Abstract
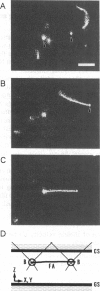
Movement of single myosin filaments, synthesized by copolymerization of intact myosin and fluorescently labeled light meromyosin, were observed along a single actin filament suspended in solution by a dual laser trap in a fluorescence microscope. The sliding velocity of the myosin filaments was 11.0 +/- 0.2 micron/s at 27 degrees C. This is similar to that of actin moving toward the center from the tip (the physiological direction) of myosin filaments bound to a glass surface but several times larger than that in the opposite direction (Ishijima and Yanagida, 1991; Yanagida, 1993). This indicates that the movement of myosin filaments is dominated by the myosin heads on one side of the myosin filament, which are correctly oriented relative to the actin filament. The incorrectly oriented myosin heads on the other side do not interfere with the fast movement. The step size (displacement produced during one ATPase cycle) of correctly oriented myosin was estimated from the minimum number of myosin heads necessary to produce the maximum velocity. This was determined by measuring the velocities of various lengths of myosin filaments. The minimum length of the myosin filaments moving near the maximum velocity was 0.30-0.40 microns, which contains 20 +/- 5 correctly oriented myosin heads. This number leads to a myosin step size of 71 +/- 22 nm. This value probably represents the lower limit, because all of the myosin heads on the filament would not always interact with the actin filament. Thus, the myosin step size is considerably larger than the length of a power stroke expected from the physical size of a myosin head, 10-20 nm (Huxley, 1957, 1969).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkin A., Dziedzic J. M. Internal cell manipulation using infrared laser traps. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7914–7918. doi: 10.1073/pnas.86.20.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkin A., Dziedzic J. M. Optical trapping and manipulation of viruses and bacteria. Science. 1987 Mar 20;235(4795):1517–1520. doi: 10.1126/science.3547653. [DOI] [PubMed] [Google Scholar]
- Ashkin A., Dziedzic J. M., Yamane T. Optical trapping and manipulation of single cells using infrared laser beams. Nature. 1987 Dec 24;330(6150):769–771. doi: 10.1038/330769a0. [DOI] [PubMed] [Google Scholar]
- Ashkin A., Schütze K., Dziedzic J. M., Euteneuer U., Schliwa M. Force generation of organelle transport measured in vivo by an infrared laser trap. Nature. 1990 Nov 22;348(6299):346–348. doi: 10.1038/348346a0. [DOI] [PubMed] [Google Scholar]
- Block S. M., Blair D. F., Berg H. C. Compliance of bacterial flagella measured with optical tweezers. Nature. 1989 Apr 6;338(6215):514–518. doi: 10.1038/338514a0. [DOI] [PubMed] [Google Scholar]
- Block S. M., Goldstein L. S., Schnapp B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990 Nov 22;348(6299):348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- Brenner B. Rapid dissociation and reassociation of actomyosin cross-bridges during force generation: a newly observed facet of cross-bridge action in muscle. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10490–10494. doi: 10.1073/pnas.88.23.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K. Myosin step size: estimates from motility assays and shortening muscle. J Muscle Res Cell Motil. 1992 Dec;13(6):590–607. doi: 10.1007/BF01738249. [DOI] [PubMed] [Google Scholar]
- Chaen S., Oiwa K., Shimmen T., Iwamoto H., Sugi H. Simultaneous recordings of force and sliding movement between a myosin-coated glass microneedle and actin cables in vitro. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1510–1514. doi: 10.1073/pnas.86.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S. Laser manipulation of atoms and particles. Science. 1991 Aug 23;253(5022):861–866. doi: 10.1126/science.253.5022.861. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954 May 22;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Harada Y., Noguchi A., Kishino A., Yanagida T. Sliding movement of single actin filaments on one-headed myosin filaments. Nature. 1987 Apr 23;326(6115):805–808. doi: 10.1038/326805a0. [DOI] [PubMed] [Google Scholar]
- Harada Y., Sakurada K., Aoki T., Thomas D. D., Yanagida T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J Mol Biol. 1990 Nov 5;216(1):49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- Higashi-Fujime S. Reconstitution of active movement in vitro based on the actin-myosin interaction. Int Rev Cytol. 1991;125:95–138. doi: 10.1016/s0074-7696(08)61217-6. [DOI] [PubMed] [Google Scholar]
