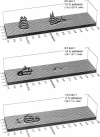
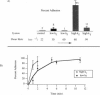
Abstract
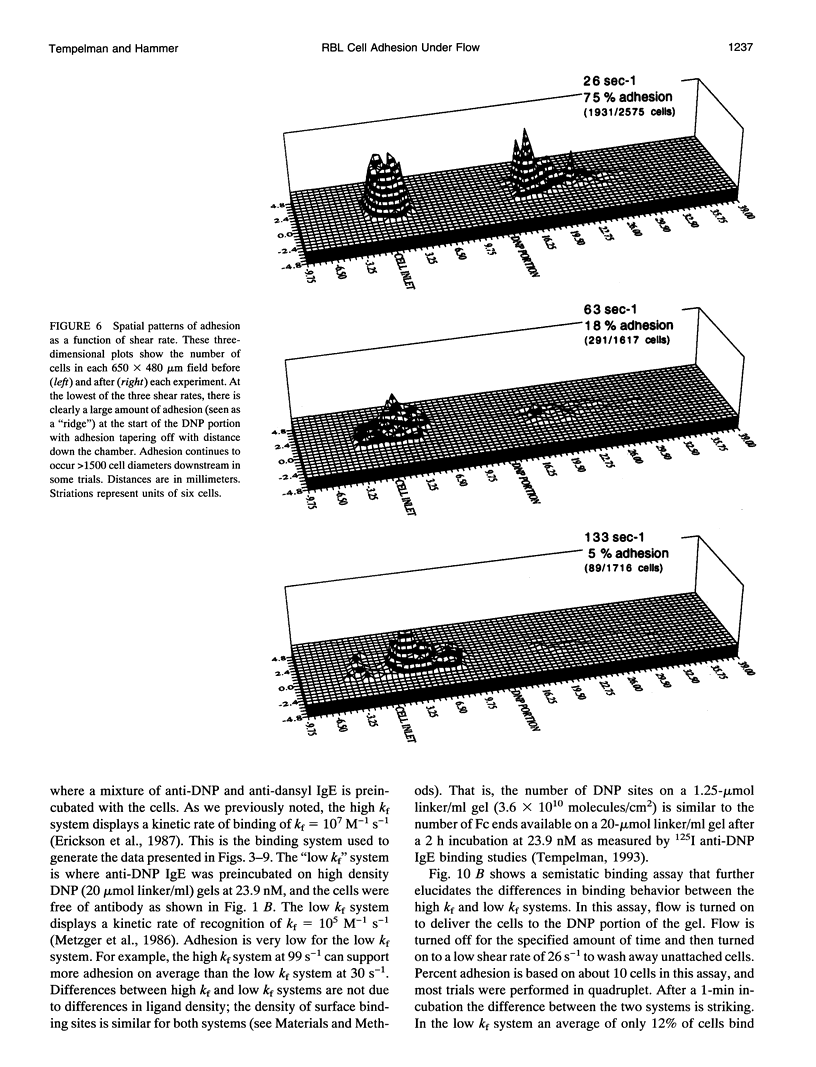
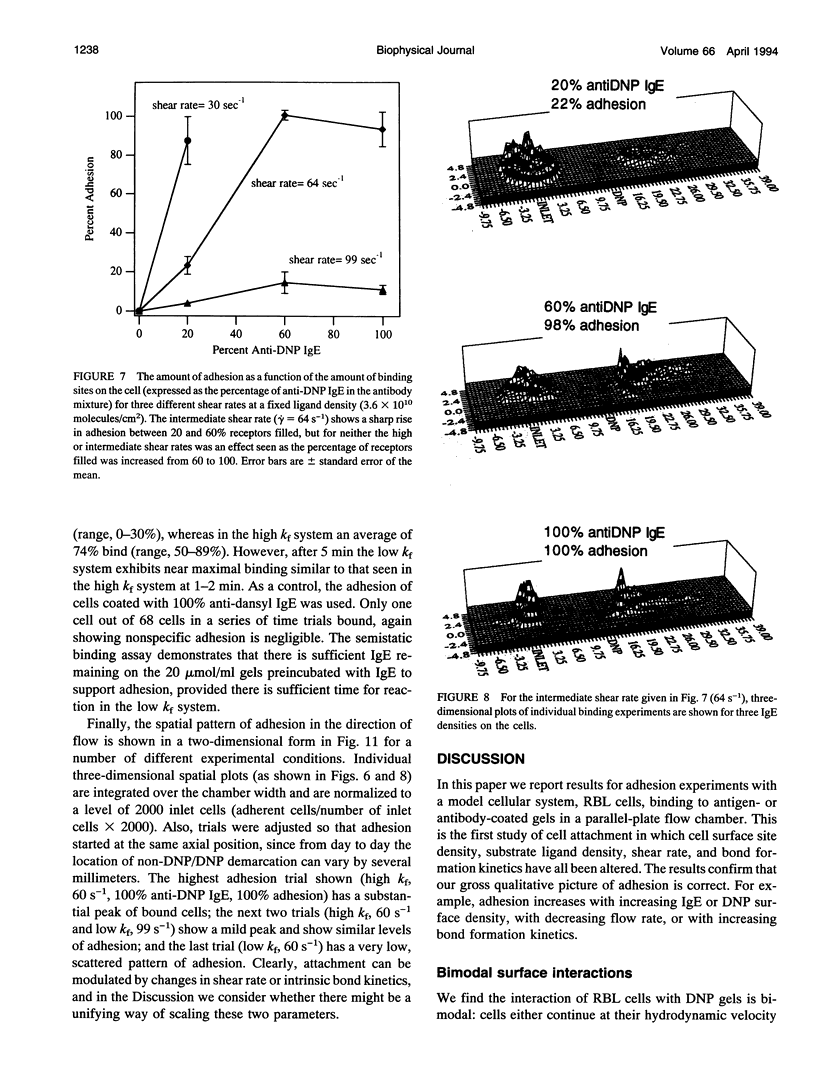
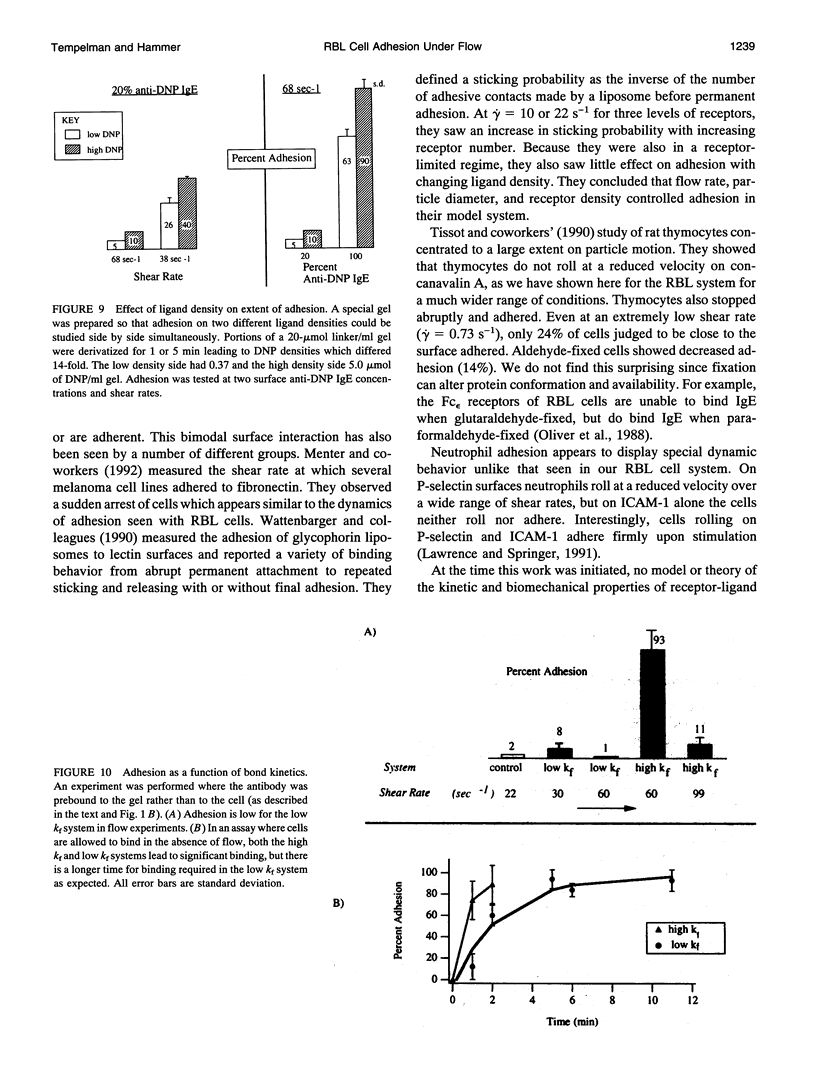
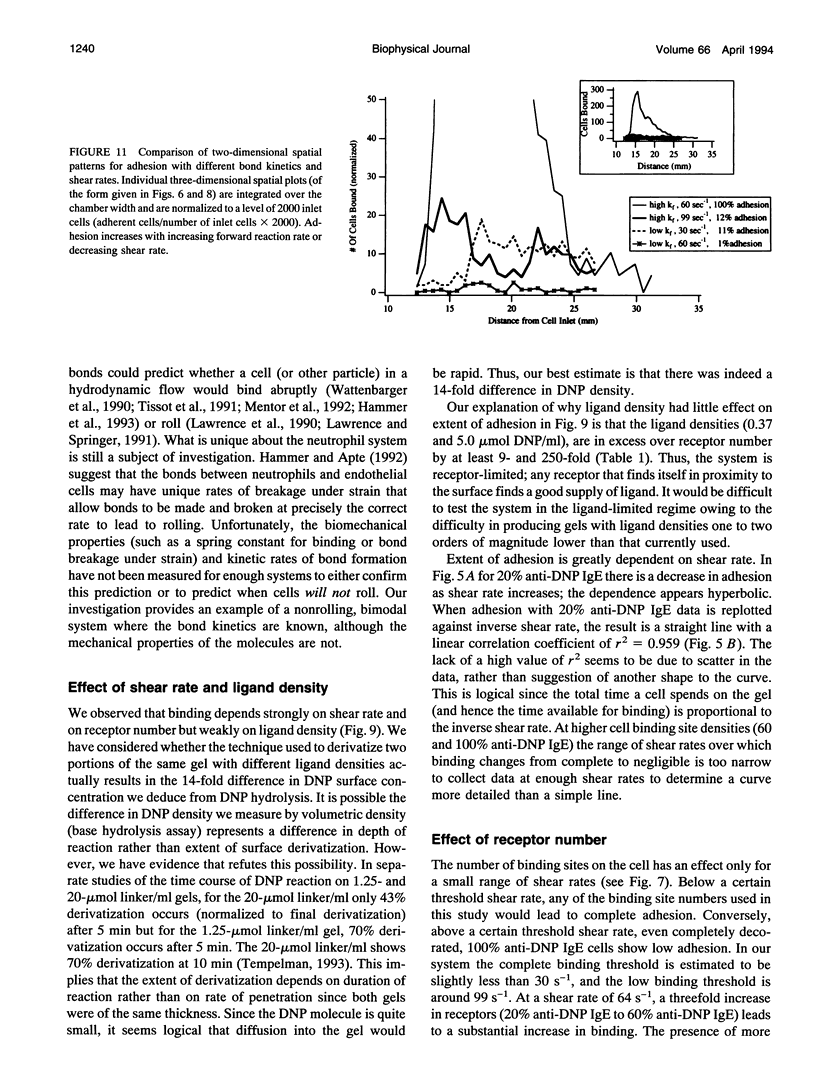
The physiological function of many cells is dependent on their ability to adhere via receptors to ligand-coated surfaces under fluid flow. We have developed a model experimental system to measure cell adhesion as a function of cell and surface chemistry and fluid flow. Using a parallel-plate flow chamber, we measured the binding of rat basophilic leukemia cells preincubated with anti-dinitrophenol IgE antibody to polyacrylamide gels covalently derivatized with 2,4-dinitrophenol. The rat basophilic leukemia cells' binding behavior is binary: cells are either adherent or continue to travel at their hydrodynamic velocity, and the transition between these two states is abrupt. The spatial location of adherent cells shows cells can adhere many cell diameters down the length of the gel, suggesting that adhesion is a probabilistic process. The majority of experiments were performed in the excess ligand limit in which adhesion depends strongly on the number of receptors but weakly on ligand density. Only 5-fold changes in IgE surface density or in shear rate were necessary to change adhesion from complete to indistinguishable from negative control. Adhesion showed a hyperbolic dependence on shear rate. By performing experiments with two IgE-antigen configurations in which the kinetic rates of receptor-ligand binding are different, we demonstrate that the forward rate of reaction of the receptor-ligand pair is more important than its thermodynamic affinity in the regulation of binding under hydrodynamic flow. In fact, adhesion increases with increasing receptor-ligand reaction rate or decreasing shear rate, and scales with a single dimensionless parameter which compares the relative rates of reaction to fluid shear.
