Abstract
We have synthesized a number of 1,2-diacyl phosphatidylcholines with hydrophobic substituents adjacent to the carbonyl group of the fatty acyl chain and studied their thermotropic phase behavior by differential scanning calorimetry, 31P-nuclear magnetic resonance spectroscopy, and x-ray diffraction. Our results indicate that the hydrocarbon chain-melting phase transition temperatures of these lipids are lower than those of the n-saturated diacylphosphatidylcholines of similar chain length. In the gel phase, the 2-alkyl substituents on the fatty acyl chains seem to inhibit the formation of tightly packed, partially dehydrated, quasi-crystalline bilayers (Lc phases), although possibly promoting the formation of chain-interdigitated bilayers. In the liquid-crystalline state, however, these 2-alkyl substituents destabilize the lamellar phase with respect to one or more inverted nonlamellar structures. In general, increases in the length, bulk, or rigidity of the alkyl substituent result in an increased destabilization of the lamellar gel and liquid-crystalline phases and a greater tendency to form inverted nonlamellar phases, the nature of which depends upon the size of the 2-alkyl substituent. Unlike normal non-lamella-forming lipids such as the phosphatidylethanolamines, increases in the length of the main acyl chain stabilize the lamellar phases and reduce the tendency to form nonlamellar structures. Our results establish that with a judicious choice of a 2-alkyl substituent and hydrocarbon chain length, phosphatidylcholines (and probably most other so-called "bilayer-preferring" lipids) can be induced to form a range of inverted nonlamellar structures at relatively low temperatures. The ability to vary the