- Higashi-Fujime S. Unidirectional sliding of myosin filaments along the bundle of F-actin filaments spontaneously formed during superprecipitation. J Cell Biol. 1985 Dec;101(6):2335–2344. doi: 10.1083/jcb.101.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H., Goldman Y. E. Sliding distance between actin and myosin filaments per ATP molecule hydrolysed in skinned muscle fibres. Nature. 1991 Jul 25;352(6333):352–354. doi: 10.1038/352352a0. [DOI] [PubMed] [Google Scholar]
- Honda H., Nagashima H., Asakura S. Directional movement of F-actin in vitro. J Mol Biol. 1986 Sep 5;191(1):131–133. doi: 10.1016/0022-2836(86)90428-6. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Sliding filaments and molecular motile systems. J Biol Chem. 1990 May 25;265(15):8347–8350. [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Irving M., Lombardi V., Piazzesi G., Ferenczi M. A. Myosin head movements are synchronous with the elementary force-generating process in muscle. Nature. 1992 May 14;357(6374):156–158. doi: 10.1038/357156a0. [DOI] [PubMed] [Google Scholar]
- Ishijima A., Doi T., Sakurada K., Yanagida T. Sub-piconewton force fluctuations of actomyosin in vitro. Nature. 1991 Jul 25;352(6333):301–306. doi: 10.1038/352301a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa R., Okagaki T., Higashi-Fujime S., Kohama K. Stimulation of the interaction between actin and myosin by Physarum caldesmon-like protein and smooth muscle caldesmon. J Biol Chem. 1991 Nov 15;266(32):21784–21790. [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. Studies on the formation and physical chemical properties of synthetic myosin filaments. Biochemistry. 1966 Nov;5(11):3474–3487. doi: 10.1021/bi00875a013. [DOI] [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Kron S. J., Spudich J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucik D. F., Kuo S. C., Elson E. L., Sheetz M. P. Preferential attachment of membrane glycoproteins to the cytoskeleton at the leading edge of lamella. J Cell Biol. 1991 Sep;114(5):1029–1036. doi: 10.1083/jcb.114.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Sheetz M. P. Force of single kinesin molecules measured with optical tweezers. Science. 1993 Apr 9;260(5105):232–234. doi: 10.1126/science.8469975. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lombardi V., Piazzesi G., Linari M. Rapid regeneration of the actin-myosin power stroke in contracting muscle. Nature. 1992 Feb 13;355(6361):638–641. doi: 10.1038/355638a0. [DOI] [PubMed] [Google Scholar]
- Manstein D. J., Ruppel K. M., Spudich J. A. Expression and characterization of a functional myosin head fragment in Dictyostelium discoideum. Science. 1989 Nov 3;246(4930):656–658. doi: 10.1126/science.2530629. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Substructure of the myosin molecule. IV. Interactions of myosin and its subfragments with adenosine triphosphate and F-actin. J Mol Biol. 1973 Mar 5;74(3):313–330. doi: 10.1016/0022-2836(73)90376-8. [DOI] [PubMed] [Google Scholar]
- Mitsui T., Ohshima H. A self-induced translation model of myosin head motion in contracting muscle. I. Force-velocity relation and energy liberation. J Muscle Res Cell Motil. 1988 Jun;9(3):248–260. doi: 10.1007/BF01773895. [DOI] [PubMed] [Google Scholar]
- Nagashima H. Active movement of synthetic myosin filaments observed by dark-field light microscopy. J Biochem. 1986 Oct;100(4):1023–1029. doi: 10.1093/oxfordjournals.jbchem.a121781. [DOI] [PubMed] [Google Scholar]
- Ohno T., Kodama T. Kinetics of adenosine triphosphate hydrolysis by shortening myofibrils from rabbit psoas muscle. J Physiol. 1991 Sep;441:685–702. doi: 10.1113/jphysiol.1991.sp018773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagaki T., Higashi-Fujime S., Ishikawa R., Takano-Ohmuro H., Kohama K. In vitro movement of actin filaments on gizzard smooth muscle myosin: requirement of phosphorylation of myosin light chain and effects of tropomyosin and caldesmon. J Biochem. 1991 Jun;109(6):858–866. doi: 10.1093/oxfordjournals.jbchem.a123471. [DOI] [PubMed] [Google Scholar]
- Oplatka A., Tirosh R. Active streaming in actomyosin solutions. Biochim Biophys Acta. 1973 Jun 28;305(3):684–688. doi: 10.1016/0005-2728(73)90093-5. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Reedy M. C., Beall C., Fyrberg E. Formation of reverse rigor chevrons by myosin heads. Nature. 1989 Jun 8;339(6224):481–483. doi: 10.1038/339481a0. [DOI] [PubMed] [Google Scholar]
- Reisler E. Kinetic studies with synthetic myosin minifilaments show the equivalence of actomyosin and acto-HMM ATPases. J Biol Chem. 1980 Oct 25;255(20):9541–9544. [PubMed] [Google Scholar]
