Abstract
This paper describes a quantitative and sensitive chemical assay for cereulide, the heat-stable emetic toxin produced by Bacillus cereus. The methods previously available for measuring cereulide are bioassays that give a toxicity titer, but not an accurate concentration. The dose of cereulide causing illness in humans is therefore not known, and thus safety limits for cereulide cannot be indicated. We developed a quantitative and sensitive chemical assay for cereulide based on high-performance liquid chromatography (HPLC) connected to ion trap mass spectrometry. This chemical assay and a bioassay based on boar sperm motility inhibition were calibrated with purified cereulide and with valinomycin, a structurally similar cyclic depsipeptide. The boar spermatozoan motility assay and chemical assay gave uniform results over a wide range of cereulide concentrations, ranging from 0.02 to 230 μg ml−1. The detection limit for cereulide and valinomycin by HPLC-mass spectrometry was 10 pg per injection. The combined chemical and biological assays were used to define conditions and concentrations of cereulide formation by B. cereus strains F4810/72, NC7401, and F5881. Cereulide production commenced at the end of logarithmic growth, but was independent of sporulation. Production of cereulide was enhanced by incubation with shaking compared to static conditions. The three emetic B. cereus strains accumulated 80 to 166 μg of cereulide g−1 (wet weight) when grown on solid medium. Strain NC7401 accumulated up to 25 μg of cereulide ml−1 in liquid medium at room temperature (21 ± 1°C) in 1 to 3 days, during the stationary growth phase when cell density was 2 × 108 to 6 × 108 CFU ml−1. Cereulide production at temperatures at and below 8°C or at 40°C was minimal.
Bacillus cereus is frequently diagnosed as a cause of gastrointestinal disorders (10, 18). The heat-stable toxin of B. cereus, cereulide, results in vomiting within 1 to 5 h of ingestion and has been linked to illness leading to fatalities or requiring hospitalization (27). Cereulide is a mitochondriotoxin (22, 28) and causes emesis in primates (30, 32). Cereulide present in food may represent the most serious food safety risk linked to B. cereus. B. cereus spores occur widely in foods and survive extended storage (29). The levels of B. cereus reported in food poisonings range from 102 to 108 CFU g−1 (8). It is generally believed that any food exceeding 104 to 105 cells or spores per g may not be safe for consumption (8, 15, 24). This number is often exceeded in a wide range of foods that are actually consumed. Nonetheless, illness is relatively rare (23) considering the high levels (>105 CFU) of B. cereus that are consumed (24). This probably reflects the wide variation of pathogenic potential and overall diversity among B. cereus strains (16).
Several studies indicated that only a minority of B. cereus isolates may produce cereulide (4, 21, 26). Cereulide is a small stable depsipeptide that is resistant to inactivation by heat (2, 4), proteases, acid, or alkali (21, 28). Bioassays that are currently used for measuring cereulide give an approximate toxicity titer, but not an accurate concentration (6, 13, 21). The dose of cereulide causing illness in humans is therefore not known. Safety limits for cereulide in foods thus cannot be indicated, such as have been set for the common fungally produced toxins aflatoxin B1, ochratoxin A, and trichothecenes (1).
In this paper, we describe a quantitative and sensitive chemical assay for cereulide [cyclo(l-O-Val-l-Val-d-O-Leu-d-Ala-)3] and valinomycin [cyclo(l-Val-d-HylVa-d-Val-l-Lac-)3], based on liquid chromatography connected to ion trap mass spectrometry (MS). In addition, we demonstrate the calibration between the chemical assay and a bioassay based on boar sperm motility inhibition (6). The boar spermatozoan motility assay and chemical assay gave uniform results over a wide range of cereulide concentrations, ranging from 0.02 to 230 μg ml−1. We used the combined chemical and biological assays to define conditions and concentrations of cereulide production by B. cereus.
MATERIALS AND METHODS
Strains.
B. cereus strain NC7401 was obtained from N. Agata (Nagoya City Public Health Institute, Nagoya, Japan). Strains F5881 and F528 were obtained from A. C. Scoging (Public Health Laboratory Service, London, United Kingdom). F4810/72 (SMR 178) was obtained from A. Christiansson (Swedish Dairy Association; strain originally from Public Health Laboratory Service) OH599 was obtained from M. Haapasalo (Institute of Dentistry, University of Helsinki, Helsinki, Finland). ATCC 14579 was obtained from the American Type Culture Collection, Manassas, Va.
Cultivation and preparation of cell extracts.
The strains were grown on tryptic soy agar (Difco) plates or in Trypticase soy broth (BBL). Biomass from plates was collected, and cells were lysed by three repeated freeze-thaw cycles and extracted with 98% methanol (10 ml per g of biomass [wet weight]) overnight. The mixture was centrifuged at 3,800 rpm (2,500 × g) in a tabletop centrifuge, and the supernatant was collected. The sample was evaporated to dryness, weighed, and dissolved in methanol. Cells grown in liquid culture were extracted two times with an equal volume of pentane for 1 h. The combined pentane phases were evaporated to dryness under a stream of nitrogen, and the residue was dissolved in 1 ml of methanol. Samples were stored at −20°C until analyzed. The extraction efficiency of spiked valinomycin was >80% (data not shown).
Analytical methods.
Valinomycin (Fluka, Buchs, Switzerland) and cereulide purified from B. cereus (6), used as standards, were dissolved in methanol and diluted in methanol as needed. High-performance liquid chromatography (HPLC)-MS analysis was performed on an Agilent series 1100 HPLC (Wilmington, Del.) equipped with a Supelco Discovery C8 column (100 by 2.1 mm, 5-μm particle size). The solvent was a mixture of 95% acetonitrile, 4.9% H2O, and 0.1% trifluoroacetic acid at a flow rate of 0.15 ml min−1, with a sample injection volume of 1.0 μl. A210 was monitored with a Diode array detector. The sample was introduced after chromatographic separation to a Bruker ESI electrospray ion trap mass analyzer (Bruker Daltonik GmbH). The source parameters were as follows: capillary, −3,700 V; end plate offset, −500 V; Nebulizer, 30.0 lb/in2; dry gas, 7.0 liters min−1; and dry temperature, 300°C. The ion trap had an ion target of 50,000, with a maximum accumulation time of 200 ms. A mass range from 500 to 1,300 m/z was collected.
The total ion chromatogram was smoothed with a Gaussian function and peak areas integrated with the Bruker data analysis software. For detection and quantification of low concentrations (<100 pg per injection) close to the detection limit, single ions 1129 and 1171 (NH4+ adducts) were monitored for valinomycin and cereulide, respectively. Standard curves were fitted by using the SigmaPlot 5.0 program.
Toxicity bioassay.
The methanol extract of the B. cereus strains was diluted in twofold steps, and a boar spermatozoan motility assay was performed as described previously (6). The extended boar semen (Super; AI Jalostuspalvelu, Rauhalinna, Finland) used is a standardized commercial product with optimized fertility properties obtained as composite semen from five boars, containing 27 × 106 spermatozoa ml−1. The extender was X-cell (IMV, Minneapolis, Minn.). The extended semen was used for toxicity testing within 2 days of collection. Briefly, 20-μl samples from each dilution and 2 ml of extended semen were incubated at room temperature overnight. For analysis of the motility of the spermatozoa, 200-μl subsamples were incubated at 37°C for 5 min, shaken for 5 s to oxygenate the extended semen, and inspected by phase-contrast microscopy. Motility was subjectively recorded and compared to that of a negative control and a positive control. The motility test was originally calibrated with purified cereulide from strain F4810/72 as described previously (6) and expressed as the endpoint dilution causing a >50% change in motility. The 50% effective concentration (EC50) values (defined as the effective concentration causing a 50% decrease in sperm motility compared to a methanol control) with Super extended semen were 0.3 to 0.5 ng ml−1 for cereulide and 0.7 to 1.4 ng ml−1 for valinomycin. Valinomycin was used for routine verification of the assay.
Effect of growth and incubation conditions on cereulide production.
