Abstract
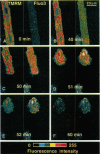
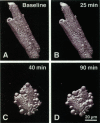
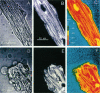
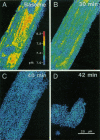
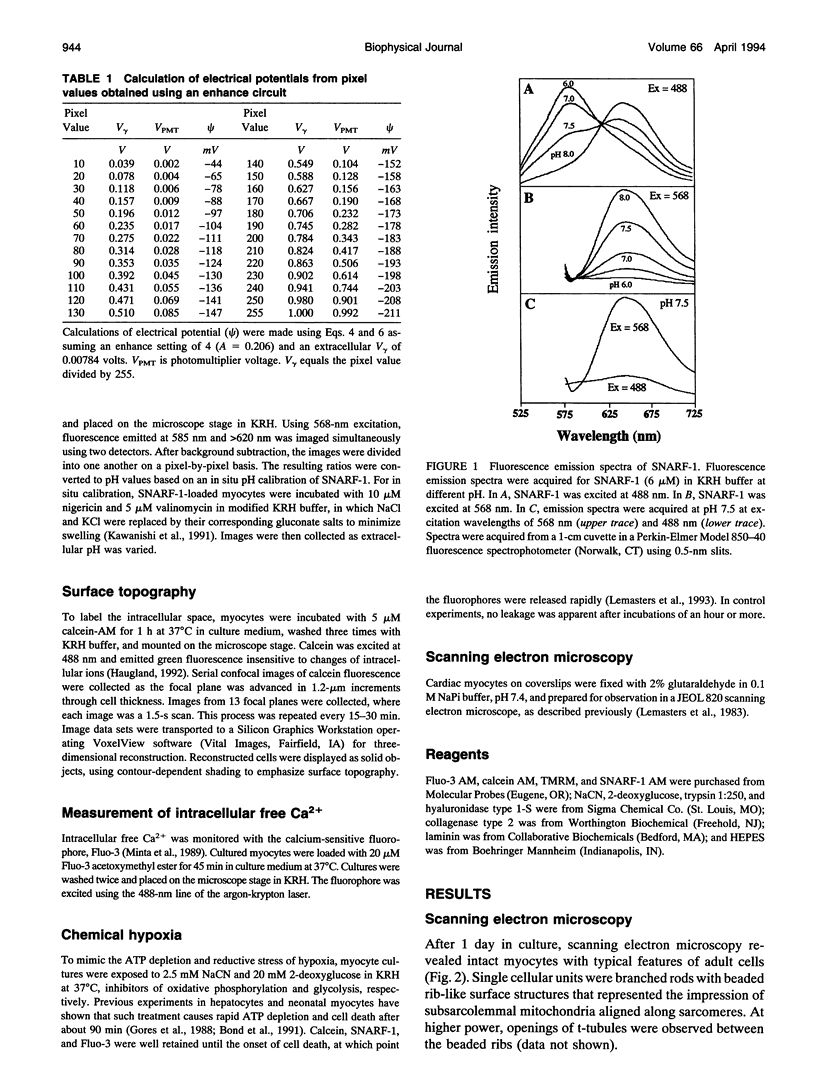
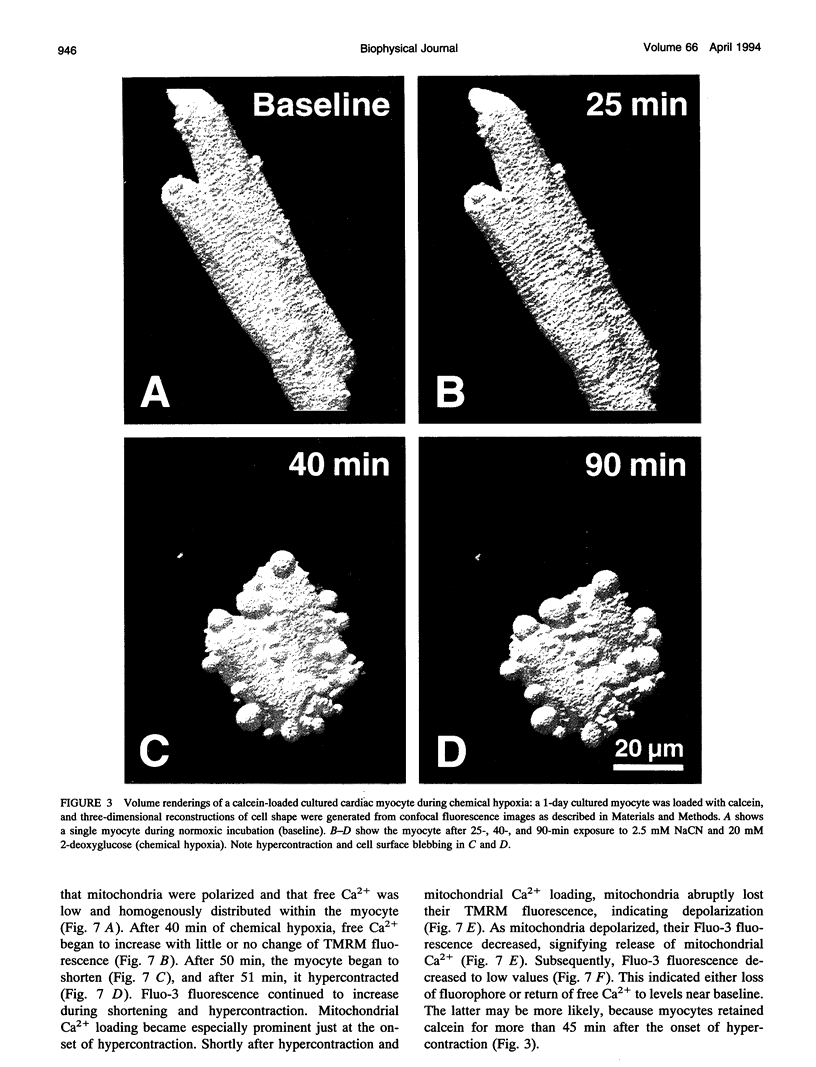
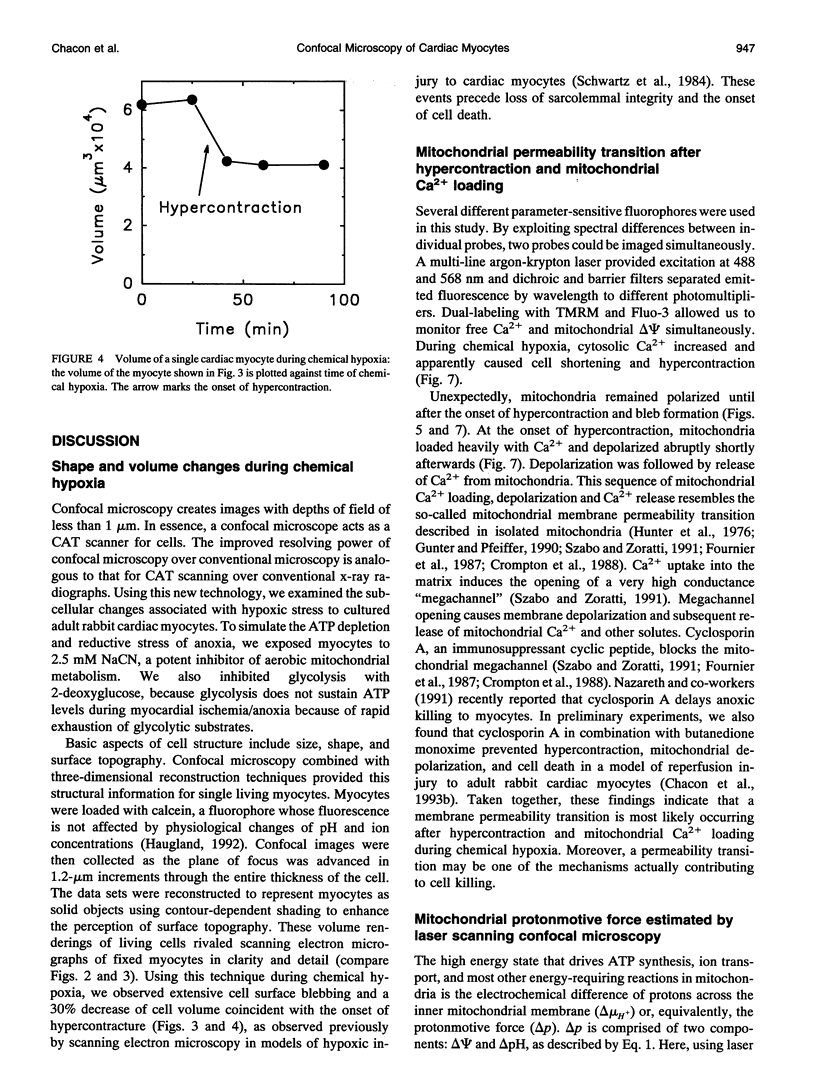
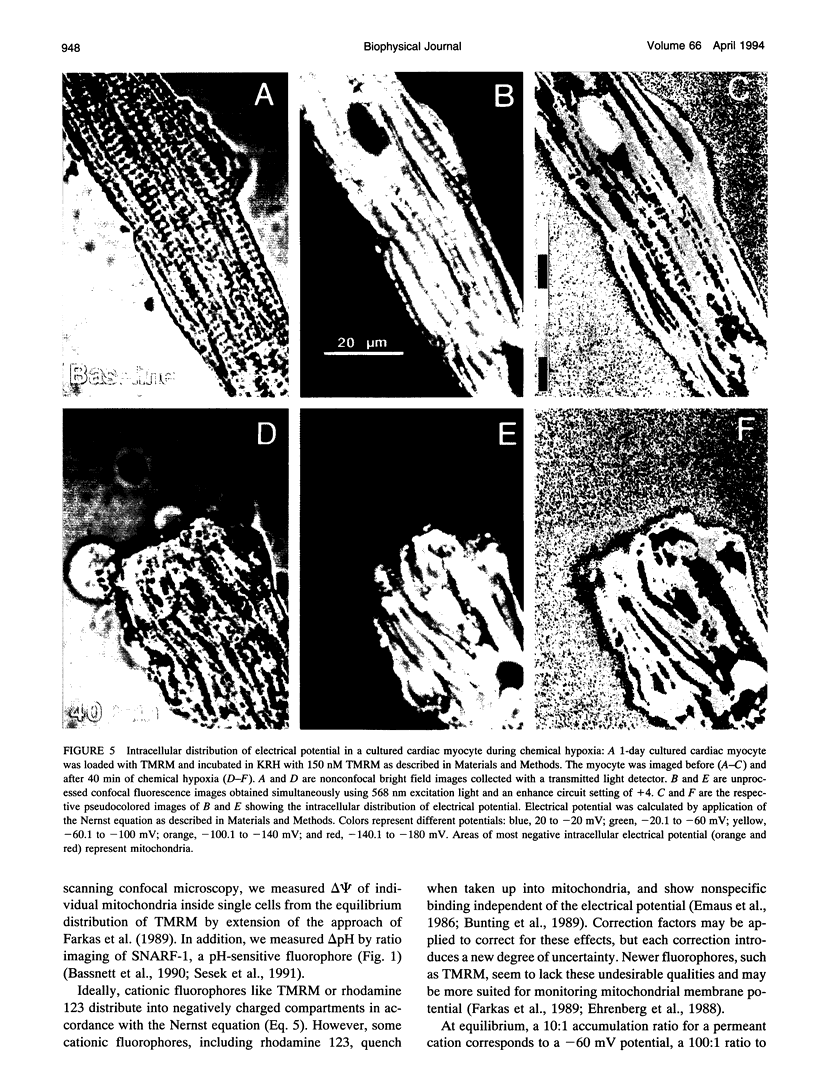
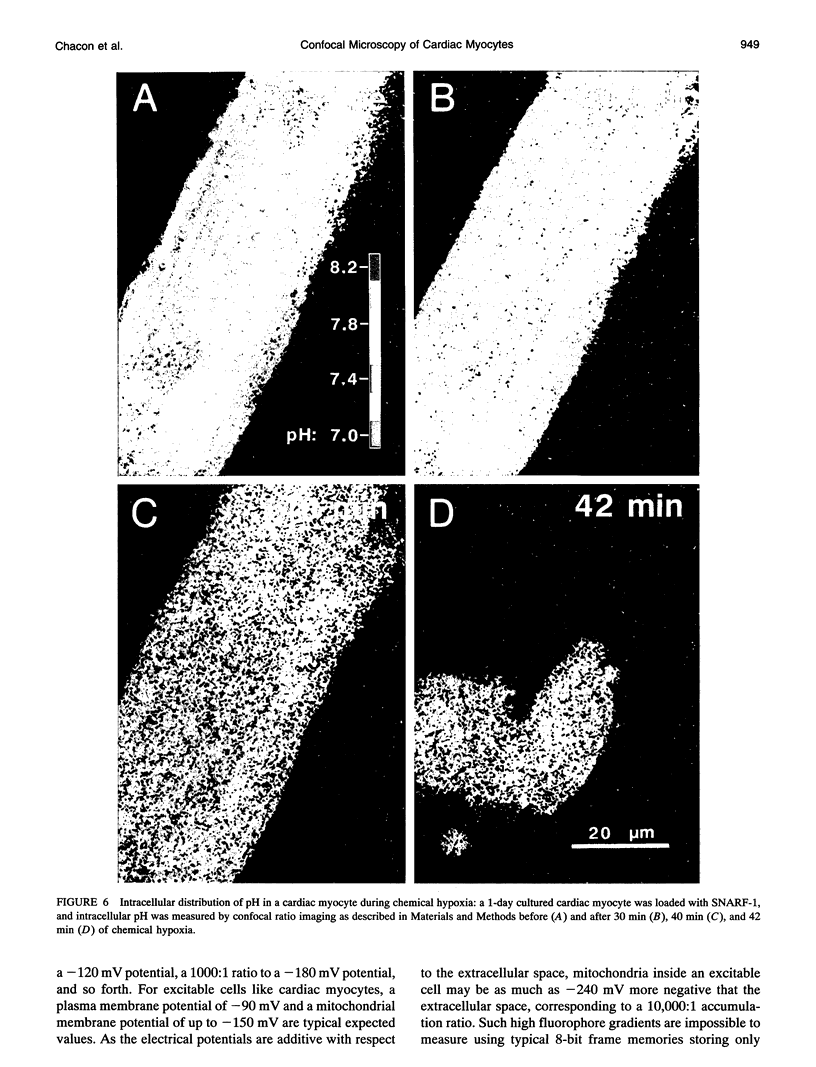
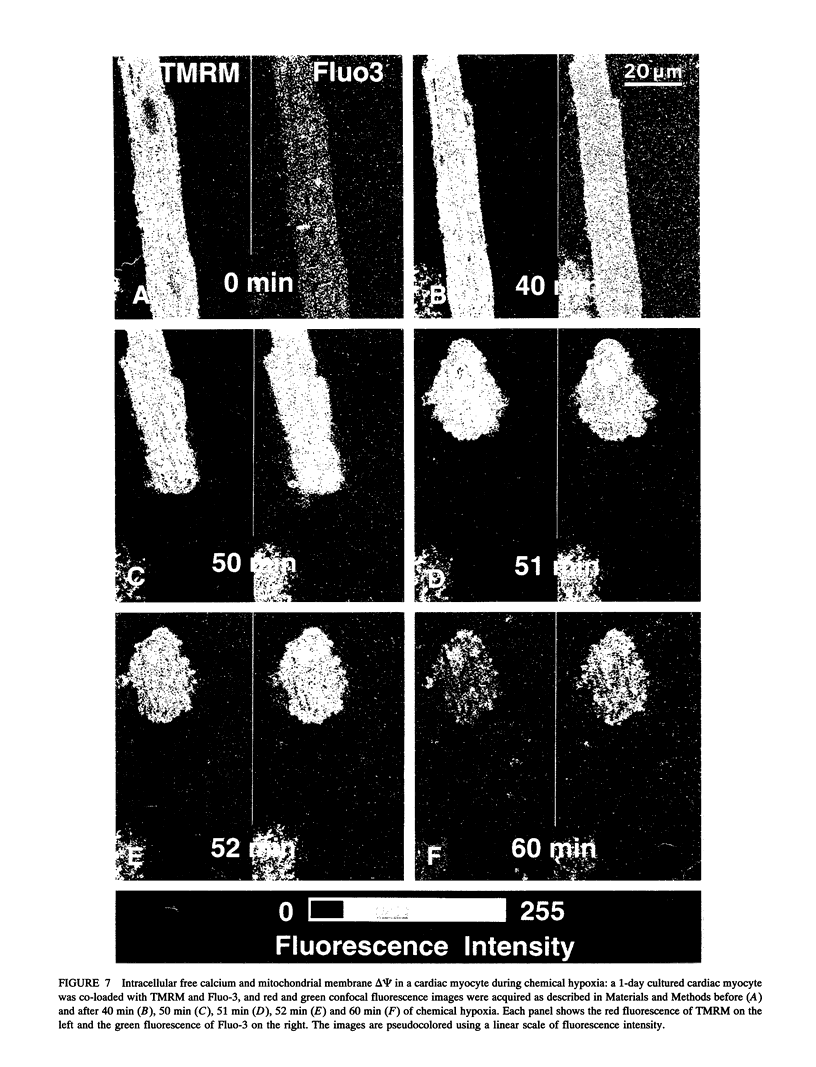
Exploiting the optical sectioning capabilities of laser scanning confocal microscopy and using parameter-specific fluorescent probes, we determined the distribution of pH, free Ca2+, electrical potential, and volume inside cultured adult rabbit cardiac myocytes during ATP depletion and reductive stress with cyanide and 2-deoxyglucose ("chemical hypoxia"). During normoxic incubations, myocytes exhibited a cytosolic pH of 7.1 and a mitochondrial pH of 8.0 (delta pH = 0.9 units). Sarcolemmal membrane potential (delta psi) was -80 mV, and mitochondrial delta psi was as high as -100 mV, yielding a mitochondrial protonmotive force (delta p) of -155 mV (delta P = delta