Abstract
Bovine brain phosphatidylserine (BBPS) vesicles were prepared with traces of dioleoylglycerol (18:1, 18:1 DAG) or hexadecane (HD) to determine the influence of changes in headgroup or acyl chain packing on divalent cation-induced lipid mixing rates. A stopped-flow apparatus was used to combine vesicles with 3 mM Ca2+ or Ba2+. Aggregation was monitored by light scattering and lipid mixing by lipid probe dilution. Neither 3-6 mol% 18:1, 18:1 DAG nor up to 10 mol % HD significantly altered the BBPS chain melting temperature, vesicle diameter, or vesicle aggregation rates. Lipid mixing rates doubled by adding either 3 mol % 18:1, 18:1 DAG or 6 mol % HD to BBPS with no change in the Ca2+ concentration threshold. The Arrhenius slopes of the lipid mixing rates for control, 3 mol % 18:1, 18:1 DAG, and 6 mol % HD vesicles were identical. 2H-nuclear magnetic resonance spectra of perdeuterated dipalmitoylglycerol and HD in BBPS in the absence and presence of Ca2+ and Ba2+ showed that the solutes occupied different time-averaged positions in the bilayer under each condition. These data suggest that: 1) the enhanced lipid mixing rate is related to the volume of the added alkyl chains; 2) 18:1, 18:1 DAG and HD may alter the activation entropy or the attempt frequency at one or more steps in the lipid mixing process; 3) 18:1, 18:1 DAG and HD are likely to act at a different spatial or temporal point than the divalent cation; and 4) it is unlikely that the effect of these solutes on lipid mixing is due to their equilibrium time-averaged positions in the bilayer. Others have shown that apolar lipids accelerate fusion in nonbilayer phase-forming systems, but BBPS does not form these phases under these conditions. Therefore, we propose that the effect of very small amounts of apolar substances may be very general, e.g., stabilizing the hydrophobic interstices associated with a variety of proposed intermediate structures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
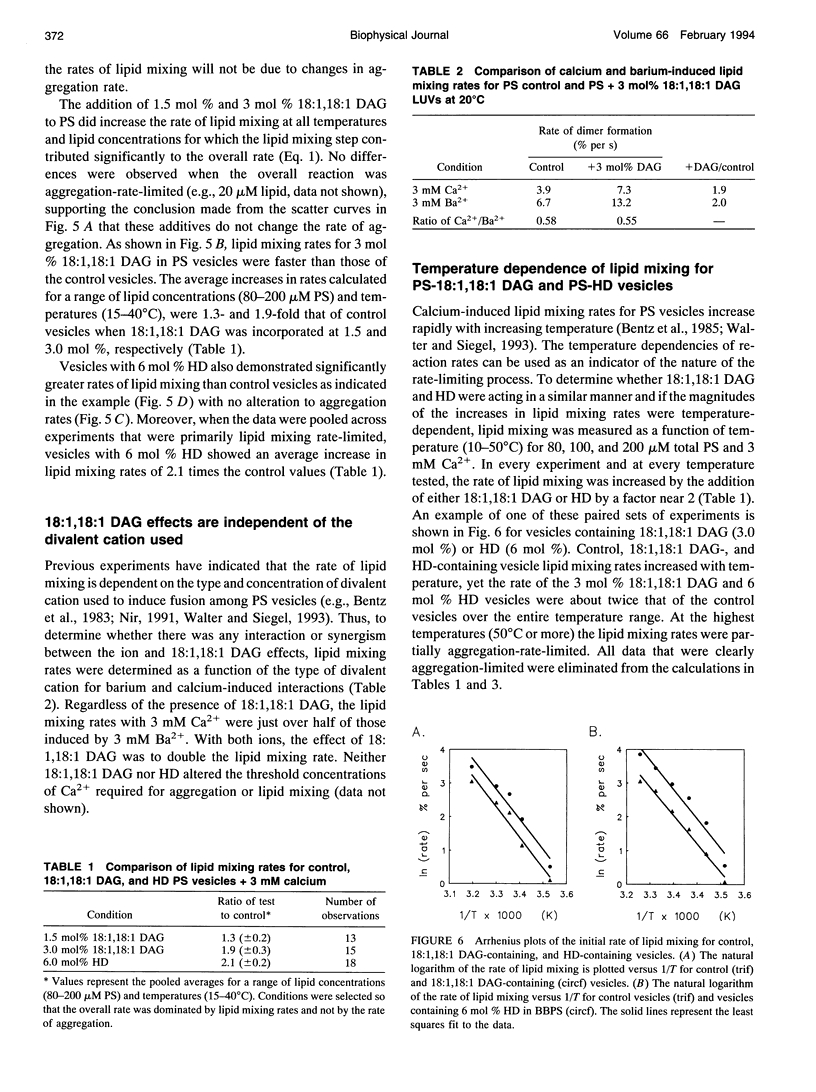
- Bentz J., Düzgüneş N., Nir S. Temperature dependence of divalent cation induced fusion of phosphatidylserine liposomes: evaluation of the kinetic rate constants. Biochemistry. 1985 Feb 12;24(4):1064–1072. doi: 10.1021/bi00325a039. [DOI] [PubMed] [Google Scholar]
- Bentz J., Ellens H., Alford D. An architecture for the fusion site of influenza hemagglutinin. FEBS Lett. 1990 Dec 10;276(1-2):1–5. doi: 10.1016/0014-5793(90)80492-2. [DOI] [PubMed] [Google Scholar]
- Bentz J. Intermediates and kinetics of membrane fusion. Biophys J. 1992 Aug;63(2):448–459. doi: 10.1016/S0006-3495(92)81622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal H. L., Martin A., Mantsch H. H., Paltauf F., Hauser H. Infrared studies of fully hydrated unsaturated phosphatidylserine bilayers. Effect of Li+ and Ca2+. Biochemistry. 1987 Nov 17;26(23):7395–7401. doi: 10.1021/bi00397a030. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Tsujita T., Brockman H. L. Enzymatic and physical characterization of diacylglycerol-phosphatidylcholine interactions in bilayers and monolayers. Biochemistry. 1989 Jan 10;28(1):32–40. doi: 10.1021/bi00427a006. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. H+- and Ca2+-induced fusion and destabilization of liposomes. Biochemistry. 1985 Jun 18;24(13):3099–3106. doi: 10.1021/bi00334a005. [DOI] [PubMed] [Google Scholar]
- Gruner S. M. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Bhamidipati S. P., Kodali D. R., Small D. M. The interfacial conformation and transbilayer movement of diacylglycerols in phospholipid bilayers. J Biol Chem. 1991 Jan 15;266(2):1177–1186. [PubMed] [Google Scholar]