Full text
PDF



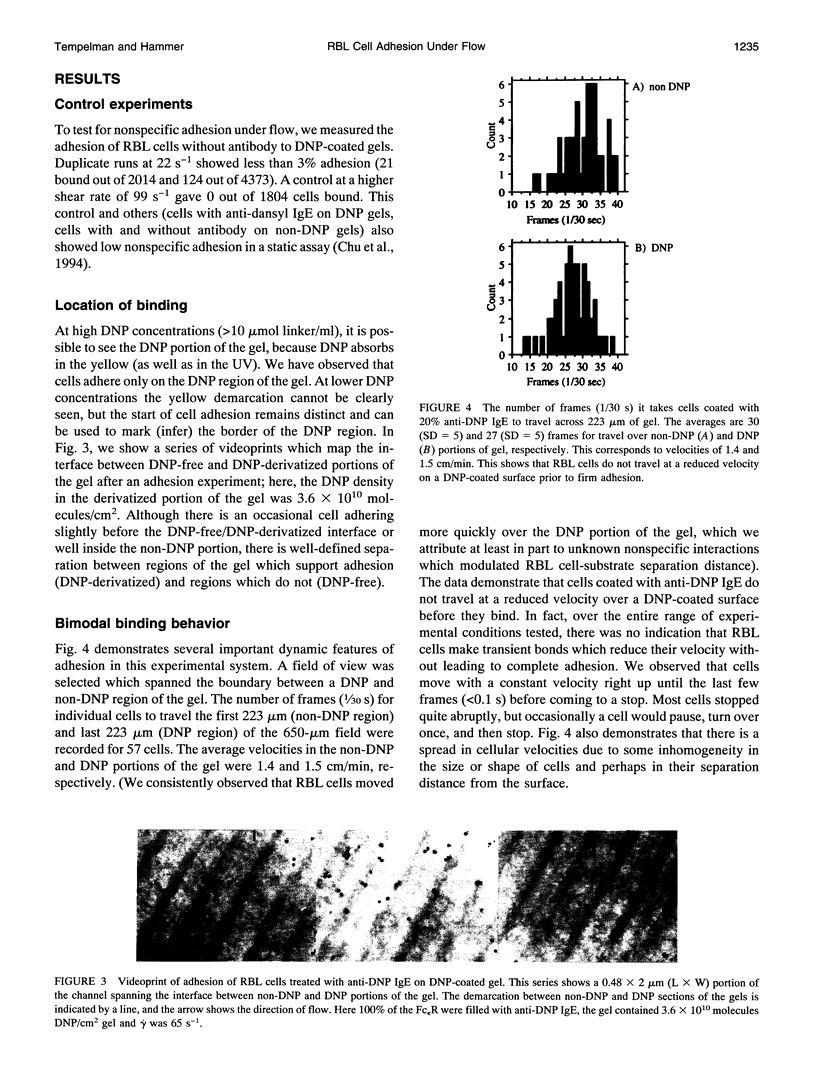
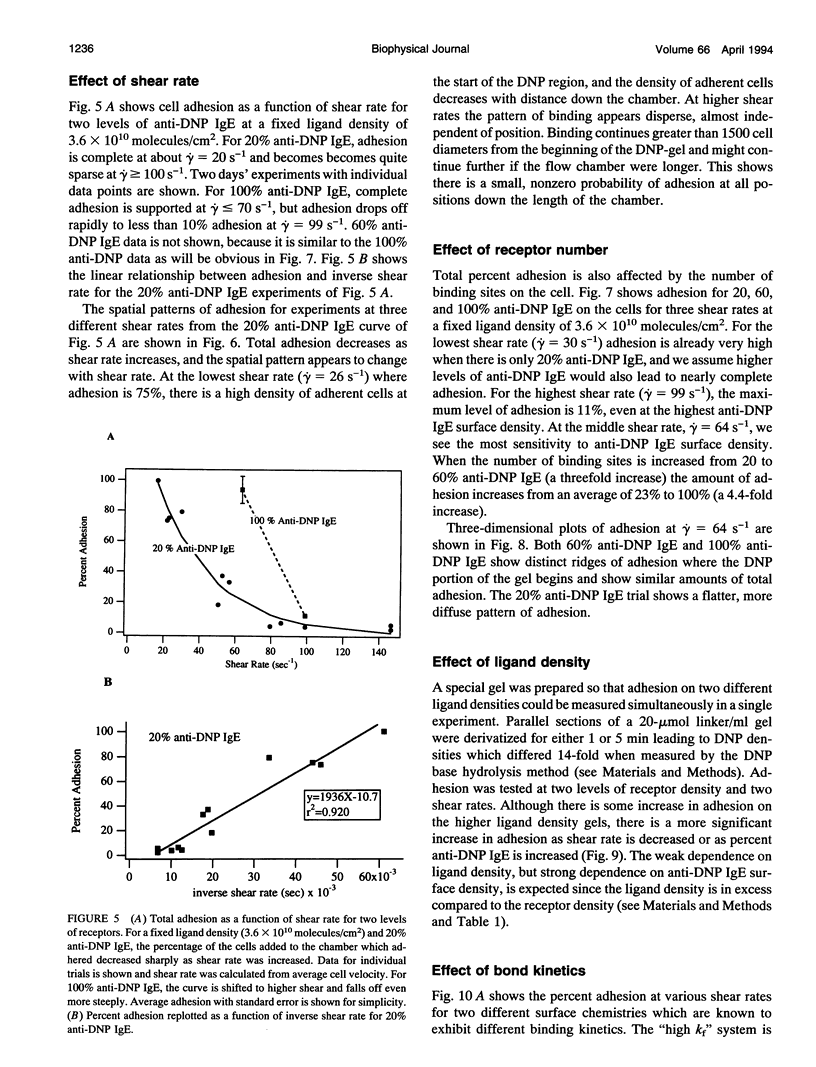



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens-Roberts C., Lauffenburger D. A., Quinn J. A. Receptor-mediated cell attachment and detachment kinetics. I. Probabilistic model and analysis. Biophys J. 1990 Oct;58(4):841–856. doi: 10.1016/S0006-3495(90)82430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens-Roberts C., Quinn J. A., Lauffenberger D. A. Receptor-mediated adhesion phenomena. Model studies with the Radical-Flow Detachment Assay. Biophys J. 1990 Jul;58(1):107–125. doi: 10.1016/S0006-3495(90)82357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens-Roberts C., Quinn J. A., Lauffenburger D. A. Receptor-mediated cell attachment and detachment kinetics. II. Experimental model studies with the radial-flow detachment assay. Biophys J. 1990 Oct;58(4):857–872. doi: 10.1016/S0006-3495(90)82431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi C. The biophysics of ligand-receptor interactions. Q Rev Biophys. 1980 May;13(2):201–230. doi: 10.1017/s0033583500001657. [DOI] [PubMed] [Google Scholar]
- Erickson J., Goldstein B., Holowka D., Baird B. The effect of receptor density on the forward rate constant for binding of ligands to cell surface receptors. Biophys J. 1987 Oct;52(4):657–662. doi: 10.1016/S0006-3495(87)83258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989 Jul;56(1):151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer D. A., Apte S. M. Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys J. 1992 Jul;63(1):35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer D. A., Lauffenburger D. A. A dynamical model for receptor-mediated cell adhesion to surfaces. Biophys J. 1987 Sep;52(3):475–487. doi: 10.1016/S0006-3495(87)83236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer D. A. Simulation of cell rolling and adhesion on surfaces in shear flow. Microvilli-coated hard spheres with adhesive springs. Cell Biophys. 1991 Apr;18(2):145–182. doi: 10.1007/BF02989811. [DOI] [PubMed] [Google Scholar]
- Hammer D. A., Tempelman L. A., Apte S. M. Statistics of cell adhesion under hydrodynamic flow: simulation and experiment. Blood Cells. 1993;19(2):261–277. [PubMed] [Google Scholar]
- Lawrence M. B., Smith C. W., Eskin S. G., McIntire L. V. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990 Jan 1;75(1):227–237. [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- McClay D. R., Wessel G. M., Marchase R. B. