Abstract
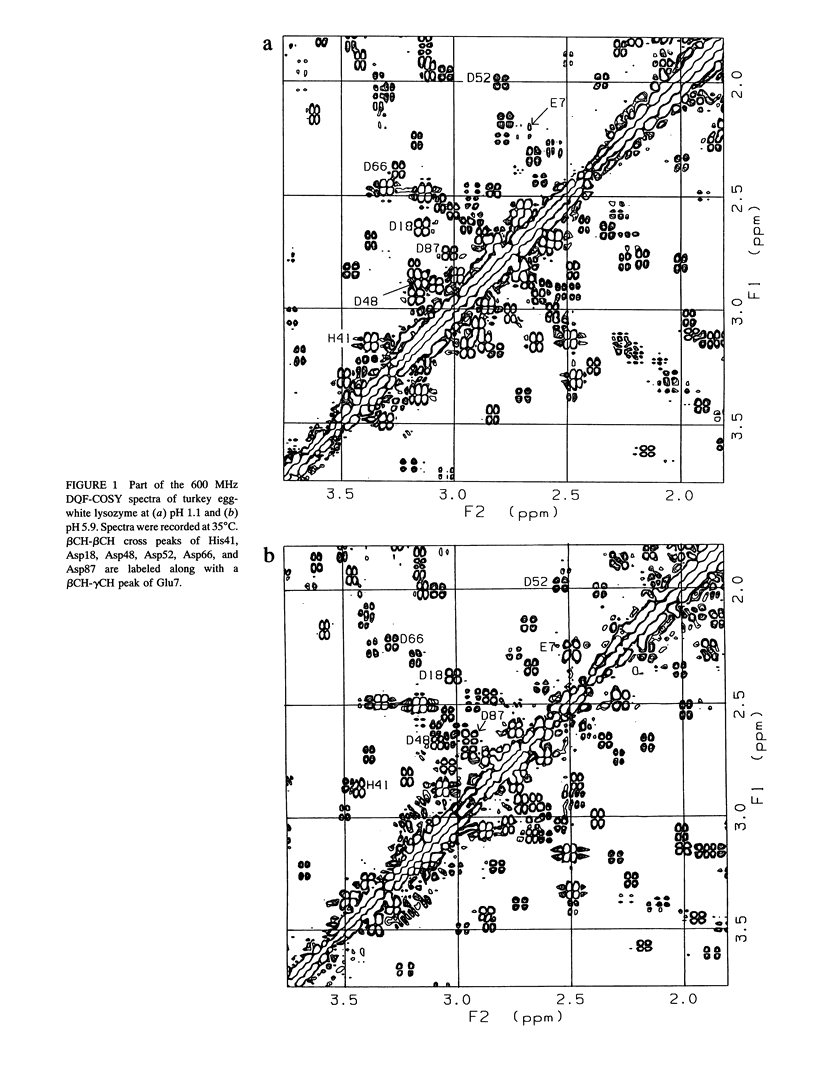
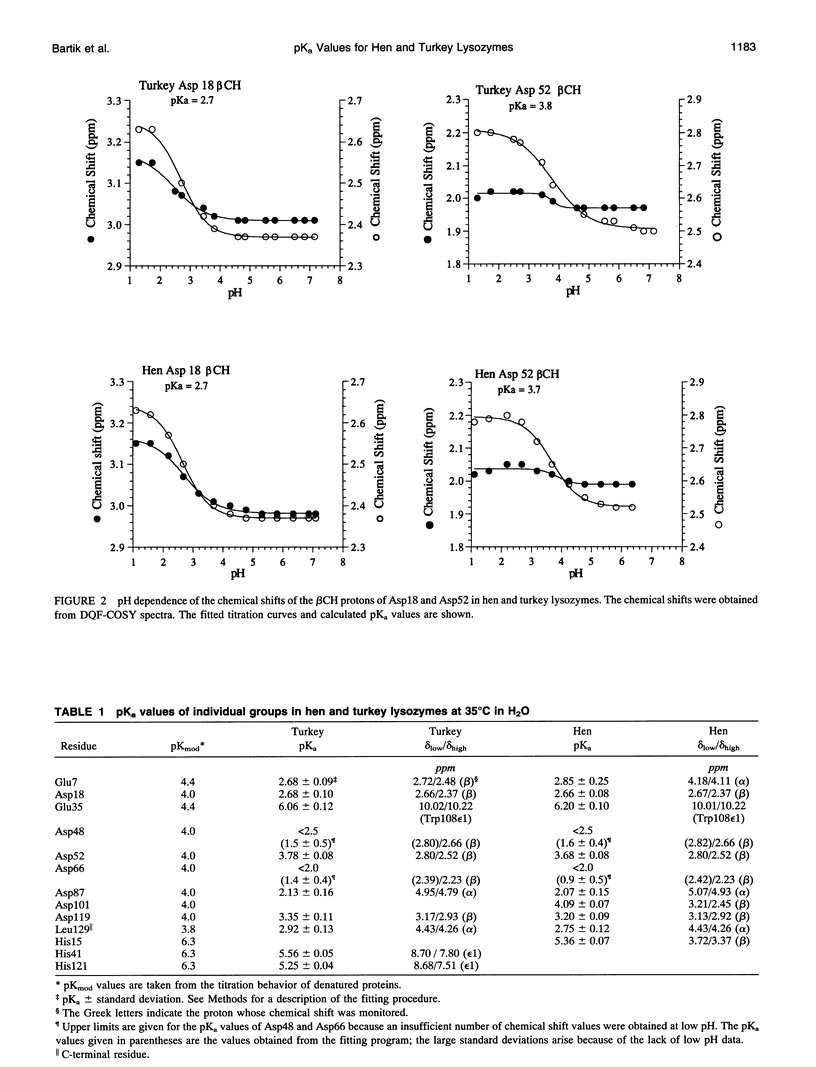
The pH dependence of the two-dimensional 1H nuclear magnetic resonance spectra of hen and turkey egg-white lysozymes has been recorded over the pH range 1-7. By monitoring the chemical shifts of the resonances of the various protons of ionizable residues, individual pKa values for the acidic residues have been determined for both proteins. The pKa values are displaced, with the exception of those of the residues in the active site cleft, by an average of 1 unit to low pH compared to model compounds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartik K., Dobson C. M., Redfield C. 1H-NMR analysis of turkey egg-white lysozyme and comparison with hen egg-white lysozyme. Eur J Biochem. 1993 Jul 15;215(2):255–266. doi: 10.1111/j.1432-1033.1993.tb18030.x. [DOI] [PubMed] [Google Scholar]
- Bashford D., Karplus M. pKa's of ionizable groups in proteins: atomic detail from a continuum electrostatic model. Biochemistry. 1990 Nov 6;29(44):10219–10225. doi: 10.1021/bi00496a010. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Cassels R., Dobson C. M., Poulsen F. M., Williams R. J. Study of the tryptophan residues of lysozyme using 1H nuclear magnetic resonance. Eur J Biochem. 1978 Dec 1;92(1):81–97. doi: 10.1111/j.1432-1033.1978.tb12725.x. [DOI] [PubMed] [Google Scholar]
- Cooper A., Eyles S. J., Radford S. E., Dobson C. M. Thermodynamic consequences of the removal of a disulphide bridge from hen lysozyme. J Mol Biol. 1992 Jun 20;225(4):939–943. doi: 10.1016/0022-2836(92)90094-z. [DOI] [PubMed] [Google Scholar]
