Abstract
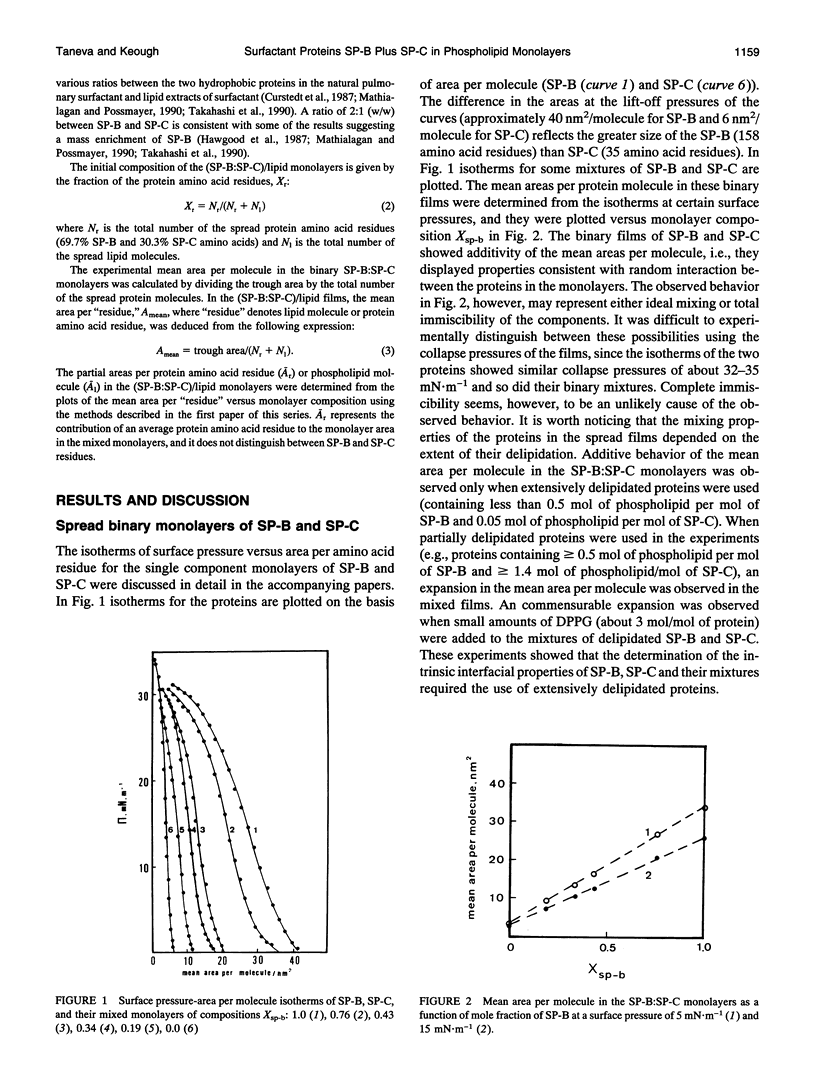
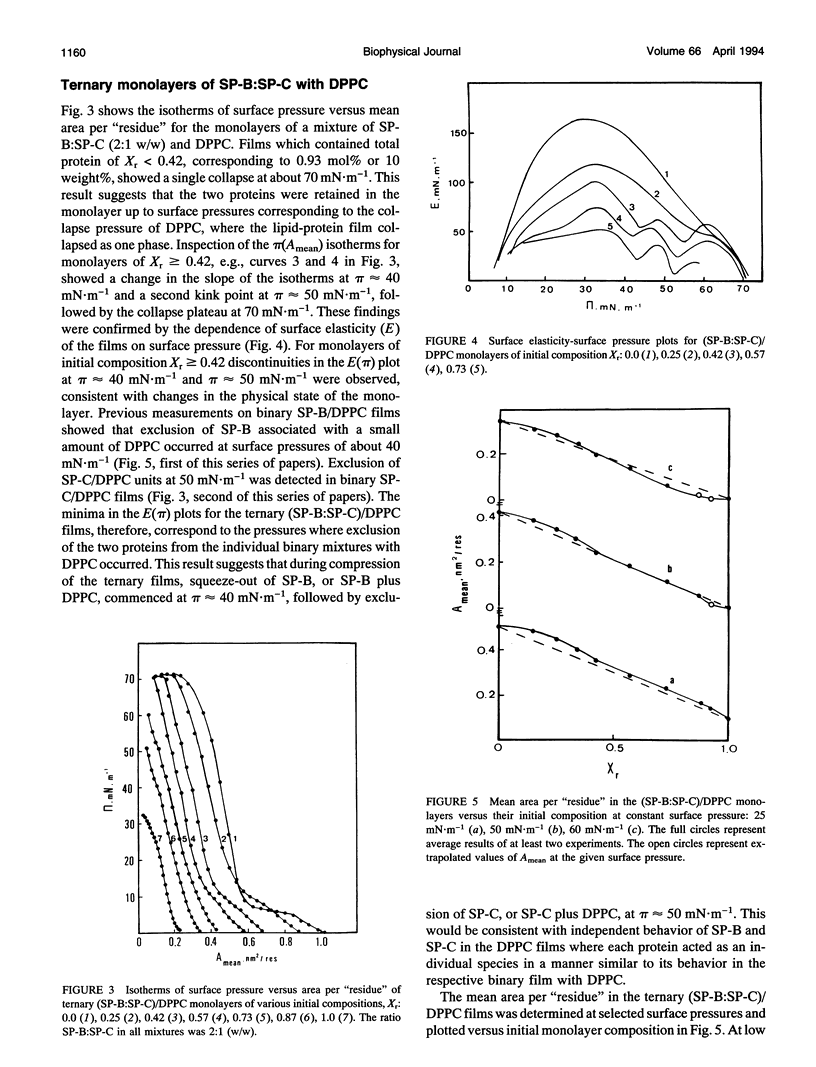
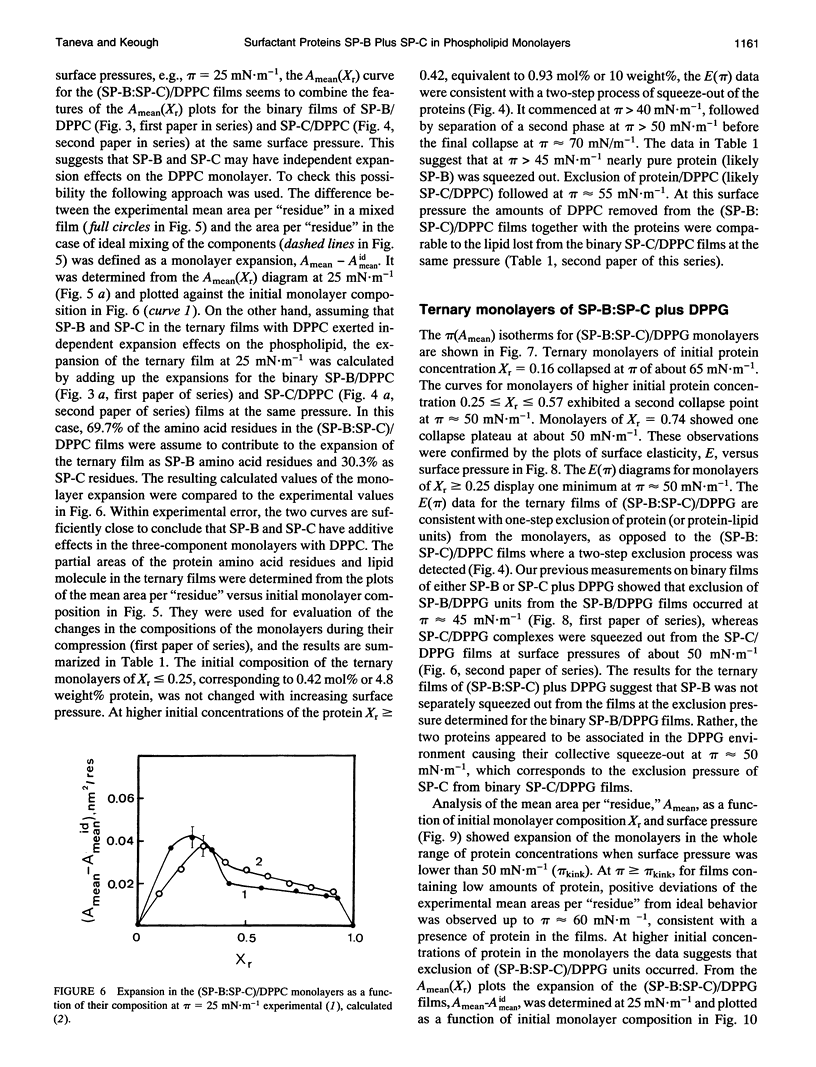
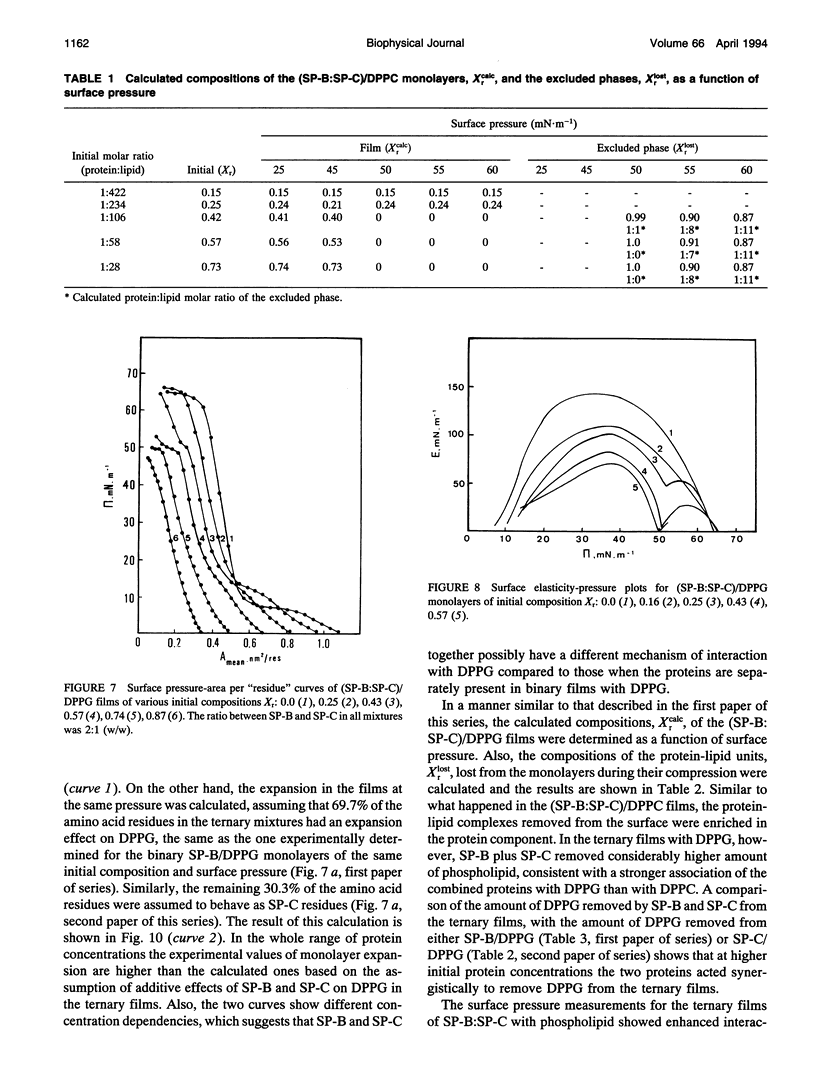
Spread binary monolayers of surfactant-associated proteins SP-B and SP-C were formed at the air-water interface. Surface pressure measurements showed no interactions between the hydrophobic proteins. The effects of a mixture of SP-B plus SP-C (2:1, w/w) on the properties of monolayers of dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylglycerol (DPPG), and DPPC:DPPG (7:3, mol:mol) were studied. During compression