Abstract
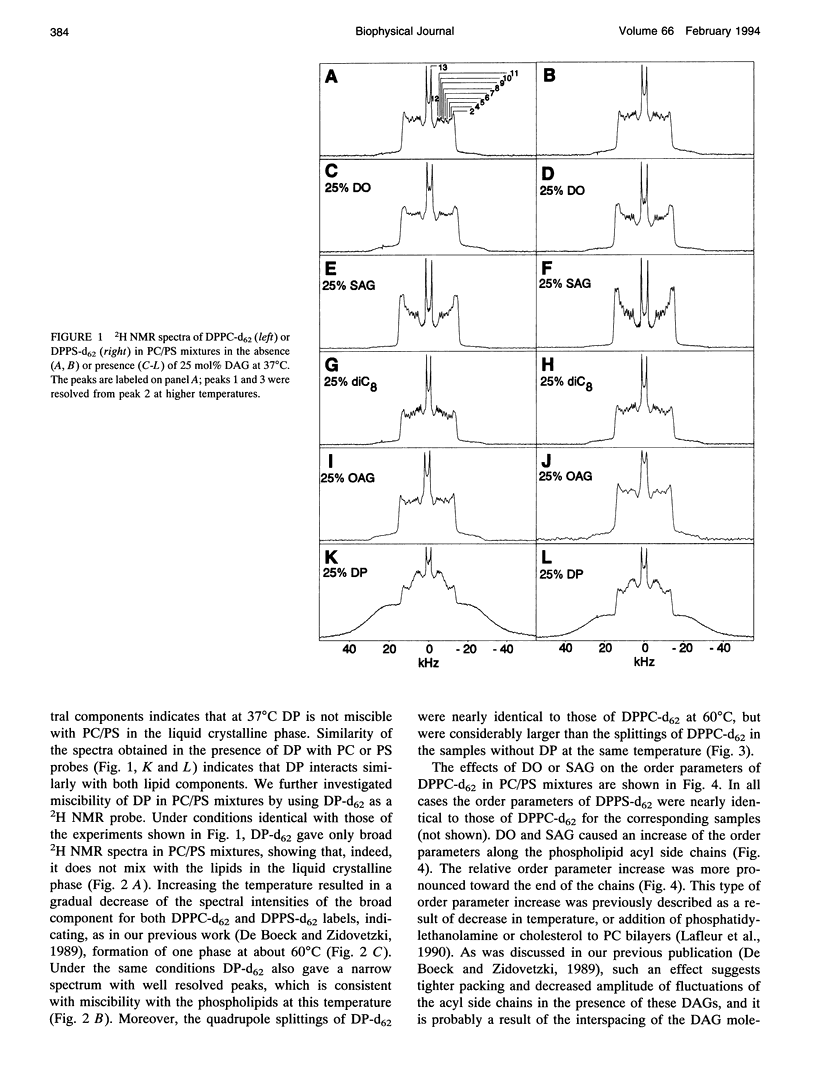
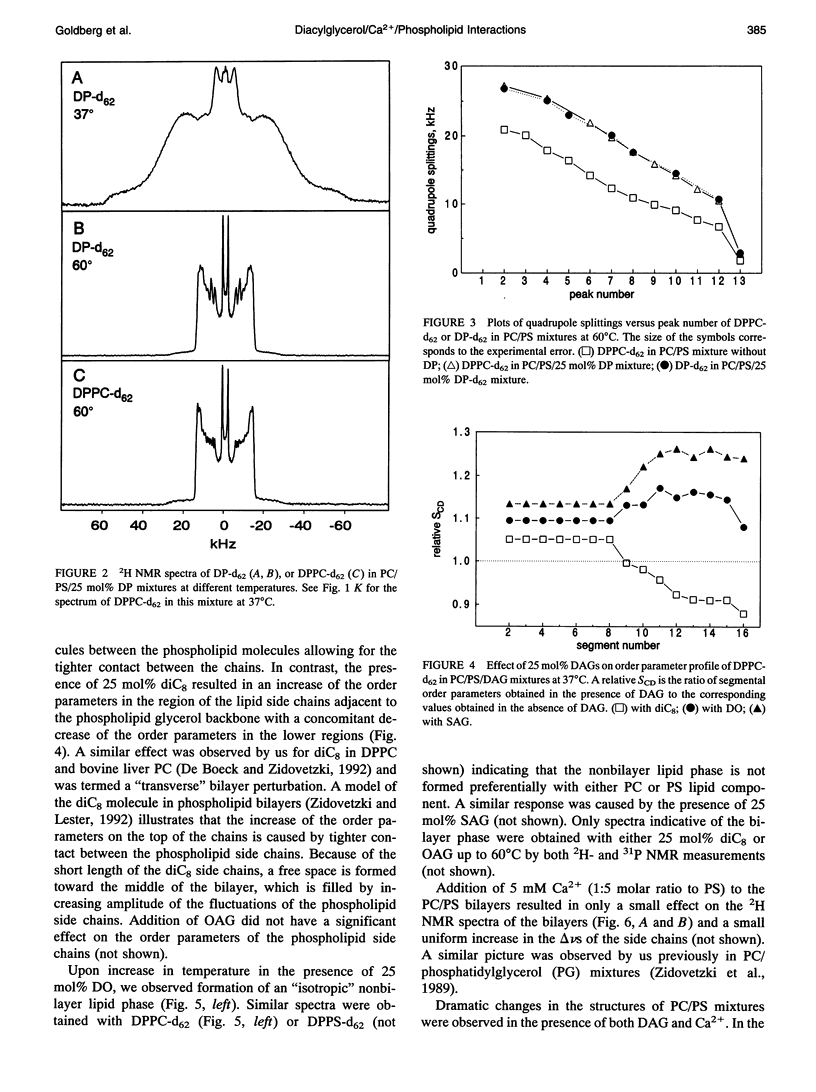
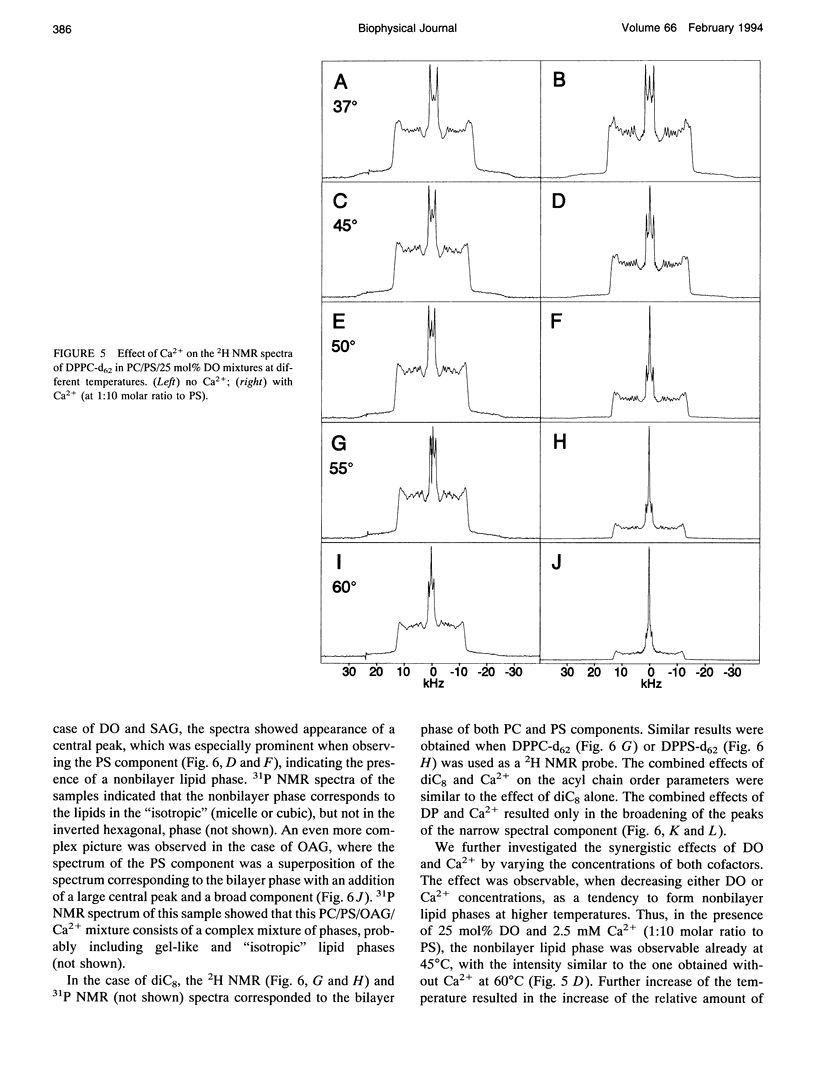
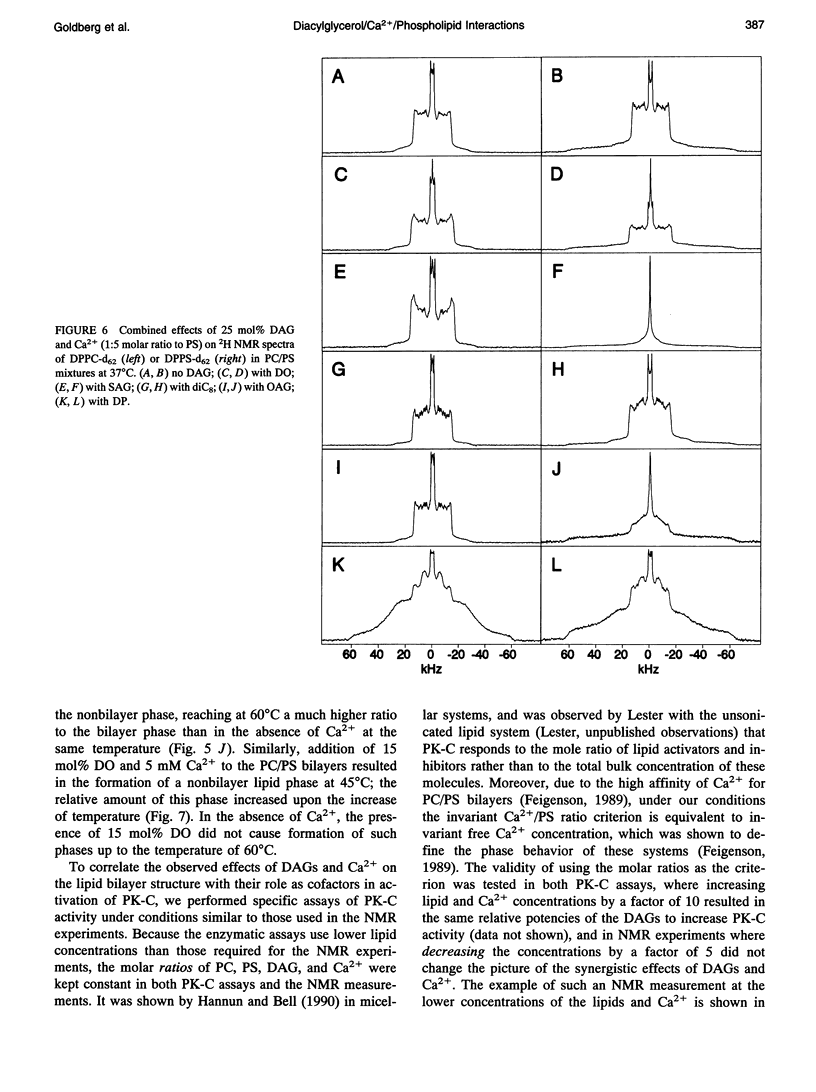
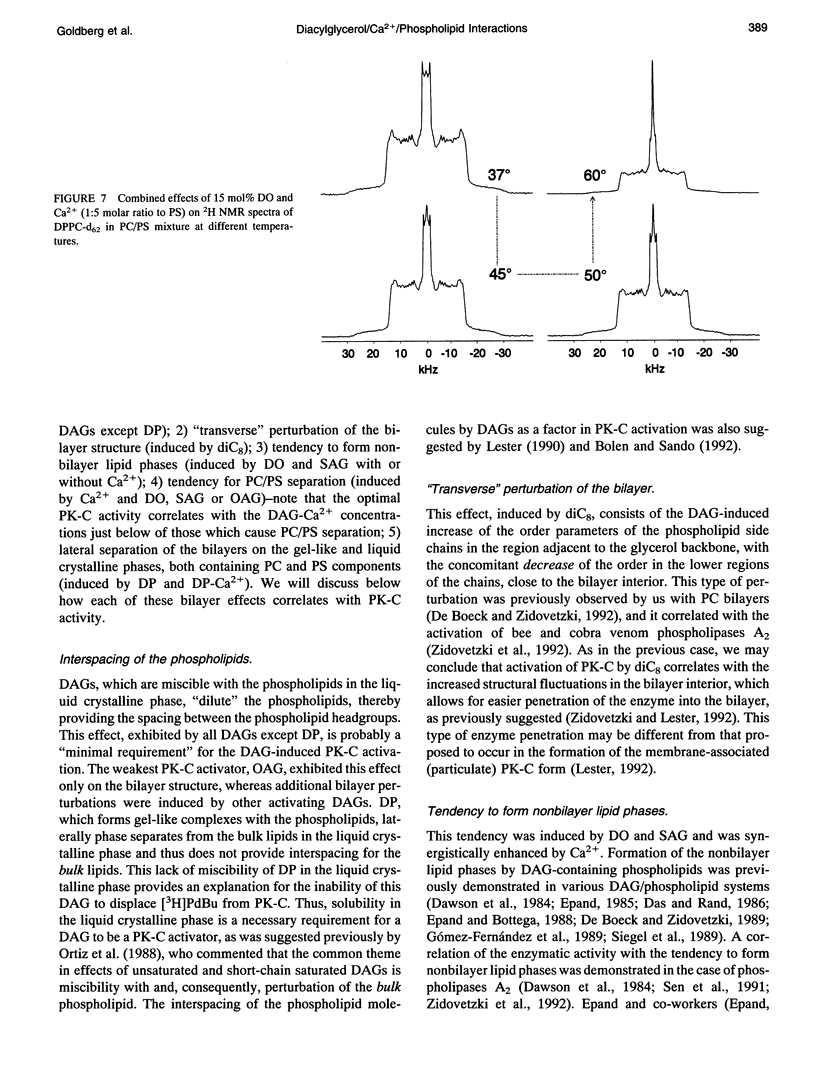
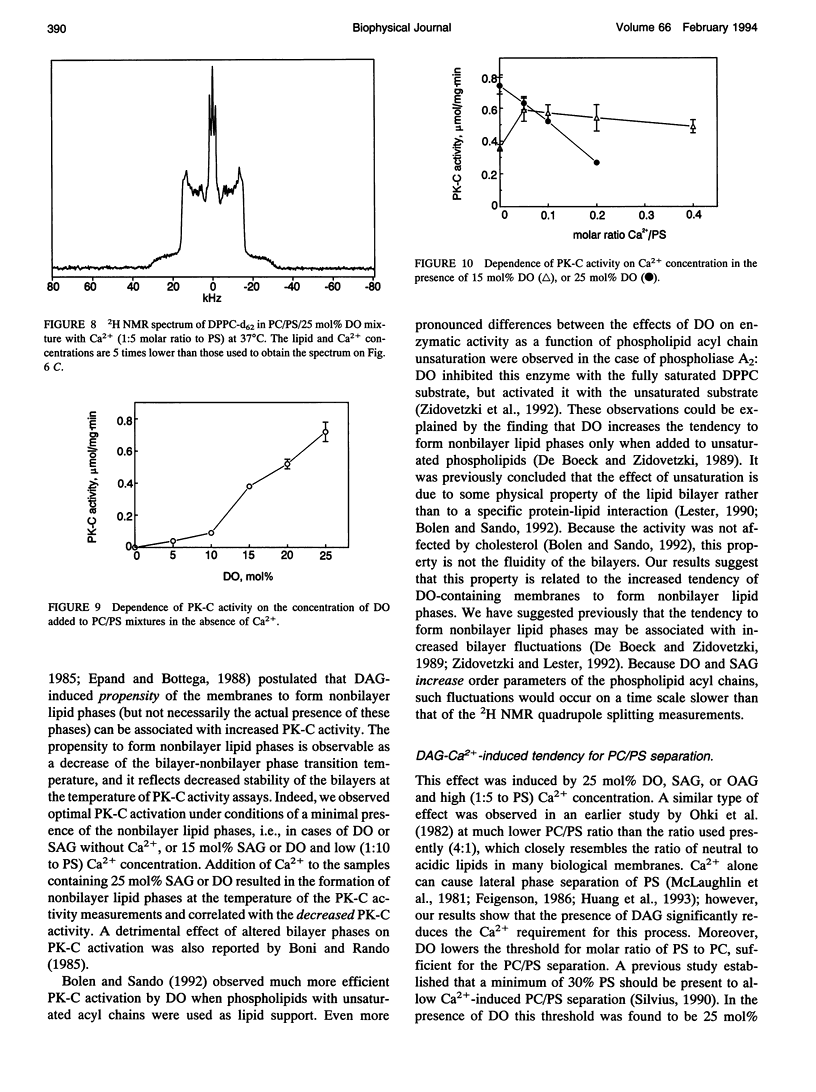
The combined effects of the diacylglycerols (DAGs) with the various acyl chains and Ca2+ on the structure of phosphatidylcholine/phosphatidylserine (4:1 mole/mole) bilayers were studied using 2H- and 31P NMR. The following DAG- and Ca(2+)-induced bilayer perturbations were identified. 1) Increased tendency to form nonbilayer lipid phases was induced by diolein or stearoylarachidonoylglycerol, and was synergistically enhanced by the addition of Ca2+. 2) "Transverse" bilayer perturbation was induced by dioctanoylglycerol. The addition of this DAG caused increased ordering of the phospholipid acyl side chains in the region adjacent to the headgroup, with the concomitant decrease of the order toward the bilayer interior. 3) Separation of the phosphatidylcholine and phosphatidylserine bilayer components was induced by combinations of relatively high (1:5 mole/mole to phosphatidylserine) Ca2+ and 25 mol% (to the phospholipids) of diolein, stearoylarachidonoylglycerol, or oleoylacetylglycerol. 4) Lateral phase separation of the bilayers on the regions of different fluidities was induced by dipalmitin. These physicochemical effects were correlated with the effects of these DAGs and Ca2+ on the activity of protein kinase C. The increased tendency to form nonbilayer lipid phases and the transverse bilayer perturbations correlated with the increased protein kinase C activity, whereas the actual presence of the nonbilayer lipid phases, as well as the separation of the phosphatidylcholine and phosphatidylserine components, was associated with the decrease in the protein kinase C activity. The lateral phase separation of the bilayer on gel-like and liquid crystalline regions did not have an effect on the activity of the enzyme. These results demonstrate the importance of the physicochemical properties of the membranes in the process of activation of protein kinase C.