- Delepierre M., Dobson C. M., Karplus M., Poulsen F. M., States D. J., Wedin R. E. Electrostatic effects and hydrogen exchange behaviour in proteins. The pH dependence of exchange rates in lysozyme. J Mol Biol. 1987 Sep 5;197(1):111–130. doi: 10.1016/0022-2836(87)90613-9. [DOI] [PubMed] [Google Scholar]
- Fukamizo T., Torikata T., Nagayama T., Minematsu T., Hayashi K. Enzymatic activity of avian egg-white lysozymes. J Biochem. 1983 Jul;94(1):115–122. doi: 10.1093/oxfordjournals.jbchem.a134319. [DOI] [PubMed] [Google Scholar]
- Kuramitsu S., Hamaguchi K. Analysis of the acid-base titration curve of hen lysozyme. J Biochem. 1980 Apr;87(4):1215–1219. [PubMed] [Google Scholar]
- Matthew J. B. Electrostatic effects in proteins. Annu Rev Biophys Biophys Chem. 1985;14:387–417. doi: 10.1146/annurev.bb.14.060185.002131. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Electrostatic effects in proteins. Science. 1978 Sep 29;201(4362):1187–1191. doi: 10.1126/science.694508. [DOI] [PubMed] [Google Scholar]
- Redfield C., Dobson C. M. Sequential 1H NMR assignments and secondary structure of hen egg white lysozyme in solution. Biochemistry. 1988 Jan 12;27(1):122–136. doi: 10.1021/bi00401a020. [DOI] [PubMed] [Google Scholar]
- Sharp K. A., Honig B. Electrostatic interactions in macromolecules: theory and applications. Annu Rev Biophys Biophys Chem. 1990;19:301–332. doi: 10.1146/annurev.bb.19.060190.001505. [DOI] [PubMed] [Google Scholar]