The minimum and maximum growth temperatures of the B. cereus strains were determined after a 10-day incubation on tryptic soy agar plates at temperatures from 10 to 52°C. The effect of growth and incubation temperature on cereulide production was tested with both cells grown on solid medium and cells grown in broth. B. cereus strains were grown on tryptic soy agar plates at temperatures of 11, 21, 40, and 42°C. Cells were harvested after 11 days of incubation, extracted with methanol, and analyzed by HPLC-MS as described above. The effect of incubation temperature on cells pregrown at 21°C was tested with strain NC7401. A 24-h-old culture grown in Trypticase soy broth at 21°C was dispensed into aliquots of 10 ml in 100-ml flasks. Triplicate cultures were then incubated on shakers at temperatures of 4, 8, and 21°C for 7 days. The cultures (including a set of reference cultures frozen at time 0) were extracted with pentane, and cereulide was analyzed by HPLC-MS as described above.
The effect of static versus shaken (150 rpm on a rotary shaker) incubation conditions was tested with strains NC7401 and F4810/72. One milliliter of Trypticase soy broth was inoculated (1/1,000) with a culture grown overnight. Duplicate cultures were incubated for either 24 or 70 h with or without shaking and then extracted for determination of the cereulide concentration.
Time course for cereulide production.
Replicate bottles of 10 ml of Trypticase soy broth were inoculated with an overnight culture of strain NC7401 and cultivated on a rotary shaker (150 rpm) at room temperature. At each time point, two culture bottles were used for determination of CFU, spore count, and cereulide concentration. A serial dilution series before and after heat treatment (15 min at 83°C) was plated on Trypticase soy agar plates, and CFU were counted after 24 h and 3 days of incubation at 28°C for viable and spore counts, respectively. Turbidity was also measured with a Klett-Summerson colorimeter. Cereulide concentrations were determined after extraction with pentane as described previously.
RESULTS
Analysis of cereulide and valinomycin.
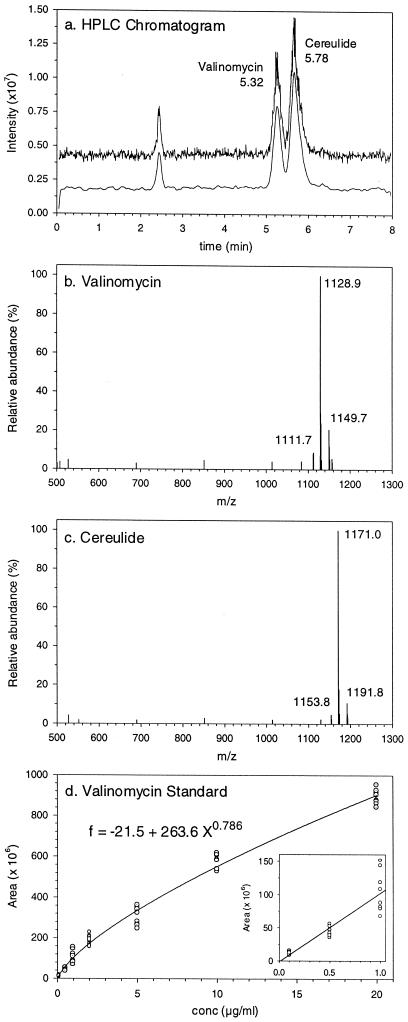
An HPLC method for the separation and analysis of the two structurally similar depsipeptide ionophores cereulide and valinomycin was developed, with detection by ion trap MS. With a C8 column and a solvent made up of 95% acetonitrile, 4.9% H2O, and 0.1% trifluoroacetic acid, the retention times of valinomycin and cereulide were 5.32 and 5.78 min, respectively (Fig. 1a). The typical mass spectra of cereulide and valinomycin are shown in Fig. 1b and c. The NH4+ adducts of valinomycin and cereulide had molecular weights of 1,128.9 and 1,171.1, respectively.
FIG. 1.
HPLC-MS analysis of cereulide and valinomycin. (a) HPLC chromatogram (500 to 1,300 m/z) showing separation of cereulide and valinomycin. (b) Mass spectrum of valinomycin. (c) Mass spectrum of cereulide. (d) Calibration curve for valinomycin determined from four injections of two separate dilution series.
