Abstract
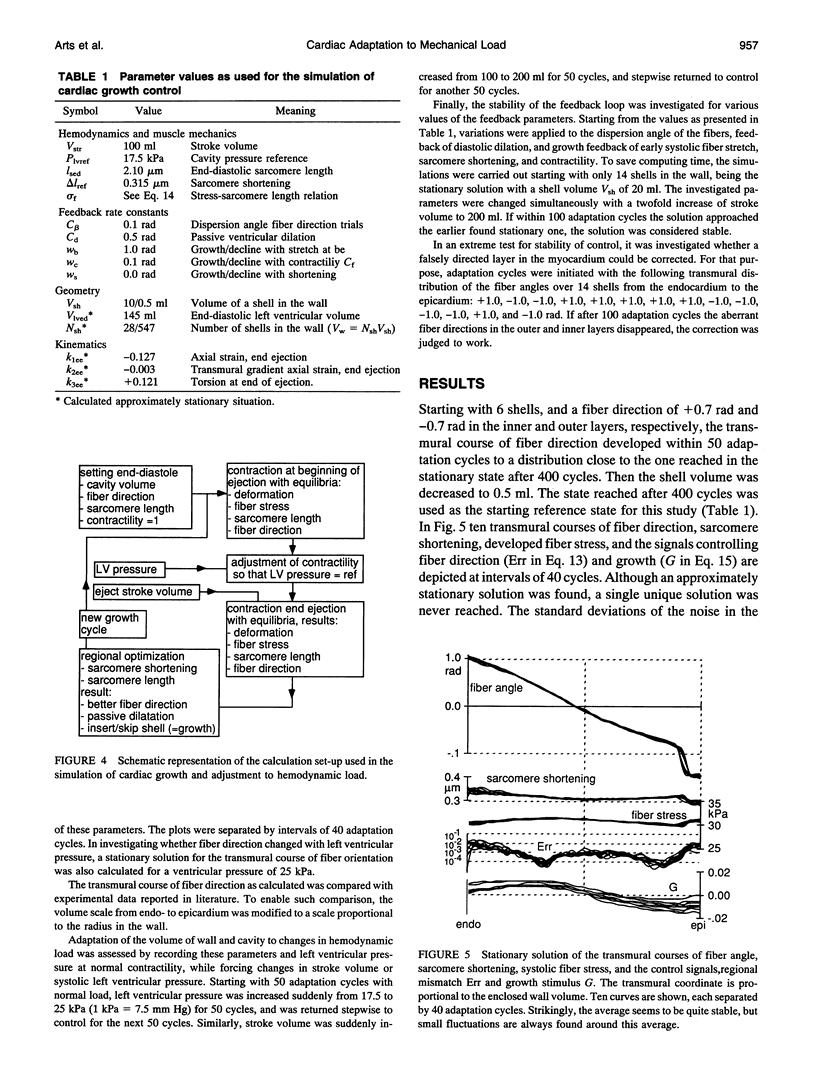
In the cardiac left ventricle during systole mechanical load of the myocardial fibers is distributed uniformly. A mechanism is proposed by which control of mechanical load is distributed over many individual control units acting in the environment of the cell. The mechanics of the equatorial region of the left ventricle was modeled by a thick-walled cylinder composed of 6-1500 shells of myocardial fiber material. In each shell a separate control unit was simulated. The direction of the cells was varied so that systolic fiber shortening approached a given optimum of 15%. End-diastolic sarcomere length was maintained at 2.1 microns. Regional early-systolic stretch and global contractility stimulated growth of cellular mass. If systolic shortening was more than normal the passive extracellular matrix stretched. The design of the load-controlling mechanism was derived from biological experiments showing that cellular processes are sensitive to mechanical deformation. After simulating a few hundred adaptation cycles, the macroscopic anatomical arrangement of helical pathways of the myocardial fibers formed automatically. If pump load of the ventricle was changed, wall thickness and cavity volume adapted physiologically. We propose that the cardiac anatomy may be defined and maintained by a multitude of control units for mechanical load, each acting in the cellular environment. Interestingly, feedback through fiber stress is not a compelling condition for such control.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi T., Mirsky I., Flanagan M. F., Currier J. J., Colan S. D., Fujii A. M. Myocardial function in immature and mature sheep with pressure-overload hypertrophy. Am J Physiol. 1992 Apr;262(4 Pt 2):H1036–H1048. doi: 10.1152/ajpheart.1992.262.4.H1036. [DOI] [PubMed] [Google Scholar]
- Arts T., Bovendeerd P. H., Prinzen F. W., Reneman R. S. Relation between left ventricular cavity pressure and volume and systolic fiber stress and strain in the wall. Biophys J. 1991 Jan;59(1):93–102. doi: 10.1016/S0006-3495(91)82201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts T., Hunter W. C., Douglas A., Muijtjens A. M., Reneman R. S. Description of the deformation of the left ventricle by a kinematic model. J Biomech. 1992 Oct;25(10):1119–1127. doi: 10.1016/0021-9290(92)90068-c. [DOI] [PubMed] [Google Scholar]
- Arts T., Reneman R. S. Dynamics of left ventricular wall and mitral valve mechanics--a model study. J Biomech. 1989;22(3):261–271. doi: 10.1016/0021-9290(89)90093-6. [DOI] [PubMed] [Google Scholar]
- Arts T., Reneman R. S., Veenstra P. C. A model of the mechanics of the left ventricle. Ann Biomed Eng. 1979;7(3-4):299–318. doi: 10.1007/BF02364118. [DOI] [PubMed] [Google Scholar]
- Bovendeerd P. H., Arts T., Huyghe J. M., van Campen D. H., Reneman R. S. Dependence of local left ventricular wall mechanics on myocardial fiber orientation: a model study. J Biomech. 1992 Oct;25(10):1129–1140. doi: 10.1016/0021-9290(92)90069-d. [DOI] [PubMed] [Google Scholar]
- Campbell S. E., Korecky B., Rakusan K. Remodeling of myocyte dimensions in hypertrophic and atrophic rat hearts. Circ Res. 1991 Apr;68(4):984–996. doi: 10.1161/01.res.68.4.984. [DOI] [PubMed] [Google Scholar]
- Chadwick R. S. Mechanics of the left ventricle. Biophys J. 1982 Sep;39(3):279–288. doi: 10.1016/S0006-3495(82)84518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D., Weber K. T., Eghbali M. Regulation of fibrillar collagen types I and III and basement membrane type IV collagen gene expression in pressure overloaded rat myocardium. Circ Res. 1990 Oct;67(4):787–794. doi: 10.1161/01.res.67.4.787. [DOI] [PubMed] [Google Scholar]
- Contard F., Koteliansky V., Marotte F., Dubus I., Rappaport L., Samuel J. L. Specific alterations in the distribution of extracellular matrix components within rat myocardium during the development of pressure overload. Lab Invest. 1991 Jan;64(1):65–75. [PubMed] [Google Scholar]
- Flaherty J. T., Pierce J. E., Ferrans V. J., Patel D. J., Tucker W. K., Fry D. L. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ Res. 1972 Jan;30(1):23–33. doi: 10.1161/01.res.30.1.23. [DOI] [PubMed] [Google Scholar]
- Gaasch W. H., Andrias C. W., Levine H. J. Chronic aortic regurgitation: the effect of aortic valve replacement on left ventricular volume, mass and function. Circulation. 1978 Nov;58(5):825–836. doi: 10.1161/01.cir.58.5.825. [DOI] [PubMed] [Google Scholar]
- Gelpi R. J., Pasipoularides A., Lader A. S., Patrick T. A., Chase N., Hittinger L., Shannon R. P., Bishop S. P., Vatner S. F. Changes in diastolic cardiac function in developing and stable perinephritic hypertension in conscious dogs. Circ Res. 1991 Feb;68(2):555–567. doi: 10.1161/01.res.68.2.555. [DOI] [PubMed] [Google Scholar]
- Grimm A. F., Lin H. L., Grimm B. R. Left ventricular free wall and intraventricular pressure-sarcomere length distributions. Am J Physiol. 1980 Jul;239(1):H101–H107. doi: 10.1152/ajpheart.1980.239.1.H101. [DOI] [PubMed] [Google Scholar]
- HILL A. V., WEBER H. H., ASTBURY W. T., DUBUISSON M., BAILEY K., PRYOR M. G. M., LUNDSGAARD E., NEEDHAM D., ELLIOTT A., BARER R. A discussion on muscular contraction and relaxation: their physical and chemical basis. Proc R Soc Lond B Biol Sci. 1950;137(886):40–87. doi: 10.1098/rspb.1950.0015. [DOI] [PubMed] [Google Scholar]
- Hammond G. L., Wieben E., Markert C. L. Molecular signals for initiating protein synthesis in organ hypertrophy. Proc Natl Acad Sci U S A. 1979 May;76(5):2455–2459. doi: 10.1073/pnas.76.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghe J. M., van Campen D. H., Arts T., Heethaar R. M. A two-phase finite element model of the diastolic left ventricle. J Biomech. 1991;24(7):527–538. doi: 10.1016/0021-9290(91)90286-v. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro I., Kaida T., Shibazaki Y., Kurabayashi M., Katoh Y., Hoh E., Takaku F., Yazaki Y. Stretching cardiac myocytes stimulates protooncogene expression. J Biol Chem. 1990 Mar 5;265(7):3595–3598. [PubMed] [Google Scholar]
- Mann D. L., Kent R. L., Cooper G., 4th Load regulation of the properties of adult feline cardiocytes: growth induction by cellular deformation. Circ Res. 1989 Jun;64(6):1079–1090. doi: 10.1161/01.res.64.6.1079. [DOI] [PubMed] [Google Scholar]
- McDermott P. J., Rothblum L. I., Smith S. D., Morgan H. E. Accelerated rates of ribosomal RNA synthesis during growth of contracting heart cells in culture. J Biol Chem. 1989 Oct 25;264(30):18220–18227. [PubMed] [Google Scholar]
- Monrad E. S., Hess O. M., Murakami T., Nonogi H., Corin W. J., Krayenbuehl H. P. Time course of regression of left ventricular hypertrophy after aortic valve replacement. Circulation. 1988 Jun;77(6):1345–1355. doi: 10.1161/01.cir.77.6.1345. [DOI] [PubMed] [Google Scholar]
- Mukherjee D., Sen S. Collagen phenotypes during development and regression of myocardial hypertrophy in spontaneously hypertensive rats. Circ Res. 1990 Dec;67(6):1474–1480. doi: 10.1161/01.res.67.6.1474. [DOI] [PubMed] [Google Scholar]
- Omens J. H., Fung Y. C. Residual strain in rat left ventricle. Circ Res. 1990 Jan;66(1):37–45. doi: 10.1161/01.res.66.1.37. [DOI] [PubMed] [Google Scholar]
- Parker T. G., Schneider M. D. Growth factors, proto-oncogenes, and plasticity of the cardiac phenotype. Annu Rev Physiol. 1991;53:179–200. doi: 10.1146/annurev.ph.53.030191.001143. [DOI] [PubMed] [Google Scholar]
- Pollack G. H., Krueger J. W. Sarcomere dynamics in intact cardiac muscle. Eur J Cardiol. 1976 May;4 (Suppl):53–65. [PubMed] [Google Scholar]
- Prinzen F. W., Augustijn C. H., Arts T., Allessie M. A., Reneman R. S. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol. 1990 Aug;259(2 Pt 2):H300–H308. doi: 10.1152/ajpheart.1990.259.2.H300. [DOI] [PubMed] [Google Scholar]
- Ross M. A., Streeter D. D., Jr Nonuniform subendocardial fiber orientation in the normal macaque left ventricle. Eur J Cardiol. 1975 Oct;3(3):229–247. [PubMed] [Google Scholar]
- Rubanyi G. M., Freay A. D., Kauser K., Johns A., Harder D. R. Mechanoreception by the endothelium: mediators and mechanisms of pressure- and flow-induced vascular responses. Blood Vessels. 1990;27(2-5):246–257. doi: 10.1159/000158816. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Jahn L., Takahashi T., Kulik T. J., Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem. 1992 May 25;267(15):10551–10560. [PubMed] [Google Scholar]
- Sadoshima J., Takahashi T., Jahn L., Izumo S. Roles of mechano-sensitive ion channels, cytoskeleton, and contractile activity in stretch-induced immediate-early gene expression and hypertrophy of cardiac myocytes. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9905–9909. doi: 10.1073/pnas.89.20.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel J. L., Barrieux A., Dufour S., Dubus I., Contard F., Koteliansky V., Farhadian F., Marotte F., Thiéry J. P., Rappaport L. Accumulation of fetal fibronectin mRNAs during the development of rat cardiac hypertrophy induced by pressure overload. J Clin Invest. 1991 Nov;88(5):1737–1746. doi: 10.1172/JCI115492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama S., Ross J., Jr, Franklin D., Bloor C. M., Bishop S., Dilley R. B. Adaptations of the left ventricle to chronic pressure overload. Circ Res. 1976 Mar;38(3):172–178. doi: 10.1161/01.res.38.3.172. [DOI] [PubMed] [Google Scholar]
- Shigematsu S., Hiramatsu K., Aizawa T., Yamada T., Takasu N., Niwa A., Miyahara Y., Tsujino M., Shimizu Z. Regression of left ventricular hypertrophy in patients with essential hypertension: outcome of 12 years antihypertensive treatment. Cardiology. 1990;77(4):280–286. doi: 10.1159/000174609. [DOI] [PubMed] [Google Scholar]
- Sigurdson W., Ruknudin A., Sachs F. Calcium imaging of mechanically induced fluxes in tissue-cultured chick heart: role of stretch-activated ion channels. Am J Physiol. 1992 Apr;262(4 Pt 2):H1110–H1115. doi: 10.1152/ajpheart.1992.262.4.H1110. [DOI] [PubMed] [Google Scholar]
- Waldman L. K., Fung Y. C., Covell J. W. Transmural myocardial deformation in the canine left ventricle. Normal in vivo three-dimensional finite strains. Circ Res. 1985 Jul;57(1):152–163. doi: 10.1161/01.res.57.1.152. [DOI] [PubMed] [Google Scholar]
- Watson P. A. Function follows form: generation of intracellular signals by cell deformation. FASEB J. 1991 Apr;5(7):2013–2019. doi: 10.1096/fasebj.5.7.1707019. [DOI] [PubMed] [Google Scholar]
- Weber K. T., Janicki J. S., Shroff S. G., Pick R., Chen R. M., Bashey R. I. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988 Apr;62(4):757–765. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- Yazaki Y., Komuro I., Yamazaki T., Tobe K., Maemura K., Kadowaki T., Nagai R. Role of protein kinase system in the signal transduction of stretch-mediated protooncogene expression and hypertrophy of cardiac myocytes. Mol Cell Biochem. 1993 Feb 17;119(1-2):11–16. doi: 10.1007/BF00926847. [DOI] [PubMed] [Google Scholar]
- Yoran C., Covell J. W., Ross J., Jr Structural basis for the ascending limb of left ventricular function. Circ Res. 1973 Feb;32(2):297–303. doi: 10.1161/01.res.32.2.297. [DOI] [PubMed] [Google Scholar]
- de Tombe P. P., ter Keurs H. E. Sarcomere dynamics in cat cardiac trabeculae. Circ Res. 1991 Feb;68(2):588–596. doi: 10.1161/01.res.68.2.588. [DOI] [PubMed] [Google Scholar]
- ter Keurs H. E., Rijnsburger W. H., van Heuningen R., Nagelsmit M. J. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res. 1980 May;46(5):703–714. doi: 10.1161/01.res.46.5.703. [DOI] [PubMed] [Google Scholar]
- van der Vusse G. J., Arts T., Glatz J. F., Reneman R. S. Transmural differences in energy metabolism of the left ventricular myocardium: fact or fiction. J Mol Cell Cardiol. 1990 Jan;22(1):23–37. doi: 10.1016/0022-2828(90)90969-9. [DOI] [PubMed] [Google Scholar]