psi - 60 delta pH). After 30 min of chemical hypoxia, mitochondrial delta pH decreased to 0.5 pH units, but mitochondrial delta psi remained essentially unchanged. By 40 min, delta pH was collapsed, and mitochondrial and cytosolic free Ca2+ began to increase. Mitochondrial and sarcolemmal delta psi remained high. as Ca2+ rose, myocytes shortened, hypercontracted, and blebbed with a 30% decrease of cell volume. After hypercontraction, extensive mitochondrial Ca2+ loading occurred. After another few minutes, mitochondrial depolarized completely and released their load of Ca2+. After many more minutes, the sarcolemmal permeability barrier broke down, and viability was lost. These studies demonstrate a sequence of subcellular ionic and electrical changes that may underlie the progression to irreversible hypoxic injury.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassnett S., Reinisch L., Beebe D. C. Intracellular pH measurement using single excitation-dual emission fluorescence ratios. Am J Physiol. 1990 Jan;258(1 Pt 1):C171–C178. doi: 10.1152/ajpcell.1990.258.1.C171. [DOI] [PubMed] [Google Scholar]
- Bond J. M., Herman B., Lemasters J. J. Recovery of cultured rat neonatal myocytes from hypercontracture after chemical hypoxia. Res Commun Chem Pathol Pharmacol. 1991 Feb;71(2):195–208. [PubMed] [Google Scholar]
- Bunting J. R., Phan T. V., Kamali E., Dowben R. M. Fluorescent cationic probes of mitochondria. Metrics and mechanism of interaction. Biophys J. 1989 Nov;56(5):979–993. doi: 10.1016/S0006-3495(89)82743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon E., Ulrich R., Acosta D. A digitized-fluorescence-imaging study of mitochondrial Ca2+ increase by doxorubicin in cardiac myocytes. Biochem J. 1992 Feb 1;281(Pt 3):871–878. doi: 10.1042/bj2810871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Ellinger H., Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988 Oct 1;255(1):357–360. [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg B., Montana V., Wei M. D., Wuskell J. P., Loew L. M. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J. 1988 May;53(5):785–794. doi: 10.1016/S0006-3495(88)83158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emaus R. K., Grunwald R., Lemasters J. J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986 Jul 23;850(3):436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Farkas D. L., Wei M. D., Febbroriello P., Carson J. H., Loew L. M. Simultaneous imaging of cell and mitochondrial membrane potentials. Biophys J. 1989 Dec;56(6):1053–1069. doi: 10.1016/S0006-3495(89)82754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier N., Ducet G., Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr. 1987 Jun;19(3):297–303. doi: 10.1007/BF00762419. [DOI] [PubMed] [Google Scholar]
- Gores G. J., Nieminen A. L., Fleishman K. E., Dawson T. L., Herman B., Lemasters J. J. Extracellular acidosis delays onset of cell death in ATP-depleted hepatocytes. Am J Physiol. 1988 Sep;255(3 Pt 1):C315–C322. doi: 10.1152/ajpcell.1988.255.3.C315. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Pfeiffer D. R. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990 May;258(5 Pt 1):C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Restrepo D., Gunter K. K. Conversion of esterified fura-2 and indo-1 to Ca2+-sensitive forms by mitochondria. Am J Physiol. 1988 Sep;255(3 Pt 1):C304–C310. doi: 10.1152/ajpcell.1988.255.3.C304. [DOI] [PubMed] [Google Scholar]
- Haddad J., Decker M. L., Hsieh L. C., Lesch M., Samarel A. M., Decker R. S. Attachment and maintenance of adult rabbit cardiac myocytes in primary cell culture. Am J Physiol. 1988 Jul;255(1 Pt 1):C19–C27. doi: 10.1152/ajpcell.1988.255.1.C19. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Nicholls D. G., Williamson J. R. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem. 1980 Feb 25;255(4):1458–1464. [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Southard J. H. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976 Aug 25;251(16):5069–5077. [PubMed] [Google Scholar]
- Kawanishi T., Nieminen A. L., Herman B., Lemasters J. J. Suppression of Ca2+ oscillations in cultured rat hepatocytes by chemical hypoxia. J Biol Chem. 1991 Oct 25;266(30):20062–20069. [PubMed] [Google Scholar]
- Lemasters J. J., Stemkowski C. J., Ji S., Thurman R. G. Cell surface changes and enzyme release during hypoxia and reoxygenation in the isolated, perfused rat liver. J Cell Biol. 1983 Sep;97(3):778–786. doi: 10.1083/jcb.97.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nazareth W., Yafei N., Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991 Dec;23(12):1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Schwartz P., Piper H. M., Spahr R., Spieckermann P. G. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am J Pathol. 1984 Jun;115(3):349–361. [PMC free article] [PubMed] [Google Scholar]
- Seksek O., Henry-Toulmé N., Sureau F., Bolard J. SNARF-1 as an intracellular pH indicator in laser microspectrofluorometry: a critical assessment. Anal Biochem. 1991 Feb 15;193(1):49–54. doi: 10.1016/0003-2697(91)90042-r. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Perlman M. E., London R. E., Murphy E. Mechanism of preconditioning. Ionic alterations. Circ Res. 1993 Jan;72(1):112–125. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- Szabó I., Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem. 1991 Feb 25;266(6):3376–3379. [PubMed] [Google Scholar]