Since cereulide is not commercially available, a calibration standard for routine use was made by using valinomcyin. The responses of valinomycin and cereulide in HPLC-MS analysis were very similar (within 10% [data not shown]). Mass spectra were scanned from 500 to 1,300 m/z, and the total ion chromatogram was integrated after smoothing. The variability in peak areas of replicate injections of the same sample was below ±10% for valinomycin concentrations of 1 to 10 μg ml−1. As seen in Fig. 1d, a linear curve could be used for concentrations below 1 ng per 1-μl injection, while a second-order equation (f = −21.5 + 263.6 X0.786) gave the best fit over a range of 0.1 to 20 ng per injection. Ten picograms per injection could readily be detected and quantified by using selected ions with molecular weights of 1,128.9 for valinomycin and 1,171.1 for cereulide (data not shown) without interference from other materials present in the pentane extracts (detected by A210 [data not shown]).
Comparison of HPLC-MS and toxicity bioassay.
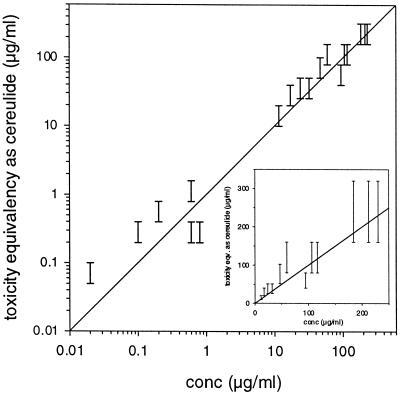
Methanol extracts from several independent preparations of the various emetic B. cereus strains were analyzed by HPLC-MS and the boar spermatozoan motility bioassay (Fig. 2). The cereulide concentrations as determined by HPLC-MS ranged from 0.02 to 230 μg ml−1. Based on twofold dilution steps, the toxicity bioassay gave a concentration range that corresponded well to the HPLC-MS assay results. The bioassay was independently calibrated with purified cereulide. The results from analysis of several different emetic strains demonstrated that the boar spermatozoan motility assay read after 1 day of incubation and the chemical assay gave uniform results over a wide range of cereulide concentrations, ranging from 0.02 to 230 μg ml−1. This also confirms that the compound responsible for the observed toxicity of the strains is cereulide.
FIG. 2.
Comparison of chemical and biological assays for determining cereulide concentrations in methanol extracts from independent preparations of different B. cereus strains. The line indicates a 1:1 correlation. The inset shows the same data drawn to a linear scale.
Effect of growth and incubation conditions on cereulide production.
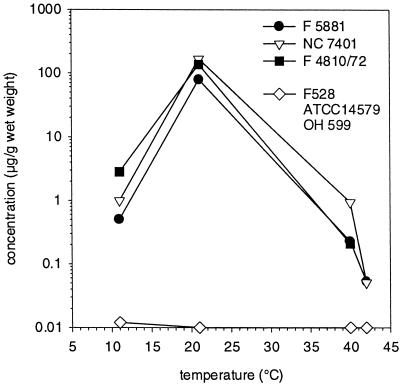
The HPLC-MS and toxicity assays were used in combination to determine the effect of different growth and incubation conditions on cereulide production by the emetic B. cereus strains. The minimum growth temperature of the tested strains was 10 to 11°C, while the maximum growth temperature ranged from 42 to 47°C. The effect of temperature on cereulide production by B. cereus strains was tested after growth for 11 days at temperatures of 11, 21, 40, and 42°C (Fig. 3). The levels of cereulide production by the three emetic strains F5881, F4810/72, and NC7401 were very similar. At 21°C, the strains accumulated 80 to 166 μg of cereulide g−1 (wet weight). Assuming that dry weight is approximately 20% of wet weight, cereulide corresponded to approximately 0.1% of cell dry weight. At 11°C, cereulide accumulation ranged from 0.5 to 2.8 μg g−1 (wet weight), while cereulide production at 40 and 42°C was negligible (<0.2 μg g−1 [wet weight], with the exception of strain NC7401, which accumulated 0.9 μg g−1 at 40°C). The nonemetic strains F528, OH599, and ATCC 14579 did not produce cereulide (<0.02 μg ml−1) at any temperature.
FIG. 3.
Production of cereulide by B. cereus strains NC7401, F5881, and F4810/72 compared to that by F528, ATCC 14579, and OH599 (mean) grown on tryptic soy agar plates for 11 days at different temperatures.