of ternary and quaternary films, containing less than 0.4 mol% or 5 weight% total protein, the proteins were not squeezed out and appeared to remain associated with the film until collapse at surface pressures of about 65-70 mN.m-1. At initial concentrations of total protein of about 0.9 mol% or 10 weight%, exclusion of protein-lipid complexes was observed at 40-50 mN.m-1. Larger amounts of phospholipid were removed by proteins from (SP-B:SP-C)/DPPG films than from (SP-B:SP-C)/DPPC ones. Separate squeeze-out of SP-B (or SP-B plus DPPC) at about 40 mN.m-1, followed by exclusion of SP-C (or SP-C plus DPPC) at about 50 mN.m-1, was observed in (SP-B:SP-C)/DPPC films. This led to a conclusion that there was independent behavior of SP-B and SP-C in (SP-B:SP-C)/DPPC monolayers. The quaternary (SP-B:SP-C)/(DPPC:DPPG) films showed qualitatively similar process of squeeze-out of the proteins. In the ternary mixtures of SP-B plus SP-C with DPPG separate exclusion of SP-B was not detected; rather, the data was consistent with exclusion of a (SP-B:SP-C)/DPPG complex at about 50 mN.m-1. The results imply possible interactions between SP-B and SP-C and the acidic phospholipid.