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzi M. D., Nelsestuen G. L. Extensive segregation of acidic phospholipids in membranes induced by protein kinase C and related proteins. Biochemistry. 1991 Aug 13;30(32):7961–7969. doi: 10.1021/bi00246a013. [DOI] [PubMed] [Google Scholar]
- Bazzi M. D., Youakim M. A., Nelsestuen G. L. Importance of phosphatidylethanolamine for association of protein kinase C and other cytoplasmic proteins with membranes. Biochemistry. 1992 Feb 4;31(4):1125–1134. doi: 10.1021/bi00119a022. [DOI] [PubMed] [Google Scholar]
- Bell R. M., Burns D. J. Lipid activation of protein kinase C. J Biol Chem. 1991 Mar 15;266(8):4661–4664. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bolen E. J., Sando J. J. Effect of phospholipid unsaturation on protein kinase C activation. Biochemistry. 1992 Jun 30;31(25):5945–5951. doi: 10.1021/bi00140a034. [DOI] [PubMed] [Google Scholar]
- Boni L. T., Rando R. R. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J Biol Chem. 1985 Sep 5;260(19):10819–10825. [PubMed] [Google Scholar]
- Bonser R. W., Thompson N. T., Hodson H. F., Beams R. M., Garland L. G. Evidence that a second stereochemical centre in diacylglycerols defines interaction at the recognition site on protein kinase C. FEBS Lett. 1988 Jul 18;234(2):341–344. doi: 10.1016/0014-5793(88)80112-1. [DOI] [PubMed] [Google Scholar]
- Cabot M. C., Jaken S. Structural and chemical specificity of diacylglycerols for protein kinase C activation. Biochem Biophys Res Commun. 