Cereulide production by strain NC7401 pregrown at 21°C was also tested at temperatures below the minimal growth temperature of 11°C (Table 1). Cell suspensions incubated at 21°C accumulated over 10 μg of cereulide ml−1 within 7 days, while cereulide production at temperatures at and below 8°C was minimal.
TABLE 1.
Effect of incubation temperature on cereulide production by B. cereus strain NC7401 pregrown at 21°C
| Temp (°C) | Cereulide production (μg ml of broth culture−1)a |
|---|---|
| 4 | 0.016 ± 0.002 |
| 8 | 0.071 ± 0.003 |
| 21 | 13.2 ± 2.3 |
| Inoculumb | 0.03 ± 0.003 |
Values represent the mean ± standard deviation of three replicate cultures after 7 days of incubation.
Inoculum of pregrown cells.
Initial experiments suggested that agitation of broth cultures affected cereulide production. This was tested in detail with strains NC7401 and F4810/72. Broth cultures inoculated 1/1,000 with an overnight culture were incubated with or without shaking (Table 2). Although growth levels were nearly identical based on turbidity (data not shown), cereulide production in static incubations was minimal compared to that in cultures incubated on a rotary shaker. This suggests that aeration may be required for cereulide production.
TABLE 2.
Comparison of levels of cereulide production by B. cereus strains NC7401 and F4810/72 incubated with or without shaking
| Strain | Incubation time (h) | Cereulide production (μg ml of culture−1)a
|
|
|---|---|---|---|
| Static | Shaken | ||
| NC7401 | 24 | 0.0 ± 0.0 | 1.36 ± 0.12 |
| F4810/72 | 24 | 0.0 ± 0.0 | 1.07 ± 0.02 |
| NC7401 | 70 | 0.16 ± 0.12 | 21.98 ± 2.21 |
The cereulide concentration was analyzed after 24 or 70 h of incubation at 21°C.
Cereulide production at different phases of growth.
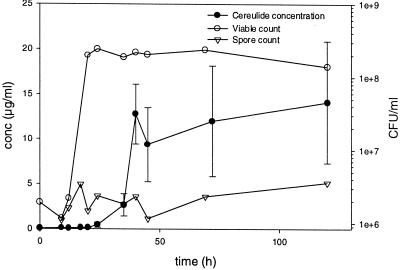
Growth, spore formation, and production of cereulide by strain NC7401 grown at room temperature in Trypticase soy broth (on a rotary shaker at 150 rpm) are shown in Fig. 4. The culture reached stationary phase within 20 h, with a cell density of 2 × 108 to 6 × 108 CFU ml−1 (corresponding to 430 ± 20 Klett units). Cereulide production did not commence until after the end of logarithmic growth, approximately 24 h into the stationary phase. Cereulide accumulated to 7 to 22 μg ml−1, which corresponds to approximately 1 to 5% of the B. cereus biomass dry weight if we assume an estimated dry weight of 1 pg per cell (the cereulide amount). The spore count did not substantially increase over 120 h, indicating that cereulide production was independent of sporulation.
FIG. 4.
Growth of B. cereus strain NC7401 in Trypticase soy broth and production of cereulide.
DISCUSSION
B. cereus is widespread (31), and due to spore formation, it is difficult to eliminate from foodstuffs. Maximum allowed levels have therefore been set in only a few countries and for few foods (e.g., see reference 25). For example, for dried baby foods, 103 CFU g−1 has been set as the legal limit (11) or limit of concern (Food and Drug Administration, cited in reference 7). For freshly cut vegetables and sprouts in France, 102 CFU g−1 (m, which is the threshold below which all results are considered satisfactory) and 104 CFU g−1 (M, which is the acceptability threshold) (12) are the limits. In Sweden for vanilla sauce powder and other dried products from nonfermented milk, 103 CFU ml−1 (m) and 104 CFU ml−1 (M) (19) are the limits. The Dutch authorities have set as a general rule that the level of B. cereus in all food products should be below 105 CFU g−1 (12).