- Hauser H., Shipley G. G. Interactions of divalent cations with phosphatidylserine bilayer membranes. Biochemistry. 1984 Jan 3;23(1):34–41. doi: 10.1021/bi00296a006. [DOI] [PubMed] [Google Scholar]
- Markin V. S., Kozlov M. M., Borovjagin V. L. On the theory of membrane fusion. The stalk mechanism. Gen Physiol Biophys. 1984 Oct;3(5):361–377. [PubMed] [Google Scholar]
- McIntosh T. J., Magid A. D., Simon S. A. Range of the solvation pressure between lipid membranes: dependence on the packing density of solvent molecules. Biochemistry. 1989 Sep 19;28(19):7904–7912. doi: 10.1021/bi00445a053. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., MacDonald R. C. The organization of n-alkanes in lipid bilayers. Biochim Biophys Acta. 1980 Apr 24;597(3):445–463. doi: 10.1016/0005-2736(80)90219-9. [DOI] [PubMed] [Google Scholar]
- Nieva J. L., Goñi F. M., Alonso A. Phospholipase C-promoted membrane fusion. Retroinhibition by the end-product diacylglycerol. Biochemistry. 1993 Feb 2;32(4):1054–1058. doi: 10.1021/bi00055a009. [DOI] [PubMed] [Google Scholar]
- Ohki S., Arnold K. Surface dielectric constant, surface hydrophobicity and membrane fusion. J Membr Biol. 1990 Apr;114(3):195–203. doi: 10.1007/BF01869214. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Aranda F. J., Villalaín J., San Martín C., Micol V., Gómez-Fernandez J. C. 1,2-Dioleoylglycerol promotes calcium-induced fusion in phospholipid vesicles. Chem Phys Lipids. 1992 Oct;62(3):215–224. doi: 10.1016/0009-3084(92)90058-w. [DOI] [PubMed] [Google Scholar]
- Pope J. M., Littlemore L. A., Westerman P. W. Chain-length dependence of n-alkane solubility in phosphatidylcholine bilayers: a 2H-NMR study. Biochim Biophys Acta. 1989 Mar 27;980(1):69–76. doi: 10.1016/0005-2736(89)90201-0. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate mechanisms. Biophys J. 1993 Nov;65(5):2124–2140. doi: 10.1016/S0006-3495(93)81256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. II. Implications for membrane-membrane interactions and membrane fusion. Biophys J. 1986 Jun;49(6):1171–1183. doi: 10.1016/S0006-3495(86)83745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölund M., Rilfors L., Lindblom G. Reversed hexagonal phase formation in lecithin-alkane-water systems with different acyl chain unsaturation and alkane length. Biochemistry. 1989 Feb 7;28(3):1323–1329. doi: 10.1021/bi00429a057. [DOI] [PubMed] [Google Scholar]
- Tate M. W., Gruner S. M. Lipid polymorphism of mixtures of dioleoylphosphatidylethanolamine and saturated and monounsaturated phosphatidylcholines of various chain lengths. Biochemistry. 1987 Jan 13;26(1):231–236. doi: 10.1021/bi00375a031. [DOI] [PubMed] [Google Scholar]
- Walter A., Siegel D. P. Divalent cation-induced lipid mixing between phosphatidylserine liposomes studied by stopped-flow fluorescence measurements: effects of temperature, comparison of barium and calcium, and perturbation by DPX. Biochemistry. 1993 Apr 6;32(13):3271–3281. doi: 10.1021/bi00064a009. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry. 1980 Dec 23;19(26):6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Curran M., Cohen F. S. A lipid/protein complex hypothesis for exocytotic fusion pore formation. Ann N Y Acad Sci. 1991;635:307–317. doi: 10.1111/j.1749-6632.1991.tb36501.x. [DOI] [PubMed] [Google Scholar]
- de Kroon A. I., Timmermans J. W., Killian J. A., de Kruijff B. The pH dependence of headgroup and acyl chain structure and dynamics of phosphatidylserine, studied by 2H-NMR. Chem Phys Lipids. 1990 Apr;54(1):33–42. doi: 10.1016/0009-3084(90)90057-x. [DOI] [PubMed] [Google Scholar]


