Abstract
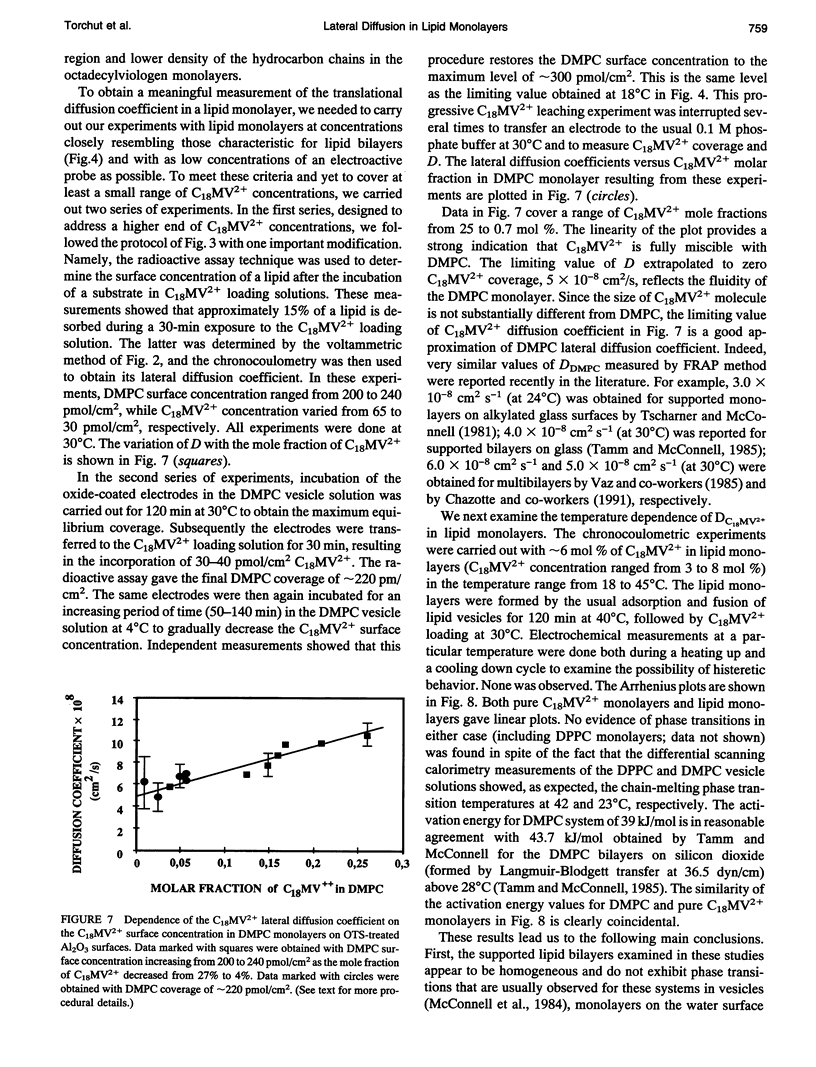
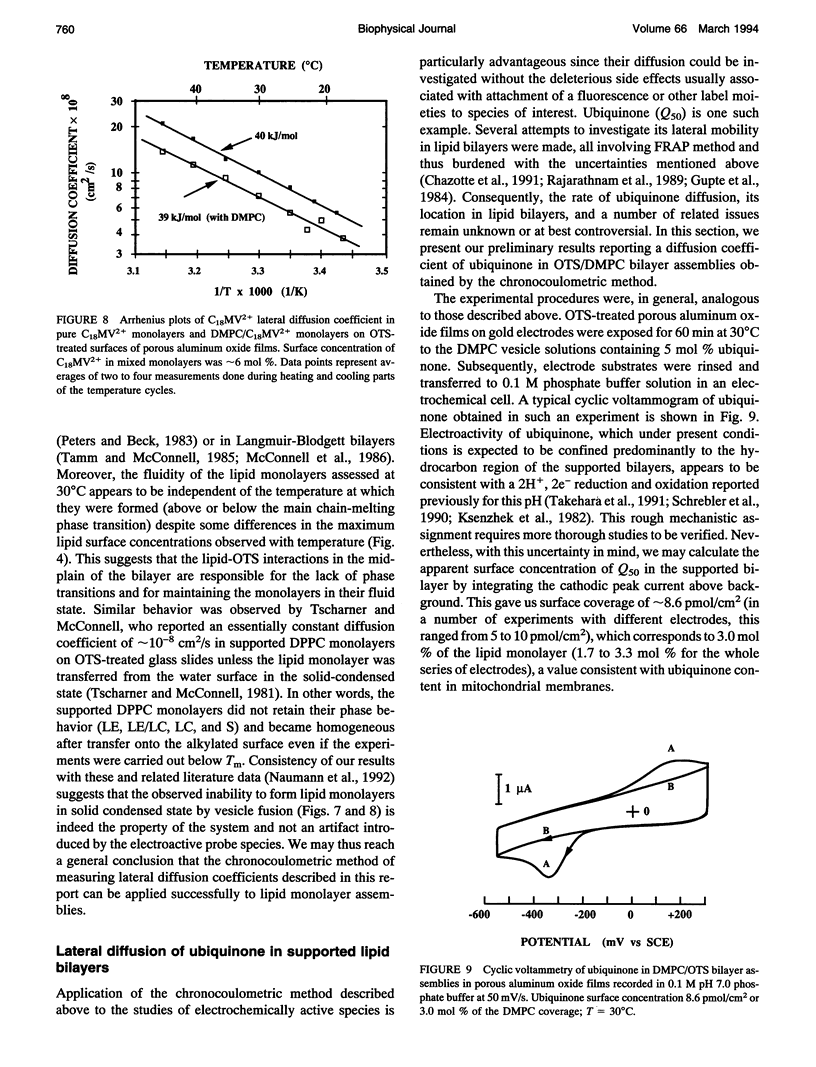
Chronocoulometry was used to characterize the fluidity and lateral diffusion coefficient of supported phospholipid bilayer assemblies. The bilayers were formed on the inner surfaces of the microporous template films of aluminum oxide on gold electrodes. The lipid monolayers were formed by adsorption and fusion of phospholipid vesicles on alkylated oxide surfaces. Octadecyltrichlorosilane (OTS) was used in the initial alkylation step. The surface concentration of the lipids in monolayer assemblies was measured by a radioactive assay method. Surface densities corresponding to 48 +/- 10 A2/molecule (DPPC) and 56 +/- 11 A2/molecule (DMPC) were obtained (for exposure times > 120 min) independent of the temperature of the vesicle's fusion (below or above chain-melting transition). Octadecylviologen (C18MV2+) was used as an electroactive probe species. Its limiting lateral diffusion coefficient in DMPC monolayers was 5 x 10(-8) cm2/s, measured as C18MV2+ mole fraction extrapolated to 0 decreasing linearly from 20 to below 1 mol%. Linear Arrhenius plots for C18MV2+ diffusion in DMPC monolayers were obtained with slopes of approximately 40 kJ/mol between 18 and 45 degrees C, demonstrating homogeneity and fluidity of the lipid monolayers. Chronocoulometry was also used to obtain lateral diffusion coefficient of ubiquinone in DMPC/OTS bilayers. A value of 1.9 x 10(-8) cm2/s at 30 degrees C was obtained.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brian A. A., McConnell H. M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6159–6163. doi: 10.1073/pnas.81.19.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazotte B., Wu E. S., Hackenbrock C. R. The mobility of a fluorescent ubiquinone in model lipid membranes. Relevance to mitochondrial electron transport. Biochim Biophys Acta. 1991 Jul 5;1058(3):400–409. doi: 10.1016/s0005-2728(05)80136-7. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Gupte S., Wu E. S., Hoechli L., Hoechli M., Jacobson K., Sowers A. E., Hackenbrock C. R. Relationship between lateral diffusion, collision frequency, and electron transfer of mitochondrial inner membrane oxidation-reduction components. Proc Natl Acad Sci U S A. 1984 May;81(9):2606–2610. doi: 10.1073/pnas.81.9.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb E., Frey S., Tamm L. K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim Biophys Acta. 1992 Jan 31;1103(2):307–316. doi: 10.1016/0005-2736(92)90101-q. [DOI] [PubMed] [Google Scholar]