Incidents of food poisoning, however, have been reported at B. cereus concentrations similar to or below these threshold values (15, 23). It thus appears that there is no clear margin of safety. B. cereus emetic syndrome is considered an intoxication caused by ingestion of the preformed vomiting-inducing toxin, cereulide (20). Identification of conditions favorable for cereulide production in foods has been difficult due to the lack of suitable assays for accurately measuring cereulide concentrations. Bioassays are error prone and when based on twofold dilution steps are able to detect only a difference in cereulide production of >50%. The work described in the present paper was aimed at developing accurate and rapid analytical methods for measuring cereulide, so that emetic toxin-producing strains can be eliminated and/or processing conditions that prevent toxin synthesis can be identified.
We developed a sensitive and rapid chemical assay for cereulide (and valinomycin), based on separation by HPLC followed by ion trap MS detection. The ion (m/z, 1,171.1; NH4+ adduct) was chosen as specific and selective for cereulide, based on its described chemical structure (2, 6, 17). The ion range of 500 to 1,300 m/z was analyzed with minimal interference from other compounds present in the pentane or methanol extracts of biomass. One of the three emetic toxin strains chosen for the present study, F4810/72, is a proven emesis-provoking strain by the monkey feeding assay (3, 32). Strain NC7401 has been tested in Suncus murinus (house musk shrew) (3), and the third strain, F5881, has been shown to produce cereulide (26). The results in this paper show that the results obtained by the boar spermatozoan motility assay and the chemical assay matched well. The chemical assay is more accurate (standard deviation, ±10%) than the bioassay (50%, based on twofold dilution steps).
Motility of boar spermatozoa is exclusively dependent on oxidative phosphorylation in the mitochondria. Since the motility inhibition assay is sensitive also to other microbial mitochondriotoxins (e.g., gramicidin), the assay is not specific for cereulide (6). The good correlation between the chemical assay for cereulide and the biological assay measuring mitochondrial damage indicates that the mitochondrial toxicity exhibited by the emetic strain F4810/72 (monkey feeding assay) (3, 32) is caused by cereulide. This further confirms that cereulide is a mitochondrial toxin, as suspected by Sakurai et al. (28).
The emetic strain NC7401 accumulated 10 to 25 μg of cereulide ml−1 in liquid broth during the stationary growth phase, which corresponds to up to 10% of the B. cereus biomass dry weight. Cereulide production was not connected to sporulation as believed earlier. Biomass collected from solid medium was estimated to contain up to 1% (cell dry weight) cereulide. Agata et al. (5) reported cereulide concentrations of 5 μg ml−1 in 10% skim milk and 1 μg ml−1 in Trypticase soy broth cultures of B. cereus strain NC7401 grown at 30°C to similar cell densities. Cereulide production at a temperature below 8°C or above 40°C was minimal. Recently, Finlay et al. (14) reported that the production of heat-stable cytotoxin by several strains of B. cereus was higher at +12 to +15°C than at +30°C. Most likely, this assay also measured cereulide, and the temperature optimum for cereulide production may thus be different (lower) from that for growth. Based on our results, food poisoning risk cannot directly be evaluated based on resident CFU of B. cereus; the toxin content has to be measured as well. It is also possible that storage (e.g., in the cafeteria) of food at a lukewarm (30 or 40°C) dispensing desk involves less poisoning risk by emetic B. cereus than storage in a poorly operating cold room.
The dose that induces emesis in humans is not known, but in Suncus murinus, it was 8 μg kg−1 (3, 17), and for rhesus monkeys of 7 to 8 kg of body weight, 70 μg per animal was reported (30). If humans are similarly sensitive, as little as 10 to 50 g of food, contaminated by a dose of an emetic strain of B. cereus of 108 CFU g−1, could lead to emetic illness. Cereulide production in broth cultures commenced at the end of logarithmic growth and was enhanced by shaking conditions. Christianson et al. (9) demonstrated a similar enhanced production of a cytotoxic protein by B. cereus in aerated compared to static cultures. We did not attempt to optimize cereulide production by the selected strains in the present study. However, it is possible that at optimal conditions, concentrations of cereulide even higher than those discussed above will be found.
Acknowledgments
We acknowledge financial support of the Academy of Finland (grant no. 50733).
The work was performed while M.M.H. was at the University of Helsinki on sabbatical leave from Rutgers University.
REFERENCES
- 1.Abramson, D. 2000. Mycotoxins. Toxicology, p. 1539-1547. In R. K. Robinson, C. A. Batt, and P. D. Patel (ed.), Encyclopedia of food microbiology, vol. 2. Academic Press, San Diego, Calif. [Google Scholar]
- 2.Agata, N., M. Mori, M. Ohta, S. Suwan, I. Ohtani, and M. Isobe. 1994. A novel dodecadepsipeptide, cereulide, isolated from Bacillus cereus causes vacuole formation in Hep-2 cells. FEMS Microbiol. Lett. 121:31-34. [DOI] [PubMed] [Google Scholar]
- 3.Agata, N., M. Ohta, M. Mori, and M. Isobe. 1995. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 129:17-20. [DOI] [PubMed] [Google Scholar]
- 4.Agata, N., M. Ohta, and M. Mori. 1996. Production of an emetic toxin, cereulide, is associated with a specific class of Bacillus cereus. Curr. Microbiol. 33:67-69. [DOI] [PubMed] [Google Scholar]
- 5.Agata, N., M. Ohta, M. Mori, and K. Shibayama. 1999. Growth conditions of and emetic toxin production by Bacillus cereus in a defined medium with amino acids. Microbiol. Immunol. 43:15-18. [DOI] [PubMed] [Google Scholar]
- 6.Andersson, M. A., R. Mikkola, J. Helin, M. C. Andersson, and M. Salkinoja-Salonen. 1998. A novel sensitive bioassay for detection of Bacillus cereus emetic toxin and related depsipeptide ionophores. Appl. Environ. Microbiol. 64:1338-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batt, C. A. 2000. Bacillus cereus, p. 119-124. In R. K. Robinson, C. A. Batt, and P. D. Patel (ed.), Encyclopedia of food microbiology, vol. 1. Academic Press, San Diego, Calif. [Google Scholar]
- 8.Beattie, S. H., and A. G. Williams. 2000. Detection of toxins, p. 141-158. In R. K. Robinson, C. A. Batt, and P. D. Patel (ed.), Encyclopedia of food microbiology, vol. 1. Academic Press, San Diego, Calif. [Google Scholar]
- 9.Christianson, A., A. S. Naidu, I. Nilsson, T. Wadström, and H.-E. Pettersson. 1989. Toxin production by Bacillus cereus dairy isolates in milk at low temperatures. Appl. Environ. Microbiol. 55:2595-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drobniewski, F. A. 1993. Bacillus cereus and related species. Clin. Microbiol. Rev. 6:324-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elintarvikevirasto. 1984. Päätös lastenruokien mikrobiologisista laatuvaatimuksista 1023/84. (Decision on the microbiological safety criteria of baby foods.) Elintarvikevirasto, Helsinki, Finland.
- 12.European Commission. 1997. Harmonization of safety criteria for minimally processed foods. Inventory report FAIR concerted actions. FAIR CT96-1020. European Commission, Brussels, Belgium.
- 13.Finlay, W. J., N. A. Logan, and A. D. Sutherland. 1999. Semiautomated metabolic staining assay for Bacillus cereus emetic toxin. Appl. Environ. Microbiol. 65:1811-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finlay, W. J., N. A. Logan, and A. D. Sutherland. 2000. Bacillus cereus produces most emetic toxin at lower temperatures. Lett. Appl. Microbiol. 31:385-389. [DOI] [PubMed] [Google Scholar]
- 15.Granum, P. E. 1997. Bacillus cereus. Detection of toxins, p. 327-336. In R. K. Robinson, C. A. Batt, and P. D. Patel (ed.), Encyclopedia of food microbiology, vol. 1. Academic Press, San Diego, Calif. [Google Scholar]
- 16.Helgason, E., D. A. Caugant, I. Olsen, and A.-B. Kolstø. 2000. Genetic structure of population of Bacillus cereus and B. thuringiensis isolates associated with periodontitis and other human infections. J. Clin. Microbiol. 38:1615-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isobe, M., T. Ishikawa, S. Suwan, N. Agata, and M. Ohta. 1995. Synthesis and activity of cereulide, a cyclic dodecadepsipeptide ionophore as emetic toxin from Bacillus cereus. Bioorg. Med. Chem. Lett. 5:2855-2858. [Google Scholar]
- 18.Kotiranta, A., K. Lounatmaa, and M. Haapasalo. 2000. Epidemiology and pathogenesis of Bacillus cereus infection. Microb. Infect. 2:189-198. [DOI] [PubMed] [Google Scholar]
- 19.Livsmedelsverket. 1998. Vägledning för mikrobiologisk bedömmning av livsmedel. Livsmedelsverket, Uppsala, Sweden.
