Abstract
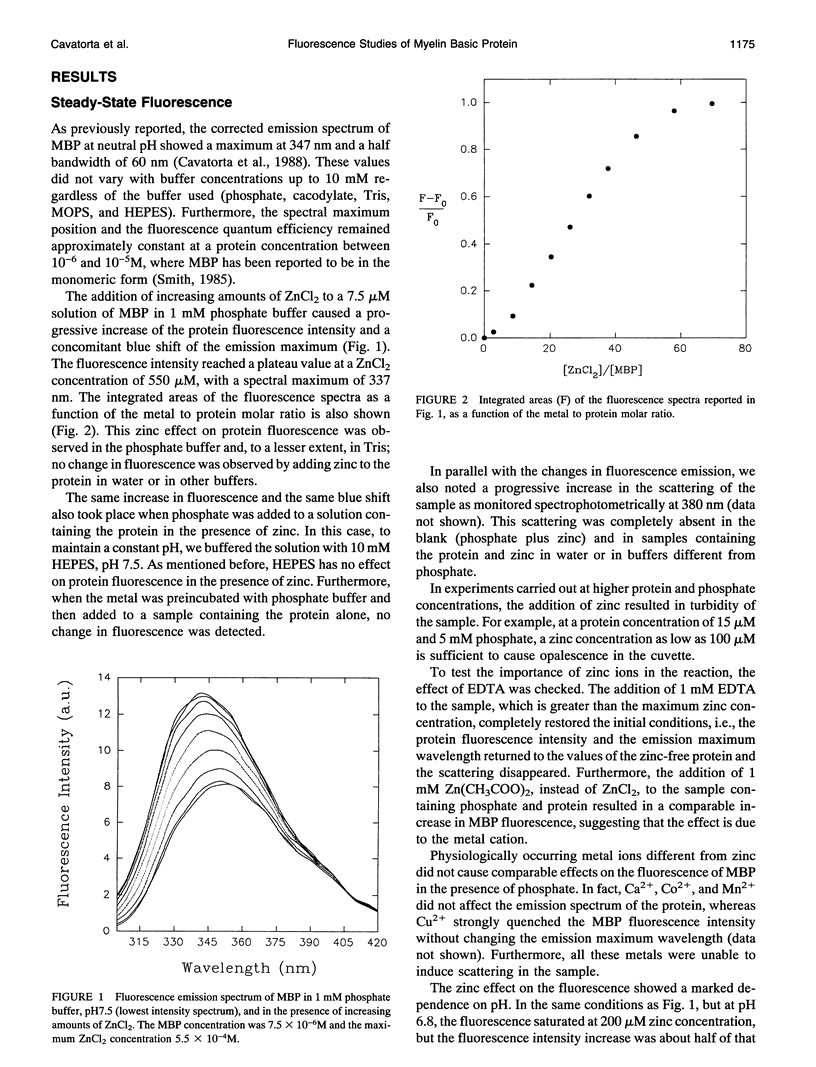
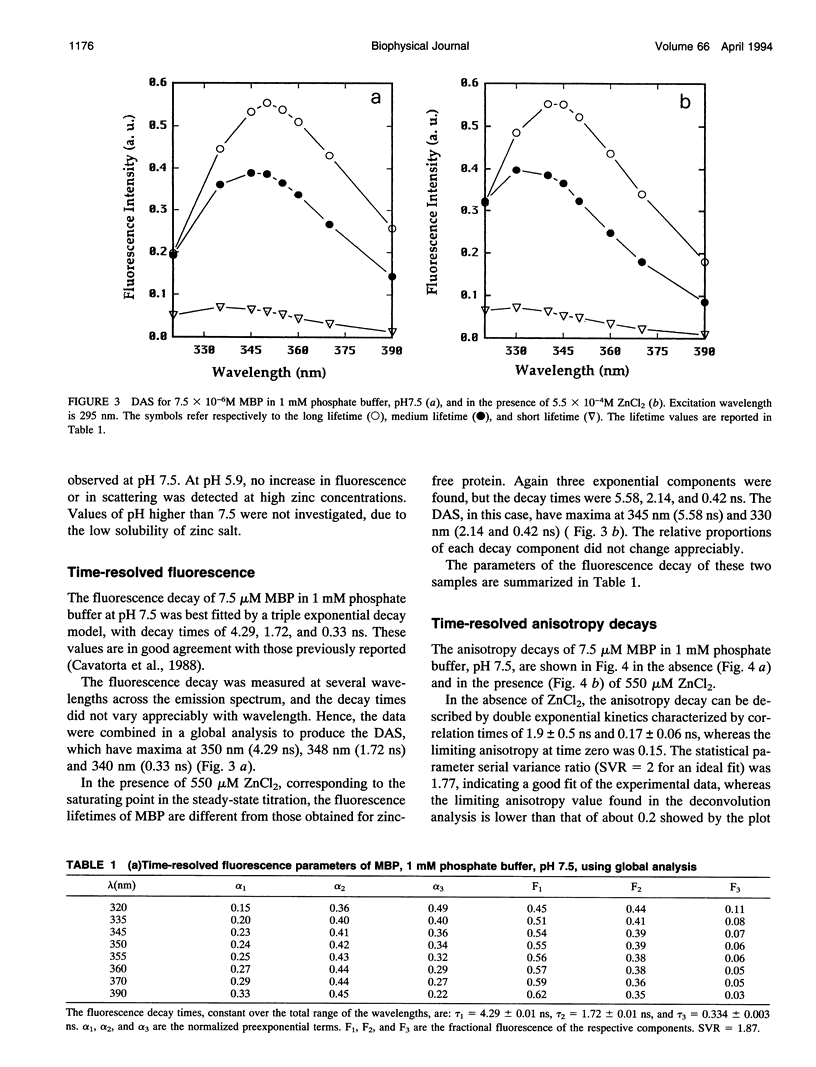
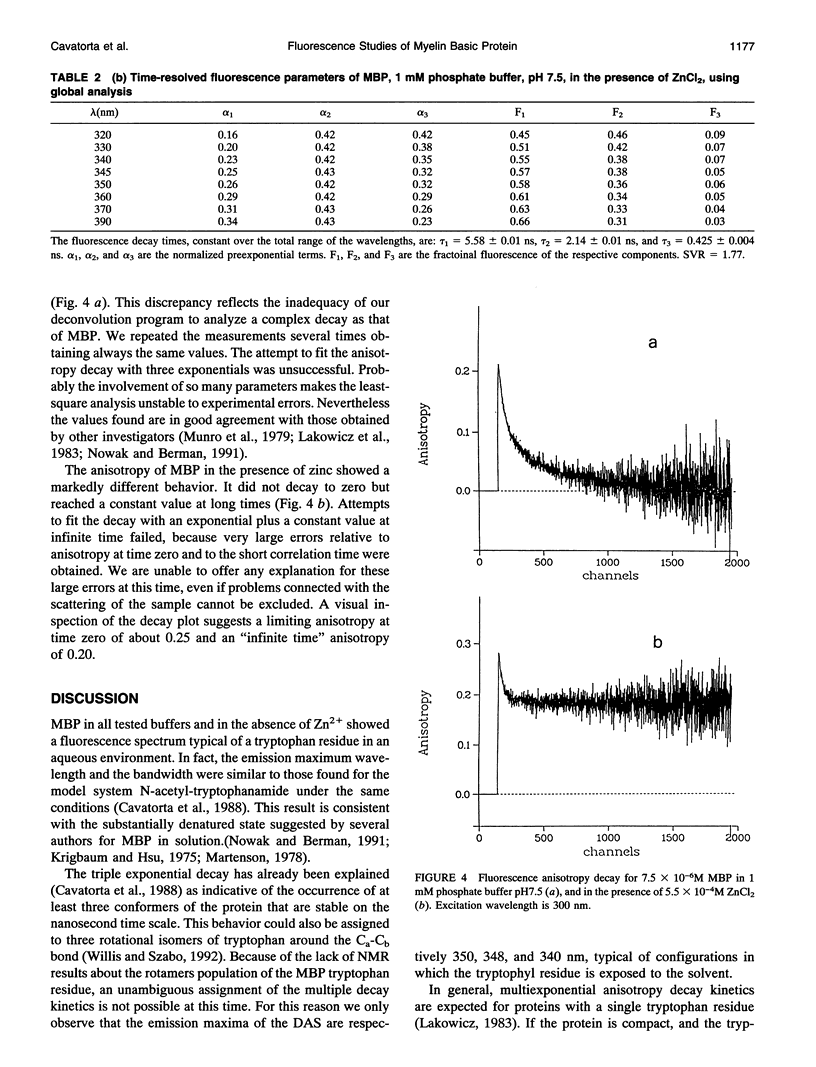
The interaction of myelin basic protein (MBP) with zinc and phosphate ions has been studied by using the emission properties of the single tryptophan residue of the protein (Trp-115). The studies have been carried out by means of both static and time-resolved fluorescence techniques. The addition of either zinc to MBP in the presence of phosphate or phosphate to MBP in the presence of zinc resulted in an increase of fluorescence intensity and a blue shift of the emission maximum wavelength. Furthermore, a concomitant increase in the scattering was also detected. Anisotropy decay experiments demonstrated that these effects are due to the formation of MBP molecules into large aggregates. A possible physiological role for such interaction is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavatorta P., Masotti L., Szabo A. G., Juretic D., Riccio P., Quagliariello E. Fluorescence spectral resolution of myelin basic protein conformers in complexes with lysophosphatidylcholine. Cell Biophys. 1988 Dec;13(3):201–215. doi: 10.1007/BF02918376. [DOI] [PubMed] [Google Scholar]
- Coleman J. E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Earl C., Chantry A., Mohammad N., Glynn P. Zinc ions stabilise the association of basic protein with brain myelin membranes. J Neurochem. 1988 Sep;51(3):718–724. doi: 10.1111/j.1471-4159.1988.tb01803.x. [DOI] [PubMed] [Google Scholar]
- Inouye H., Kirschner D. A. Effects of ZnCl2 on membrane interactions in myelin of normal and shiverer mice. Biochim Biophys Acta. 1984 Oct 3;776(2):197–208. doi: 10.1016/0005-2736(84)90209-8. [DOI] [PubMed] [Google Scholar]