Full text
PDF
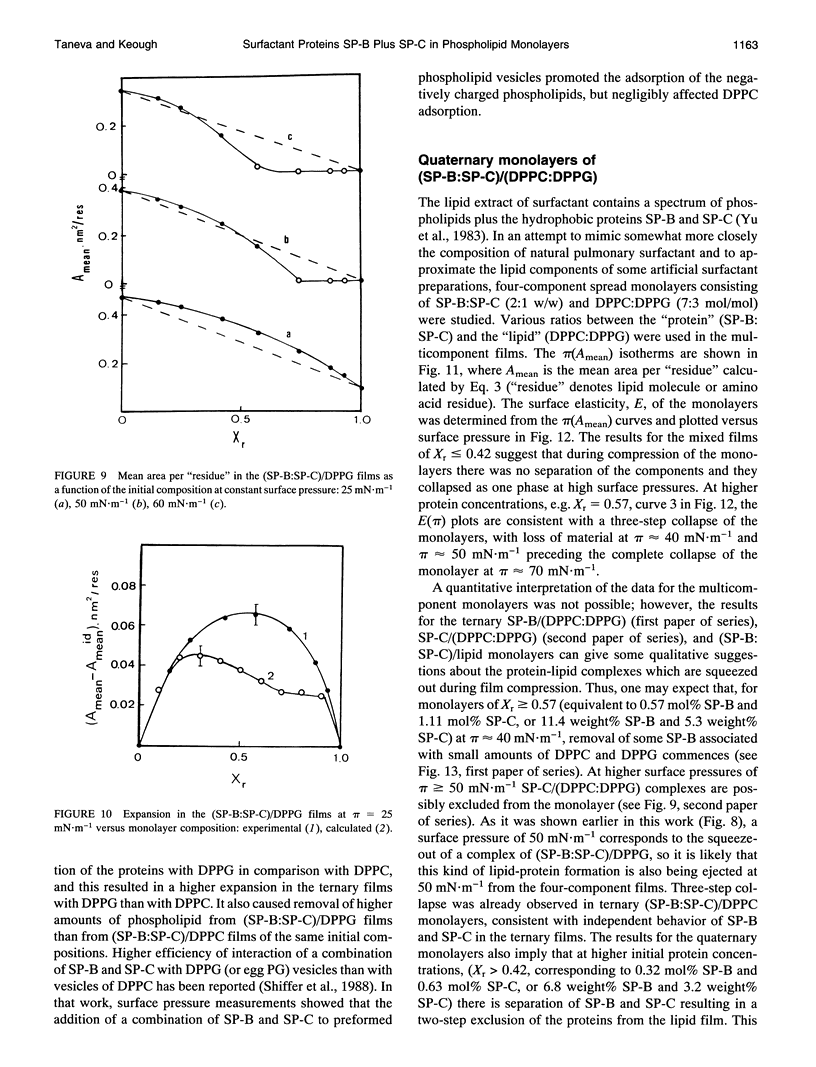
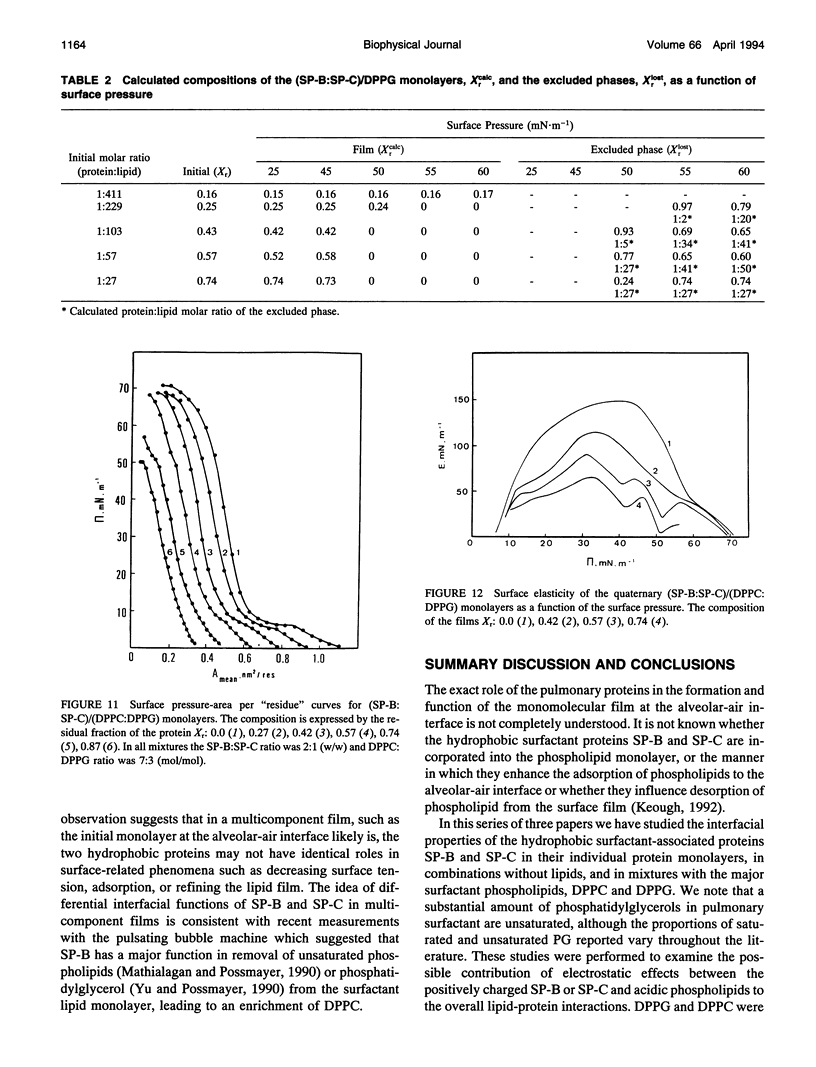


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Curstedt T., Jörnvall H., Robertson B., Bergman T., Berggren P. Two hydrophobic low-molecular-mass protein fractions of pulmonary surfactant. Characterization and biophysical activity. Eur J Biochem. 1987 Oct 15;168(2):255–262. doi: 10.1111/j.1432-1033.1987.tb13414.x. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Schilling J., Damm D., Clements J. A., White R. T. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28-36 in surfactant lipid adsorption. Proc Natl Acad Sci U S A. 1987 Jan;84(1):66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathialagan N., Possmayer F. Low-molecular-weight hydrophobic proteins from bovine pulmonary surfactant. Biochim Biophys Acta. 1990 Jul 16;1045(2):121–127. doi: 10.1016/0005-2760(90)90140-s. [DOI] [PubMed] [Google Scholar]
- Schürch S. Surface tension at low lung volumes: dependence on time and alveolar size. Respir Physiol. 1982 Jun;48(3):339–355. doi: 10.1016/0034-5687(82)90038-x. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Waring A. J., Amirkhanian J., Fan B., Taeusch H. W. Structure-function relationships of bovine pulmonary surfactant proteins: SP-B and SP-C. Biochim Biophys Acta. 1990 May 1;1044(1):43–49. doi: 10.1016/0005-2760(90)90216-k. [DOI] [PubMed] [Google Scholar]
- Yu S. H., Chung W., Olafson R. W., Harding P. G., Possmayer F. Characterization of the small hydrophobic proteins associated with pulmonary surfactant. Biochim Biophys Acta. 1987 Oct 17;921(3):437–448. doi: 10.1016/0005-2760(87)90070-1. [DOI] [PubMed] [Google Scholar]
- Yu S. H., Possmayer F. Comparative studies on the biophysical activities of the low-molecular-weight hydrophobic proteins purified from bovine pulmonary surfactant. Biochim Biophys Acta. 1988 Aug 12;961(3):337–350. doi: 10.1016/0005-2760(88)90081-1. [DOI] [PubMed] [Google Scholar]
- Yu S. H., Possmayer F. Role of bovine pulmonary surfactant-associated proteins in the surface-active property of phospholipid mixtures. Biochim Biophys Acta. 1990 Oct 1;1046(3):233–241. doi: 10.1016/0005-2760(90)90236-q. [DOI] [PubMed] [Google Scholar]
- Yu S., Harding P. G., Smith N., Possmayer F. Bovine pulmonary surfactant: chemical composition and physical properties. Lipids. 1983 Aug;18(8):522–529. doi: 10.1007/BF02535391. [DOI] [PubMed] [Google Scholar]