- 20.McKillip, J. L. 2000. Prevalence and expression of enterotoxins in Bacillus cereus and other Bacillus spp.: a literature review. Antonie Leeuwenhoek 77:393-399. [DOI] [PubMed] [Google Scholar]
- 21.Mikami, T., T. Horikawa, T. Murakami, T. Matsumoto, A. Yamakawa, S. Murayama, S. Katagiri, K. Shinagawa, and M. Suzuki. 1994. An improved method for detecting cytostatic toxin (emetic toxin) of Bacillus cereus and its application to food samples. FEMS Microbiol. Lett. 119:53-58. [DOI] [PubMed] [Google Scholar]
- 22.Mikkola, R., N.-E. L. Saris, P. A. Grigoriev, M. A. Andersson, and M. S. Salkinoja-Salonen. 1999. Ionophoretic properties and mitochondrial effects of cereulide, the emetic toxin of B. cereus. Eur. J. Biochem. 263:112-117. [DOI] [PubMed] [Google Scholar]
- 23.Notermans, S., J. Dufrenne, P. Teunis, R. Beumer, M. te Giffel, and P. P. Weem. 1997. A risk assessment study of Bacillus cereus present in pasteurised milk. Food Microbiol. 14:143-151. [Google Scholar]
- 24.Notermans, S., and C. A. Batt. 1998. A risk assessment approach for food-borne Bacillus cereus and its toxins. J. Appl. Microbiol. Symp. Suppl. 84:51S-61S. [DOI] [PubMed] [Google Scholar]
- 25.Pirttijärvi, T. 2000. Contaminant aerobic sporeforming bacteria in the manufacturing processes of food packaging board and food. Ph.D. thesis. University of Helsinki, Helsinki, Finland.
- 26.Pirttijärvi, T. S. M., M. A. Andersson, A. C. Scoging, and M. S. Salkinoja-Salonen. 1999. Evaluation of methods for recognizing strains of the Bacillus cereus group with food poisoning potential among industrial and environmental contaminants. Syst. Appl. Microbiol. 22:133-144. [DOI] [PubMed] [Google Scholar]
- 27.Rowan, N. J., J. G. Anderson, and A. Anderton. 1997. The bacteriological quality of hospital-prepared infant feeds. J. Hosp. Infect. 36:259-267. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai, N., K. A. Koike, Y. Irie, and H. Hayashi. 1994. The rice culture filtrate of Bacillus cereus isolated from emetic-type food poisoning causes mitochondrial swelling in Hep-2 cell. Microbiol. Immunol. 38:337-343. [DOI] [PubMed] [Google Scholar]
- 29.Setlow, P., and E. A. Johnson. 1997. Spores and their significance, p. 30-64. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers. ASM Press, Washington, D.C.
- 30.Shinagawa, K., H. Konuma, H. Sekita, and S. Sugii. 1995. Emesis of rhesus monkeys induced by intragastric administration with the Hep-2 vacuolation factor, cereulide, produced by Bacillus cereus. FEMS Microbiol. Lett. 130:87-90. [DOI] [PubMed] [Google Scholar]
- 31.te Giffel, M. C., R. R. Beumer, S. Leijendekkers, and F. M. Rombouts. 1996. Incidence of Bacillus cereus and Bacillus subtilis in foods in The Netherlands. Food Microbiol. 13:53-58. [Google Scholar]
- 32.Turnbull, P. C. B., J. M. Kramer, K. Jorgensen, R. J. Gilbert, and J. Melling. 1979. Properties and production characteristics of vomiting, diarrheal and necrotizing toxins of Bacillus cereus. Am. J. Clin. Nutr. 32:219-228. [DOI] [PubMed] [Google Scholar]