1984 Nov 30;125(1):163–169. doi: 10.1016/s0006-291x(84)80349-6. [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Chen S. Y., Butko P., Van der Meer B. W., Somerharju P. Intramolecular excimer formation of pyrene-labeled lipids in lamellar and inverted hexagonal phases of lipid mixtures containing unsaturated phosphatidylethanolamine. Biophys Chem. 1991 Feb;39(2):137–144. [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Davis J. H. Deuterium magnetic resonance study of the gel and liquid crystalline phases of dipalmitoyl phosphatidylcholine. Biophys J. 1979 Sep;27(3):339–358. doi: 10.1016/S0006-3495(79)85222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Bray J., Quinn P. J. Long-chain unsaturated diacylglycerols cause a perturbation in the structure of phospholipid bilayers rendering them susceptible to phospholipase attack. Biochem Biophys Res Commun. 1984 Dec 14;125(2):836–842. doi: 10.1016/0006-291x(84)90615-6. [DOI] [PubMed] [Google Scholar]
- De Boeck H., Zidovetzki R. Effects of diacylglycerols on the structure of phosphatidylcholine bilayers: a 2H and 31P NMR study. Biochemistry. 1989 Sep 5;28(18):7439–7446. doi: 10.1021/bi00444a043. [DOI] [PubMed] [Google Scholar]
- De Boeck H., Zidovetzki R. Interactions of saturated diacylglycerols with phosphatidylcholine bilayers: A 2H NMR study. Biochemistry. 1992 Jan 21;31(2):623–630. doi: 10.1021/bi00117a046. [DOI] [PubMed] [Google Scholar]
- Dill K. A., Flory P. J. Molecular organization in micelles and vesicles. Proc Natl Acad Sci U S A. 1981 Feb;78(2):676–680. doi: 10.1073/pnas.78.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand R. M., Bottega R. Determination of the phase behaviour of phosphatidylethanolamine admixed with other lipids and the effects of calcium chloride: implications for protein kinase C regulation. Biochim Biophys Acta. 1988 Oct 6;944(2):144–154. doi: 10.1016/0005-2736(88)90427-0. [DOI] [PubMed] [Google Scholar]
- Epand R. M. Diacylglycerols, lysolecithin, or hydrocarbons markedly alter the bilayer to hexagonal phase transition temperature of phosphatidylethanolamines. Biochemistry. 1985 Dec 3;24(25):7092–7095. doi: 10.1021/bi00346a011. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Lester D. S. The role of membrane biophysical properties in the regulation of protein kinase C activity. Trends Pharmacol Sci. 1990 Aug;11(8):317–320. doi: 10.1016/0165-6147(90)90234-y. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Stafford A. R., Lester D. S. Lipid vesicles which can bind to protein kinase C and activate the enzyme in the presence of EGTA. Eur J Biochem. 1992 Sep 1;208(2):327–332. doi: 10.1111/j.1432-1033.1992.tb17190.x. [DOI] [PubMed] [Google Scholar]
- Feigenson G. W. Calcium ion binding between lipid bilayers: the four-component system of phosphatidylserine, phosphatidylcholine, calcium chloride, and water. Biochemistry. 1989 Feb 7;28(3):1270–1278. doi: 10.1021/bi00429a048. [DOI] [PubMed] [Google Scholar]
- Feigenson G. W. On the nature of calcium ion binding between phosphatidylserine lamellae. Biochemistry. 1986 Sep 23;25(19):5819–5825. doi: 10.1021/bi00367a071. [DOI] [PubMed] [Google Scholar]
- Gheriani-Gruszka N., Almog S., Biltonen R. L., Lichtenberg D. Hydrolysis of phosphatidylcholine in phosphatidylcholine-cholate mixtures by porcine pancreatic phospholipase A2. J Biol Chem. 1988 Aug 25;263(24):11808–11813. [PubMed] [Google Scholar]
- Gómez-Fernández J. C., Aranda F. J., Micol V., Villalaín J., Ortiz A. Effect of diacylglycerols on calcium-induced fusion of phosphatidylserine/phosphatidylcholine vesicles. Biochem Soc Trans. 1989 Dec;17(6):957–960. doi: 10.1042/bst0170957. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Rat brain protein kinase C. Kinetic analysis of substrate dependence, allosteric regulation, and autophosphorylation. J Biol Chem. 1990 Feb 15;265(5):2962–2972. [PubMed] [Google Scholar]
- Huang J., Swanson J. E., Dibble A. R., Hinderliter A. K., Feigenson G. W. Nonideal mixing of phosphatidylserine and phosphatidylcholine in the fluid lamellar phase. Biophys J. 1993 Feb;64(2):413–425. doi: 10.1016/S0006-3495(93)81382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M. K., Jahagirdar D. V. Action of phospholipase A2 on bilayers. Effect of inhibitors. Biochim Biophys Acta. 1985 Apr 11;814(2):319–326. doi: 10.1016/0005-2736(85)90451-1. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Lafleur M., Cullis P. R., Bloom M. Modulation of the orientational order profile of the lipid acyl chain in the L alpha phase. Eur Biophys J. 1990;19(2):55–62. doi: 10.1007/BF00185086. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Ganong B. R., Bell R. M. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985 Feb 10;260(3):1358–1361. [PubMed] [Google Scholar]
- Lee M. H., Bell R. M. Phospholipid functional groups involved in protein kinase C activation, phorbol ester binding, and binding to mixed micelles. J Biol Chem. 1989 Sep 5;264(25):14797–14805. [PubMed] [Google Scholar]
- Lester D. S., Doll L., Brumfeld V., Miller I. R. Lipid dependence of surface conformations of protein kinase C. Biochim Biophys Acta. 1990 May 31;1039(1):33–41. doi: 10.1016/0167-4838(90)90223-3. [DOI] [PubMed] [Google Scholar]
- Lester D. S. High-pressure extraction of membrane-associated protein kinase C from rat brain. J Neurochem. 1989 Jun;52(6):1950–1953. doi: 10.1111/j.1471-4159.1989.tb07284.x. [DOI] [PubMed] [Google Scholar]
- Lester D. S. In vitro linoleic acid activation of protein kinase C. Biochim Biophys Acta. 1990 Sep 24;1054(3):297–303. doi: 10.1016/0167-4889(90)90100-r. [DOI] [PubMed] [Google Scholar]
- Lester D. S., Orr N., Brumfeld V. Structural distinction between soluble and particulate protein kinase C species. J Protein Chem. 1990 Apr;9(2):209–220. doi: 10.1007/BF01025311. [DOI] [PubMed] [Google Scholar]
- Mayer L. D., Hope M. J., Cullis P. R., Janoff A. S. Solute distributions and trapping efficiencies observed in freeze-thawed multilamellar vesicles. Biochim Biophys Acta. 1985 Jul 11;817(1):193–196. doi: 10.1016/0005-2736(85)90084-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Mulrine N., Gresalfi T., Vaio G., McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981 