- Krigbaum W. R., Hsu T. S. Molecular conformation of bovine A1 basic protein, a coiling macromolecule in aqueous solution. Biochemistry. 1975 Jun 3;14(11):2542–2546. doi: 10.1021/bi00682a038. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Maliwal B. P., Cherek H., Balter A. Rotational freedom of tryptophan residues in proteins and peptides. Biochemistry. 1983 Apr 12;22(8):1741–1752. doi: 10.1021/bi00277a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebes L. F., Zand R., Phillips W. D. Solution behavior, circular dichroism and 22 HMz PMR studies of the bovine myelin basic protein. Biochim Biophys Acta. 1975 Sep 9;405(1):27–39. doi: 10.1016/0005-2795(75)90311-6. [DOI] [PubMed] [Google Scholar]
- Liuzzi G. M., Ventola A., Riccio P., Quagliariello E. Identification of water-soluble proteases in myelin preparations. Biochem Biophys Res Commun. 1992 Jul 15;186(1):89–94. doi: 10.1016/s0006-291x(05)80779-x. [DOI] [PubMed] [Google Scholar]
- Martenson R. E. The use of gel filtration to follow conformational changes in proteins. Conformational flexibility of bovine myelin basic protein. J Biol Chem. 1978 Dec 25;253(24):8887–8893. [PubMed] [Google Scholar]
- Munro I., Pecht I., Stryer L. Subnanosecond motions of tryptophan residues in proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):56–60. doi: 10.1073/pnas.76.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M. W., Berman H. A. Fluorescence studies on the interactions of myelin basic protein in electrolyte solutions. Biochemistry. 1991 Jul 30;30(30):7642–7651. doi: 10.1021/bi00244a037. [DOI] [PubMed] [Google Scholar]
- Riccio P., Liuzzi G. M., Quagliariello E. Lipid-bound, native-like, myelin basic protein. Batch-wise preparation and perspectives for use in demyelinating diseases. Mol Chem Neuropathol. 1990 Dec;13(3):185–194. doi: 10.1007/BF03159921. [DOI] [PubMed] [Google Scholar]
- Riccio P., Masotti L., Cavatorta P., De Santis A., Juretic D., Bobba A., Pasquali-Ronchetti I., Quagliariello E. Myelin basic protein ability to organize lipid bilayers: structural transition in bilayers of lysophosphatidylcholine micelles. Biochem Biophys Res Commun. 1986 Jan 14;134(1):313–319. doi: 10.1016/0006-291x(86)90564-4. [DOI] [PubMed] [Google Scholar]