lamellar/nonlamellar phase preference of such lipids should be useful in studies of bilayer/nonbilayer phase transitions and of the molecular organization of various nonlamellar phases. Moreover, because the nonlamellar phases can easily be induced at physiologically relevant temperatures and hydration levels while avoiding changes in polar headgroup composition, this new class of 2-alkyl-substituted phosphatidylcholines should prove valuable in studies of the physiological role of non-lamella-forming lipids in reconstituted lipid-protein model membranes.
Full text
PDF


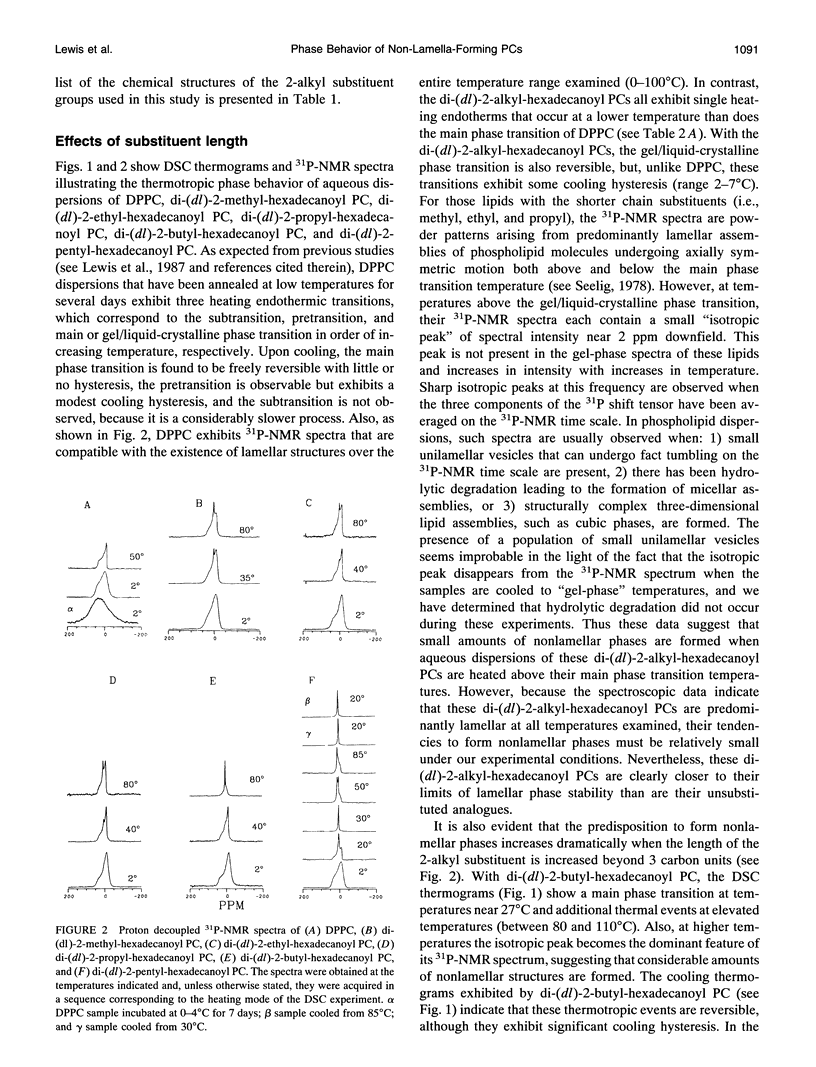
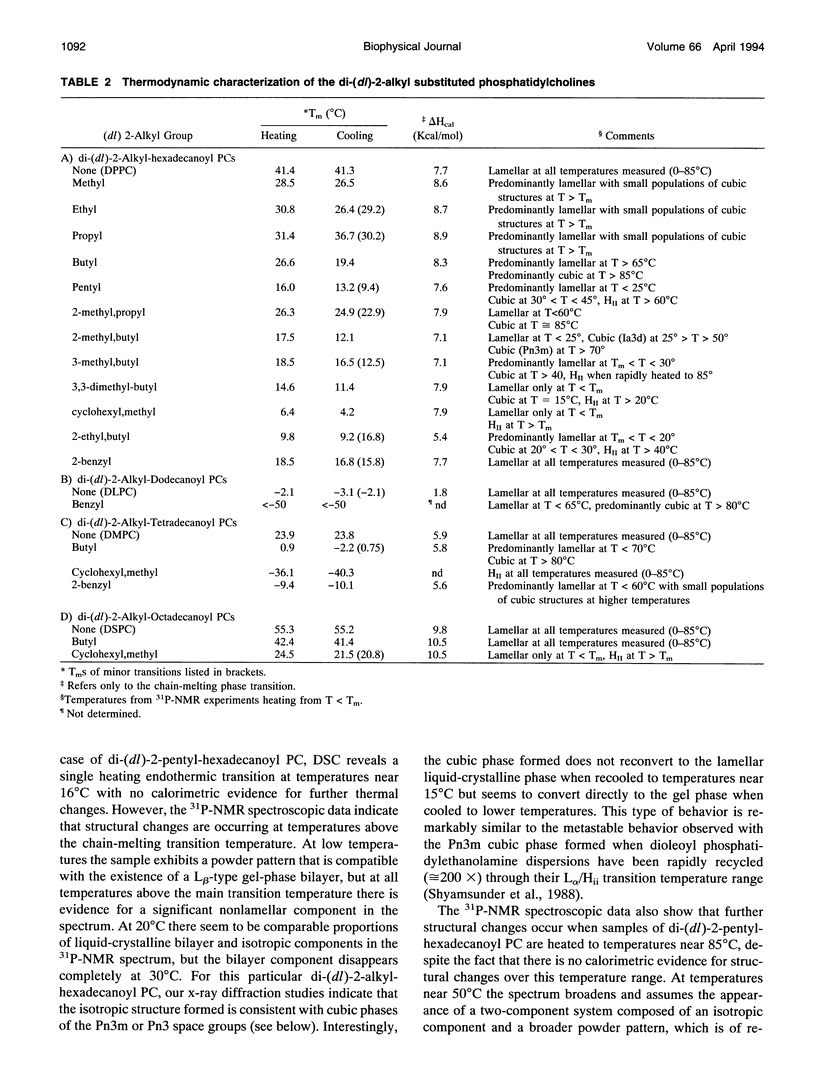
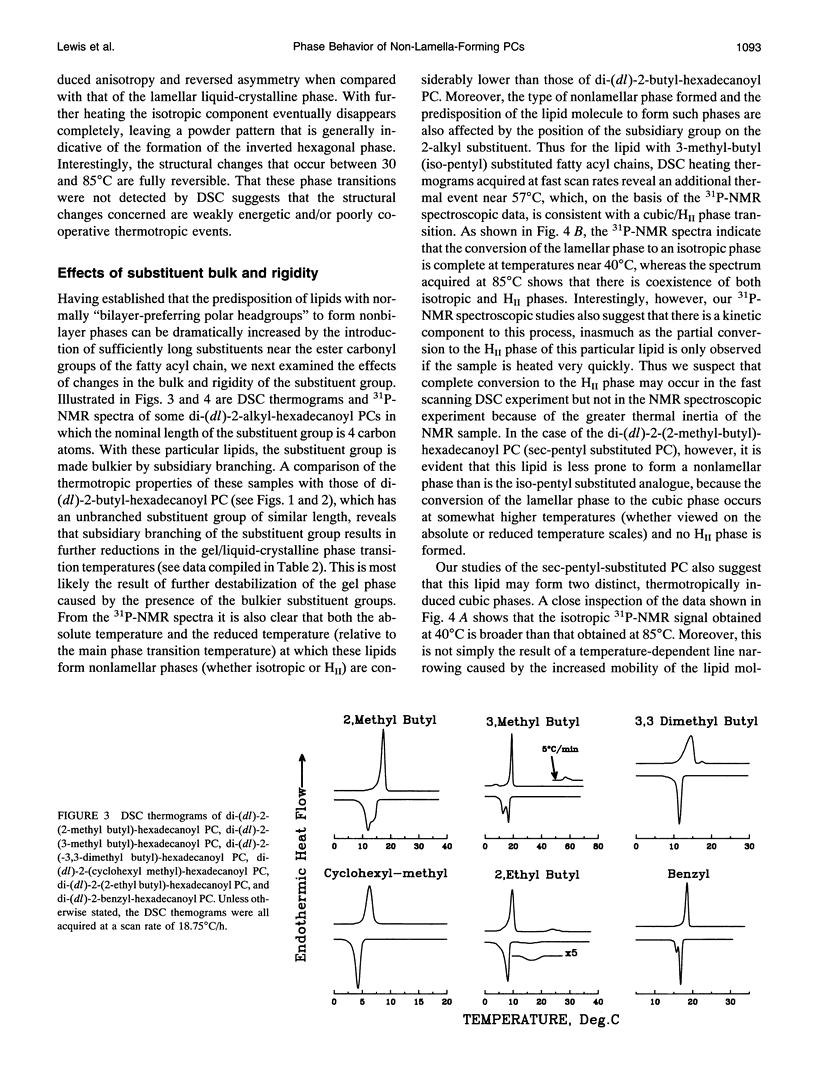
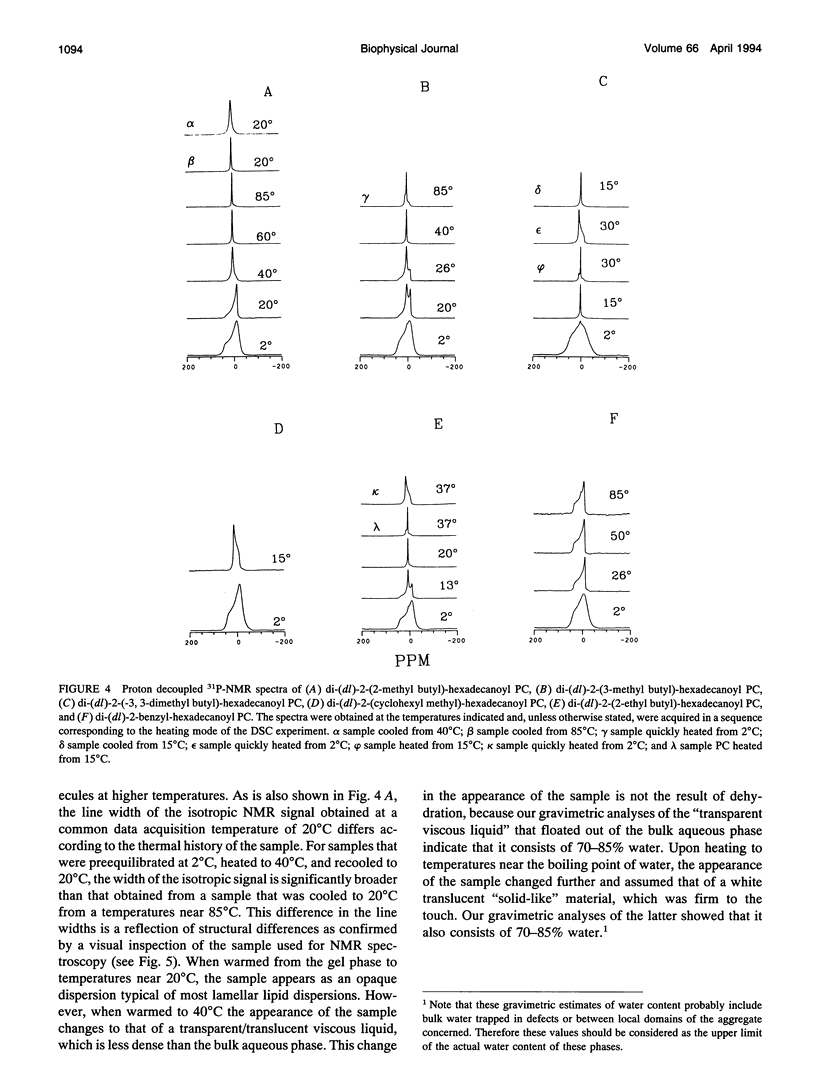

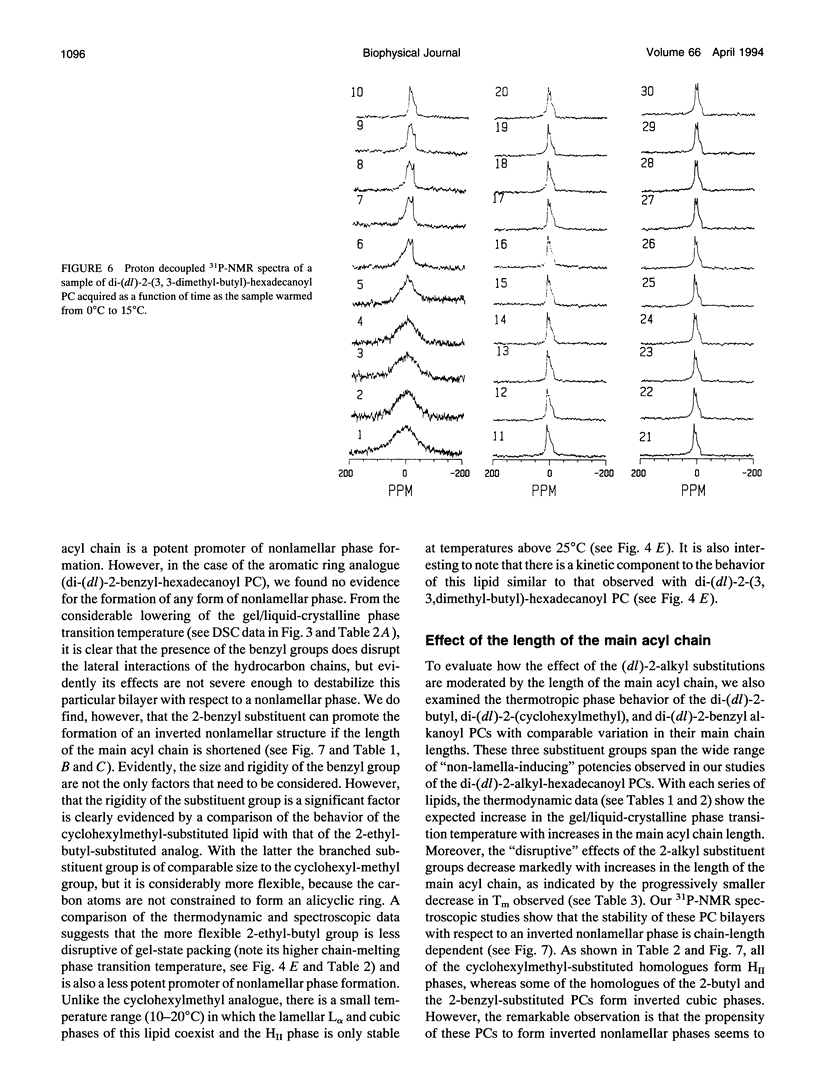
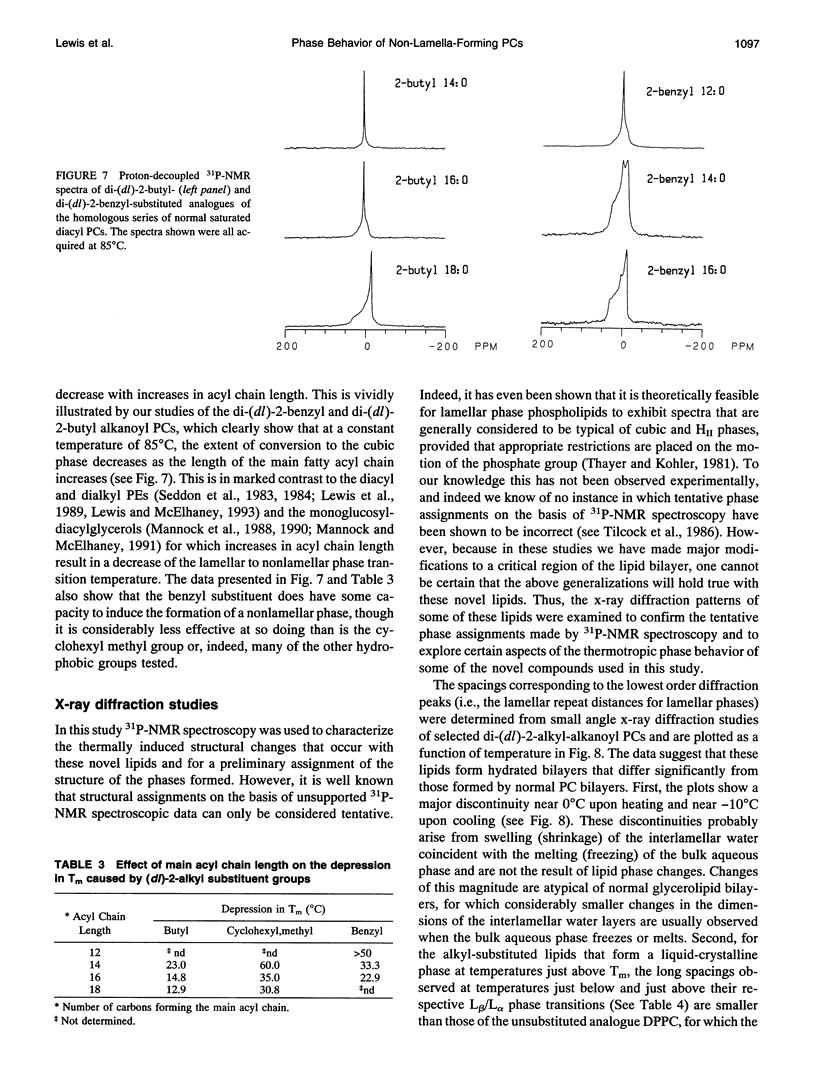
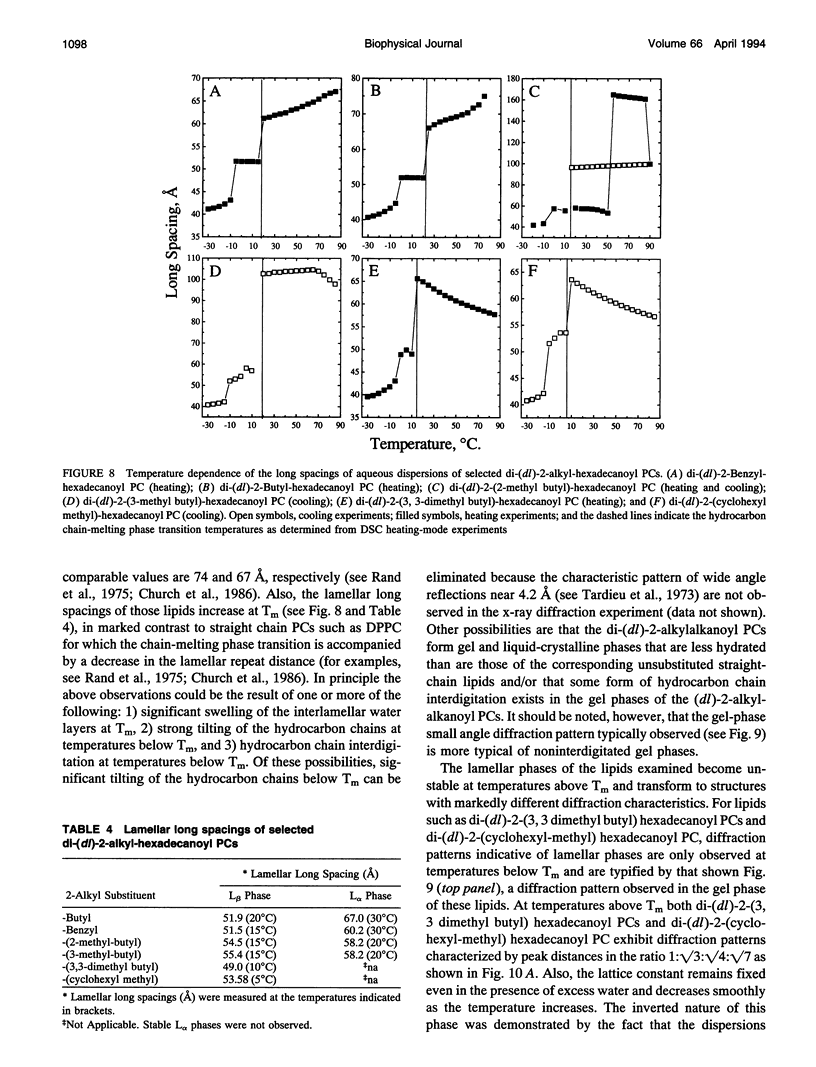
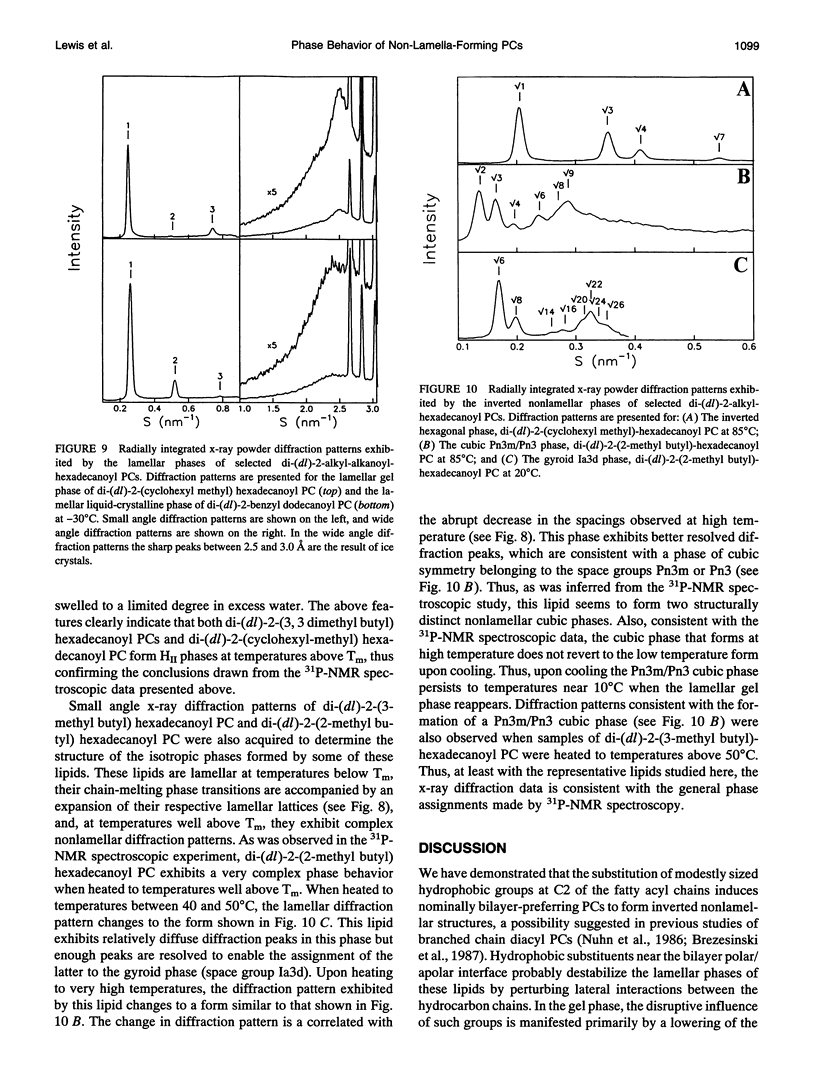



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. M., Gruner S. M., Leibler S. Geometrical aspects of the frustration in the cubic phases of lyotropic liquid crystals. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5364–5368. doi: 10.1073/pnas.85.15.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton P. G., Gunstone F. D. Hydrocarbon chain packing and molecular motion in phospholipid bilayers formed from unsaturated lecithins. Synthesis and properties of sixteen positional isomers of 1,2-dioctadecenoyl-sn-glycero-3-phosphorylcholine. J Biol Chem. 1975 Jun 25;250(12):4470–4476. [PubMed] [Google Scholar]
- Bhakoo M., Lewis R. N., McElhaney R. N. Isolation and characterization of a novel monoacylated glucopyranosyl neutral lipid from the plasma membrane of Acholeplasma laidlawii B. Biochim Biophys Acta. 1987 Oct 31;922(1):34–45. doi: 10.1016/0005-2760(87)90242-6. [DOI] [PubMed] [Google Scholar]