Apr;77(4):445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashe M., Lichtenberg D., Gutierrez-Merino C., Biltonen R. L. Relationship between the activity of pancreatic phospholipase A2 and the physical state of the phospholipid substrate. J Biol Chem. 1981 May 10;256(9):4541–4543. [PubMed] [Google Scholar]
- Nelsestuen G. L., Bazzi M. D. Activation and regulation of protein kinase C enzymes. J Bioenerg Biomembr. 1991 Feb;23(1):43–61. doi: 10.1007/BF00768838. [DOI] [PubMed] [Google Scholar]
- Ohki K., Sekiya T., Yamauchi T., Nozawa Y. Effect of phosphatidylinositol replacement by diacylglycerol on various physical properties of artificial membranes with respect to the role of phosphatidylinositol response. Biochim Biophys Acta. 1982 Dec 22;693(2):341–350. doi: 10.1016/0005-2736(82)90441-2. [DOI] [PubMed] [Google Scholar]
- Orr J. W., Keranen L. M., Newton A. C. Reversible exposure of the pseudosubstrate domain of protein kinase C by phosphatidylserine and diacylglycerol. J Biol Chem. 1992 Aug 5;267(22):15263–15266. [PubMed] [Google Scholar]
- Orr J. W., Newton A. C. Interaction of protein kinase C with phosphatidylserine. 1. Cooperativity in lipid binding. Biochemistry. 1992 May 19;31(19):4661–4667. doi: 10.1021/bi00134a018. [DOI] [PubMed] [Google Scholar]
- Orr J. W., Newton A. C. Interaction of protein kinase C with phosphatidylserine. 2. Specificity and regulation. Biochemistry. 1992 May 19;31(19):4667–4673. doi: 10.1021/bi00134a019. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Villalaín J., Gómez-Fernández J. C. Interaction of diacylglycerols with phosphatidylcholine vesicles as studied by differential scanning calorimetry and fluorescence probe depolarization. Biochemistry. 1988 Dec 13;27(25):9030–9036. doi: 10.1021/bi00425a022. [DOI] [PubMed] [Google Scholar]
- Romero G., Thompson K., Biltonen R. L. The activation of porcine pancreatic phospholipase A2 by dipalmitoylphosphatidylcholine large unilamellar vesicles. Analysis of the state of aggregation of the activated enzyme. J Biol Chem. 1987 Oct 5;262(28):13476–13482. [PubMed] [Google Scholar]