- Riccio P., Rosenbusch J. P., Quagliariello E. A new procedure for the isolation of the brain myelin basic protein in a lipid-bound form. FEBS Lett. 1984 Nov 19;177(2):236–240. doi: 10.1016/0014-5793(84)81290-9. [DOI] [PubMed] [Google Scholar]
- Smith R. The encephalitogenic protein of myelin forms hexamers in which the polypeptides have a pleated-sheet structure. FEBS Lett. 1985 Apr 22;183(2):331–334. doi: 10.1016/0014-5793(85)80804-8. [DOI] [PubMed] [Google Scholar]
- Valeur B., Weber G. Resolution of the fluorescence excitation spectrum of indole into the 1La and 1Lb excitation bands. Photochem Photobiol. 1977 May;25(5):441–444. doi: 10.1111/j.1751-1097.1977.tb09168.x. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Catz I. A correlation between cerebrospinal fluid myelin basic protein and anti-myelin basic protein in multiple sclerosis patients. Ann Neurol. 1987 Feb;21(2):183–189. doi: 10.1002/ana.410210211. [DOI] [PubMed] [Google Scholar]
- Willis K. J., Szabo A. G. Conformation of parathyroid hormone: time-resolved fluorescence studies. Biochemistry. 1992 Sep 22;31(37):8924–8931. doi: 10.1021/bi00152a032. [DOI] [PubMed] [Google Scholar]
- Willis K. J., Szabo A. G., Drew J., Zuker M., Ridgeway J. M. Resolution of heterogeneous fluorescence into component decay-associated excitation spectra. Application to subtilisins. Biophys J. 1990 Feb;57(2):183–189. doi: 10.1016/S0006-3495(90)82521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis K. J., Szabo A. G., Krajcarski D. T. The use of Stokes Raman scattering in time correlated single photon counting: application to the fluorescence lifetime of tyrosinate. Photochem Photobiol. 1990 Mar;51(3):375–377. doi: 10.1111/j.1751-1097.1990.tb01725.x. [DOI] [PubMed] [Google Scholar]