- Charp P. A., Zhou Q. Z., Wood M. G., Jr, Raynor R. L., Menger F. M., Kuo J. F. Synthetic branched-chain analogues of distearoylphosphatidylcholine: structure-activity relationship in inhibiting and activating protein kinase C. Biochemistry. 1988 Jun 28;27(13):4607–4612. doi: 10.1021/bi00413a004. [DOI] [PubMed] [Google Scholar]
- Church S. E., Griffiths D. J., Lewis R. N., McElhaney R. N., Wickman H. H. X-ray structure study of thermotropic phases in isoacylphosphatidylcholine multibilayers. Biophys J. 1986 Mar;49(3):597–605. doi: 10.1016/S0006-3495(86)83687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellens H., Siegel D. P., Alford D., Yeagle P. L., Boni L., Lis L. J., Quinn P. J., Bentz J. Membrane fusion and inverted phases. Biochemistry. 1989 May 2;28(9):3692–3703. doi: 10.1021/bi00435a011. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Bottega R. Determination of the phase behaviour of phosphatidylethanolamine admixed with other lipids and the effects of calcium chloride: implications for protein kinase C regulation. Biochim Biophys Acta. 1988 Oct 6;944(2):144–154. doi: 10.1016/0005-2736(88)90427-0. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Stafford A. R., Cheetham J. J., Bottega R., Ball E. H. The relationship between the bilayer to hexagonal phase transition temperature in membranes and protein kinase C activity. Biosci Rep. 1988 Feb;8(1):49–54. doi: 10.1007/BF01128971. [DOI] [PubMed] [Google Scholar]
- Goldfine H., Johnston N. C., Mattai J., Shipley G. G. Regulation of bilayer stability in Clostridium butyricum: studies on the polymorphic phase behavior of the ether lipids. Biochemistry. 1987 May 19;26(10):2814–2822. doi: 10.1021/bi00384a024. [DOI] [PubMed] [Google Scholar]