- Korzeniowski A, Fry JL, Orr DE, Fazleev NG. Korzeniowski et al. reply. Phys Rev Lett. 1993 Sep 27;71(13):2160–2161. doi: 10.1103/PhysRevLett.71.2160. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Tamm L. K., Weis R. M. Periodic structures in lipid monolayer phase transitions. Proc Natl Acad Sci U S A. 1984 May;81(10):3249–3253. doi: 10.1073/pnas.81.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., Watts T. H., Weis R. M., Brian A. A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta. 1986 Jun 12;864(1):95–106. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Naumann C., Brumm T., Bayerl T. M. Phase transition behavior of single phosphatidylcholine bilayers on a solid spherical support studied by DSC, NMR and FT-IR. Biophys J. 1992 Nov;63(5):1314–1319. doi: 10.1016/S0006-3495(92)81708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpaleix T., Laval J. M., Majda M., Bourdillon C. Potentiometric and voltammetric investigations of H2/H+ catalysis by periplasmic hydrogenase from Desulfovibrio gigas immobilized at the electrode surface in an amphiphilic bilayer assembly. Anal Chem. 1992 Mar 15;64(6):641–646. doi: 10.1021/ac00030a013. [DOI] [PubMed] [Google Scholar]
- Peters R., Beck K. Translational diffusion in phospholipid monolayers measured by fluorescence microphotolysis. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7183–7187. doi: 10.1073/pnas.80.23.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarathnam K., Hochman J., Schindler M., Ferguson-Miller S. Synthesis, location, and lateral mobility of fluorescently labeled ubiquinone 10 in mitochondrial and artificial membranes. Biochemistry. 1989 Apr 18;28(8):3168–3176. doi: 10.1021/bi00434a009. [DOI] [PubMed] [Google Scholar]
- Singh S., Keller D. J. Atomic force microscopy of supported planar membrane bilayers. Biophys J. 1991 Dec;60(6):1401–1410. doi: 10.1016/S0006-3495(91)82177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L. K., McConnell H. M. Supported phospholipid bilayers. Biophys J. 1985 Jan;47(1):105–113. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Almeida P. F. Microscopic versus macroscopic diffusion in one-component fluid phase lipid bilayer membranes. Biophys J. 1991 Dec;60(6):1553–1554. doi: 10.1016/S0006-3495(91)82190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., McConnell H. M. Physical properties of lipid monolayers on alkylated planar glass surfaces. Biophys J. 1981 Nov;36(2):421–427. doi: 10.1016/S0006-3495(81)84741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


