. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Hurwitz J. ATP stimulates the binding of simian virus 40 (SV40) large tumor antigen to the SV40 origin of replication. Proc Natl Acad Sci U S A. 1988 Jan;85(1):64–68. doi: 10.1073/pnas.85.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Hurwitz J. Localized melting and structural changes in the SV40 origin of replication induced by T-antigen. EMBO J. 1988 Oct;7(10):3149–3158. doi: 10.1002/j.1460-2075.1988.tb03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante C., Vesenka J., Tang C. L., Rees W., Guthold M., Keller R. Circular DNA molecules imaged in air by scanning force microscopy. Biochemistry. 1992 Jan 14;31(1):22–26. doi: 10.1021/bi00116a005. [DOI] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean F. B., Borowiec J. A., Eki T., Hurwitz J. The simian virus 40 T antigen double hexamer assembles around the DNA at the replication origin. J Biol Chem. 1992 Jul 15;267(20):14129–14137. [PubMed] [Google Scholar]
- Dean F. B., Dodson M., Echols H., Hurwitz J. ATP-dependent formation of a specialized nucleoprotein structure by simian virus 40 (SV40) large tumor antigen at the SV40 replication origin. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8981–8985. doi: 10.1073/pnas.84.24.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S. P., Tegtmeyer P. ATP enhances the binding of simian virus 40 large T antigen to the origin of replication. J Virol. 1987 Dec;61(12):3649–3654. doi: 10.1128/jvi.61.12.3649-3654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., DeLucia A. L., Baur C. P., Koff A., Tegtmeyer P. Domain structure of the simian virus 40 core origin of replication. Mol Cell Biol. 1986 May;6(5):1663–1670. doi: 10.1128/mcb.6.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Vesenka J., Siegerist C., Kelderman G., Morrett H., Sinsheimer R. L., Elings V., Bustamante C., Hansma P. K. Reproducible imaging and dissection of plasmid DNA under liquid with the atomic force microscope. Science. 1992 May 22;256(5060):1180–1184. doi: 10.1126/science.256.5060.1180. [DOI] [PubMed] [Google Scholar]
- Lyubchenko Y. L., Jacobs B. L., Lindsay S. M. Atomic force microscopy of reovirus dsRNA: a routine technique for length measurements. Nucleic Acids Res. 1992 Aug 11;20(15):3983–3986. doi: 10.1093/nar/20.15.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo I. A., Hough P. V., Wall J. S., Dodson M., Dean F. B., Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature. 1989 Apr 20;338(6217):658–662. doi: 10.1038/338658a0. [DOI] [PubMed] [Google Scholar]
- Mastrangelo I. A., Hough P. V., Wilson V. G., Wall J. S., Hainfeld J. F., Tegtmeyer P. Monomers through trimers of large tumor antigen bind in region I and monomers through tetramers bind in region II of simian virus 40 origin of replication DNA as stable structures in solution. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3626–3630. doi: 10.1073/pnas.82.11.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. N., Hansma H. G., Bezanilla M., Sano T., Ogletree D. F., Kolbe W., Smith C. L., Cantor C. R., Spengler S., Hansma P. K. Atomic force microscopy of biochemically tagged DNA. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3811–3814. doi: 10.1073/pnas.90.9.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnesorge F., Binnig G. True atomic resolution by atomic force microscopy through repulsive and attractive forces. Science. 1993 Jun 4;260(5113):1451–1456. doi: 10.1126/science.260.5113.1451. [DOI] [PubMed] [Google Scholar]
- Parsons R. E., Stenger J. E., Ray S., Welker R., Anderson M. E., Tegtmeyer P. Cooperative assembly of simian virus 40 T-antigen hexamers on functional halves of the replication origin. J Virol. 1991 Jun;65(6):2798–2806. doi: 10.1128/jvi.65.6.2798-2806.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmacher M., Tillamnn R. W., Fritz M., Gaub H. E. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 1992 Sep 25;257(5078):1900–1905. doi: 10.1126/science.1411505. [DOI] [PubMed] [Google Scholar]
- Rees W. A., Keller R. W., Vesenka J. P., Yang G., Bustamante C. Evidence of DNA bending in transcription complexes imaged by scanning force microscopy. Science. 1993 Jun 11;260(5114):1646–1649. doi: 10.1126/science.8503010. [DOI] [PubMed] [Google Scholar]
- Richards F. M. The interpretation of protein structures: total volume, group volume distributions and packing density. J Mol Biol. 1974 Jan 5;82(1):1–14. doi: 10.1016/0022-2836(74)90570-1. [DOI] [PubMed] [Google Scholar]
- Samorí B., Siligardi G., Quagliariello C., Weisenhorn A. L., Vesenka J., Bustamante C. J. Chirality of DNA supercoiling assigned by scanning force microscopy. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3598–3601. doi: 10.1073/pnas.90.8.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaiu W. L., Larson D. D., Vesenka J., Henderson E. Atomic force microscopy of oriented linear DNA molecules labeled with 5nm gold spheres. Nucleic Acids Res. 1993 Jan 11;21(1):99–103. doi: 10.1093/nar/21.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. Protein-DNA interactions at the origin of simian virus 40 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):655–661. doi: 10.1101/sqb.1979.043.01.073. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Tumor suppressor genes. Science. 1991 Nov 22;254(5035):1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- Zenhausern F., Adrian M., ten Heggeler-Bordier B., Emch R., Jobin M., Taborelli M., Descouts P. Imaging of DNA by scanning force microscopy. J Struct Biol. 1992 Jan-Feb;108(1):69–73. doi: 10.1016/1047-8477(92)90008-x. [DOI] [PubMed] [Google Scholar]