- Goldfine H., Rosenthal J. J., Johnston N. C. Lipid shape as a determinant of lipid composition in Clostridium butyricum. The effects of incorporation of various fatty acids on the ratios of the major ether lipids. Biochim Biophys Acta. 1987 Nov 13;904(2):283–289. doi: 10.1016/0005-2736(87)90377-4. [DOI] [PubMed] [Google Scholar]
- Gruner S. M., Cullis P. R., Hope M. J., Tilcock C. P. Lipid polymorphism: the molecular basis of nonbilayer phases. Annu Rev Biophys Biophys Chem. 1985;14:211–238. doi: 10.1146/annurev.bb.14.060185.001235. [DOI] [PubMed] [Google Scholar]
- Gruner S. M. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Sen A. Effects of lipid packing on polymorphic phase behavior and membrane properties. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5825–5829. doi: 10.1073/pnas.86.15.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Mitchell D. J., Ninham B. W. Theory of self-assembly of lipid bilayers and vesicles. Biochim Biophys Acta. 1977 Oct 17;470(2):185–201. doi: 10.1016/0005-2736(77)90099-2. [DOI] [PubMed] [Google Scholar]
- Johnston N. C., Goldfine H. Phospholipid aliphatic chain composition modulates lipid class composition, but not lipid asymmetry in Clostridium butyricum. Biochim Biophys Acta. 1985 Feb 28;813(1):10–18. doi: 10.1016/0005-2736(85)90339-6. [DOI] [PubMed] [Google Scholar]
- Keller S. L., Bezrukov S. M., Gruner S. M., Tate M. W., Vodyanoy I., Parsegian V. A. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys J. 1993 Jul;65(1):23–27. doi: 10.1016/S0006-3495(93)81040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., Mak N., McElhaney R. N. A differential scanning calorimetric study of the thermotropic phase behavior of model membranes composed of phosphatidylcholines containing linear saturated fatty acyl chains. Biochemistry. 