- Sellers J. R., Kachar B. Polarity and velocity of sliding filaments: control of direction by actin and of speed by myosin. Science. 1990 Jul 27;249(4967):406–408. doi: 10.1126/science.2377894. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Spudich J. A. Movement of myosin-coated fluorescent beads on actin cables in vitro. Nature. 1983 May 5;303(5912):31–35. doi: 10.1038/303031a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Sutoh K., Ando M., Sutoh K., Toyoshima Y. Y. Site-directed mutations of Dictyostelium actin: disruption of a negative charge cluster at the N terminus. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7711–7714. doi: 10.1073/pnas.88.17.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K., Schmidt C. F., Branton D., Block S. M. Conformation and elasticity of the isolated red blood cell membrane skeleton. Biophys J. 1992 Sep;63(3):784–793. doi: 10.1016/S0006-3495(92)81644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Toyoshima C., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987 Aug 6;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., Spudich J. A. The myosin step size: measurement of the unit displacement per ATP hydrolyzed in an in vitro assay. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7130–7134. doi: 10.1073/pnas.87.18.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Toyoshima C., Spudich J. A. Bidirectional movement of actin filaments along tracks of myosin heads. Nature. 1989 Sep 14;341(6238):154–156. doi: 10.1038/341154a0. [DOI] [PubMed] [Google Scholar]
- Umemoto S., Bengur A. R., Sellers J. R. Effect of multiple phosphorylations of smooth muscle and cytoplasmic myosins on movement in an in vitro motility assay. J Biol Chem. 1989 Jan 25;264(3):1431–1436. [PubMed] [Google Scholar]
- Uyeda T. Q., Kron S. J., Spudich J. A. Myosin step size. Estimation from slow sliding movement of actin over low densities of heavy meromyosin. J Mol Biol. 1990 Aug 5;214(3):699–710. doi: 10.1016/0022-2836(90)90287-V. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Warrick H. M., Kron S. J., Spudich J. A. Quantized velocities at low myosin densities in an in vitro motility assay. Nature. 1991 Jul 25;352(6333):307–311. doi: 10.1038/352307a0. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Oosawa F. Protein motors and Maxwell's demons: does mechanochemical transduction involve a thermal ratchet? Adv Biophys. 1990;26:97–134. doi: 10.1016/0065-227x(90)90009-i. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Giniger E. Calcium-sensitive binding of heavy meromyosin to regulated actin in the presence of ATP. J Biol Chem. 1981 Dec 25;256(24):12647–12650. [PubMed] [Google Scholar]
- Warshaw D. M., Desrosiers J. M., Work S. S., Trybus K. M. Effects of MgATP, MgADP, and Pi on actin movement by smooth muscle myosin. J Biol Chem. 1991 Dec 25;266(36):24339–24343. [PubMed] [Google Scholar]
- Yamada A., Ishii N., Takahashi K. Direction and speed of actin filaments moving along thick filaments isolated from molluscan smooth muscle. J Biochem. 1990 Sep;108(3):341–343. doi: 10.1093/oxfordjournals.jbchem.a123203. [DOI] [PubMed] [Google Scholar]
- Yamada A., Takahashi K. Sudden increase in speed of an actin filament moving on myosin cross-bridges of "mismatched" polarity observed when its leading end begins to interact with cross-bridges of "matched" polarity. J Biochem. 1992 May;111(5):676–680. doi: 10.1093/oxfordjournals.jbchem.a123817. [DOI] [PubMed] [Google Scholar]
- Yamada A., Wakabayashi T. Movement of actin away from the center of reconstituted rabbit myosin filament is slower than in the opposite direction. Biophys J. 1993 Feb;64(2):565–569. doi: 10.1016/S0006-3495(93)81388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Abe O., Kobayashi T., Sugi H. Myofilament sliding per ATP molecule in rabbit muscle fibres studied using laser flash photolysis of caged ATP. J Physiol. 1993 Jul;466:229–243. [PMC free article] [PubMed] [Google Scholar]
- Yanagida T., Arata T., Oosawa F. Sliding distance of actin filament induced by a myosin crossbridge during one ATP hydrolysis cycle. Nature. 1985 Jul 25;316(6026):366–369. doi: 10.1038/316366a0. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Harada Y., Ishijima A. Nano-manipulation of actomyosin molecular motors in vitro: a new working principle. Trends Biochem Sci. 1993 Sep;18(9):319–324. doi: 10.1016/0968-0004(93)90064-t. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Nakase M., Nishiyama K., Oosawa F. Direct observation of motion of single F-actin filaments in the presence of myosin. Nature. 1984 Jan 5;307(5946):58–60. doi: 10.1038/307058a0. [DOI] [PubMed] [Google Scholar]
- Yano M. Observation of steady streamings in a solution of Mg-ATP and acto-heavy meromyosin from rabbit skeletal muscle. J Biochem. 1978 Apr;83(4):1203–1204. doi: 10.1093/oxfordjournals.jbchem.a132012. [DOI] [PubMed] [Google Scholar]