- Sando J. J., Maurer M. C., Bolen E. J., Grisham C. M. Role of cofactors in protein kinase C activation. Cell Signal. 1992 Nov;4(6):595–609. doi: 10.1016/0898-6568(92)90041-6. [DOI] [PubMed] [Google Scholar]
- Schaap D., Parker P. J. Expression, purification, and characterization of protein kinase C-epsilon. J Biol Chem. 1990 May 5;265(13):7301–7307. [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Tsukuda M., Ase K., Kikkawa U., Nishizuka Y. Mode of activation and kinetic properties of three distinct forms of protein kinase C from rat brain. J Biochem. 1988 May;103(5):759–765. doi: 10.1093/oxfordjournals.jbchem.a122343. [DOI] [PubMed] [Google Scholar]
- Sen A., Isac T. V., Hui S. W. Bilayer packing stress and defects in mixed dilinoleoylphosphatidylethanolamine and palmitoyloleoylphosphatidylcholine and their susceptibility to phospholipase A2. Biochemistry. 1991 May 7;30(18):4516–4521. doi: 10.1021/bi00232a021. [DOI] [PubMed] [Google Scholar]
- Senisterra G., Epand R. M. Role of membrane defects in the regulation of the activity of protein kinase C. Arch Biochem Biophys. 1993 Jan;300(1):378–383. doi: 10.1006/abbi.1993.1051. [DOI] [PubMed] [Google Scholar]
- Silvius J. R. Calcium-induced lipid phase separations and interactions of phosphatidylcholine/anionic phospholipid vesicles. Fluorescence studies using carbazole-labeled and brominated phospholipids. Biochemistry. 1990 Mar 27;29(12):2930–2938. doi: 10.1021/bi00464a007. [DOI] [PubMed] [Google Scholar]
- Snoek G. T., Feijen A., Hage W. J., van Rotterdam W., de Laat S. W. The role of hydrophobic interactions in the phospholipid-dependent activation of protein kinase C. Biochem J. 1988 Oct 15;255(2):629–637. [PMC free article] [PubMed] [Google Scholar]
- Souvignet C., Pelosin J. M., Daniel S., Chambaz E. M., Ransac S., Verger R. Activation of protein kinase C in lipid monolayers. J Biol Chem. 1991 Jan 5;266(1):40–44. [PubMed] [Google Scholar]
- Walker J. M., Homan E. C., Sando J. J. Differential activation of protein kinase C isozymes by short chain phosphatidylserines and phosphatidylcholines. J Biol Chem. 1990 May 15;265(14):8016–8021. [PubMed] [Google Scholar]
- Westman J., Boulanger Y., Ehrenberg A., Smith I. C. Charge and pH dependent drug binding to model membranes. A 2H-NMR and light absorption study. Biochim Biophys Acta. 1982 Mar 8;685(3):315–328. doi: 10.1016/0005-2736(82)90073-6. [DOI] [PubMed] [Google Scholar]
- Wilschut J. C., Regts J., Westenberg H., Scherphof G. Action of phospholipases A2 on phosphatidylcholine bilayers. Effects of the phase transition, bilayer curvature and structural defects. Biochim Biophys Acta. 1978 Apr 4;508(2):185–196. doi: 10.1016/0005-2736(78)90324-3. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R., Atiya A. W., De Boeck H. Effect of divalent cations on the structure of dipalmitoylphosphatidylcholine and phosphatidylcholine/phosphatidylglycerol bilayers: an 2H-NMR study. Membr Biochem. 1989;8(3):177–186. doi: 10.3109/09687688909025830. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R., Laptalo L., Crawford J. Effect of diacylglycerols on the activity of cobra venom, bee venom, and pig pancreatic phospholipases A2. Biochemistry. 1992 Aug 25;31(33):7683–7691. doi: 10.1021/bi00148a032. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R., Lester D. S. The mechanism of activation of protein kinase C: a biophysical perspective. Biochim Biophys Acta. 1992 Apr 7;1134(3):261–272. doi: 10.1016/0167-4889(92)90185-e. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R., Sherman I. W., Cardenas M., Borchardt D. B. Chloroquine stabilization of phospholipid membranes against diacylglycerol-induced perturbation. Biochem Pharmacol. 1993 Jan 7;45(1):183–189. doi: 10.1016/0006-2952(93)90391-9. [DOI] [PubMed] [Google Scholar]