1987 Sep 22;26(19):6118–6126. doi: 10.1021/bi00393a026. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Mannock D. A., McElhaney R. N., Turner D. C., Gruner S. M. Effect of fatty acyl chain length and structure on the lamellar gel to liquid-crystalline and lamellar to reversed hexagonal phase transitions of aqueous phosphatidylethanolamine dispersions. Biochemistry. 1989 Jan 24;28(2):541–548. doi: 10.1021/bi00428a020. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Calorimetric and spectroscopic studies of the polymorphic phase behavior of a homologous series of n-saturated 1,2-diacyl phosphatidylethanolamines. Biophys J. 1993 Apr;64(4):1081–1096. doi: 10.1016/S0006-3495(93)81474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing iso-branched fatty acids. 1. Differential scanning calorimetric studies. Biochemistry. 1985 May 7;24(10):2431–2439. doi: 10.1021/bi00331a007. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Sykes B. D., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing cis-monounsaturated acyl chain homologues of oleic acid: differential scanning calorimetric and 31P NMR spectroscopic studies. Biochemistry. 1988 Feb 9;27(3):880–887. doi: 10.1021/bi00403a007. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Yue A. W., McElhaney R. N., Turner D. C., Gruner S. M. Thermotropic characterization of the 2-O-acyl,polyprenyl alpha-D-glucopyranoside isolated from palmitate-enriched Acholeplasma laidlawii B membranes. Biochim Biophys Acta. 1990 Jul 9;1026(1):21–28. doi: 10.1016/0005-2736(90)90327-k. [DOI] [PubMed] [Google Scholar]
- Lindblom G., Brentel I., Sjölund M., Wikander G., Wieslander A. Phase equilibria of membrane lipids from Acholeplasma laidlawii: importance of a single lipid forming nonlamellar phases. Biochemistry. 1986 Nov 18;25(23):7502–7510. doi: 10.1021/bi00371a037. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., Lewis R. N., McElhaney R. N. Physical properties of glycosyl diacylglycerols. 1. Calorimetric studies of a homologous series of 1,2-di-O-acyl-3-O-(alpha-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1990 Aug 28;29(34):7790–7799. doi: 10.1021/bi00486a003. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., Lewis R. N., Sen A., McElhaney R. N. The physical properties of glycosyldiacylglycerols. Calorimetric studies of a homologous series of 1,2-di-O-acyl-3-O-(beta-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1988 Sep 6;27(18):6852–6859. doi: 10.1021/bi00418a030. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., McElhaney R. N. Differential scanning calorimetry and X-ray diffraction studies of a series of synthetic beta-D-galactosyl diacylglycerols. Biochem Cell Biol. 1991 Dec;69(12):863–867. doi: 10.1139/o91-128. [DOI] [PubMed] [Google Scholar]
- Mariani P., Luzzati V., Delacroix H. Cubic phases of lipid-containing systems. Structure analysis and biological implications. J Mol Biol. 1988 Nov 5;204(1):165–189. doi: 10.1016/0022-2836(88)90607-9. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N. The influence of membrane lipid composition and physical properties of membrane structure and function in Acholeplasma laidlawii. Crit Rev Microbiol. 1989;17(1):1–32. doi: 10.3109/10408418909105720. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N. The structure and function of the Acholeplasma laidlawii plasma membrane. Biochim Biophys Acta. 1984 Jan 27;779(1):1–42. doi: 10.1016/0304-4157(84)90002-9. [DOI] [PubMed] [Google Scholar]
- Nuhn P., Brezesinski G., Dobner B., Förster G., Gutheil M., Dörfler H. D. Synthesis, calorimetry, and X-ray diffraction of lecithins containing branched fatty acid chains. Chem Phys Lipids. 1986 Feb;39(3):221–236. doi: 10.1016/0009-3084(86)90012-5. [DOI] [PubMed] [Google Scholar]
- Patel K. M., Morrisett J. D., Sparrow J. T. A convenient synthesis of phosphatidylcholines: acylation of sn-glycero-3-phosphocholine with fatty acid anhydride and 4-pyrrolidinopyridine. J Lipid Res. 1979 Jul;20(5):674–677. [PubMed] [Google Scholar]
- Raheja R. K., Kaur C., Singh A., Bhatia I. S. New colorimetric method for the quantitative estimation of phospholipids without acid digestion. J Lipid Res. 1973 Nov;14(6):695–697. [PubMed] [Google Scholar]
- Rand R. P., Chapman D., Larsson K. Tilted hydrocarbon chains of dipalmitoyl lecithin become perpendicular to the bilayer before melting. Biophys J. 1975 Nov;15(11):1117–1124. doi: 10.1016/S0006-3495(75)85888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Marsh D. Calorimetric studies of the gel-fluid (L beta-L alpha) and lamellar-inverted hexagonal (L alpha-HII) phase transitions in dialkyl- and diacylphosphatidylethanolamines. Biochemistry. 1983 Mar 1;22(5):1280–1289. doi: 10.1021/bi00274a045. [DOI] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Shyamsunder E., Gruner S. M., Tate M. W., Turner D. C., So P. T., Tilcock C. P. Observation of inverted cubic phase in hydrated dioleoylphosphatidylethanolamine membranes. Biochemistry. 1988 Apr 5;27(7):2332–2336. doi: 10.1021/bi00407a014. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. I. Mechanism of the L alpha----HII phase transitions. Biophys J. 1986 Jun;49(6):1155–1170. doi: 10.1016/S0006-3495(86)83744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. II. Implications for membrane-membrane interactions and membrane fusion. Biophys J. 1986 Jun;49(6):1171–1183. doi: 10.1016/S0006-3495(86)83745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. R., Mak N., McElhaney R. N. Lipid and protein composition and thermotropic lipid phase transitions in fatty acid-homogeneous membranes of Acholeplasma laidlawii B. Biochim Biophys Acta. 1980 Apr 10;597(2):199–215. doi: 10.1016/0005-2736(80)90099-1. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Tate M. W., Eikenberry E. F., Turner D. C., Shyamsunder E., Gruner S. M. Nonbilayer phases of membrane lipids. Chem Phys Lipids. 1991 Mar;57(2-3):147–164. doi: 10.1016/0009-3084(91)90073-k. [DOI] [PubMed] [Google Scholar]
- Thayer A. M., Kohler S. J. Phosphorus-31 nuclear magnetic resonance spectra characteristic of hexagonal and isotropic phospholipid phases generated from phosphatidylethanolamine in the bilayer phase. Biochemistry. 1981 Nov 24;20(24):6831–6834. doi: 10.1021/bi00527a014. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Mombers C., Gerritsen W. J., Leunissen-Bijvelt L., Cullis P. R. Fusion of phospholipid vesicles in association with the appearance of lipidic particles as visualized by freeze fracturing. Biochim Biophys Acta. 1979 Aug 7;555(2):358–361. doi: 10.1016/0005-2736(79)90175-5. [DOI] [PubMed] [Google Scholar]
- Wieslander A., Christiansson A., Rilfors L., Lindblom G. Lipid bilayer stability in membranes. Regulation of lipid composition in Acholeplasma laidlawii as governed by molecular shape. Biochemistry. 1980 Aug 5;19(16):3650–3655. doi: 10.1021/bi00557a002. [DOI] [PubMed] [Google Scholar]



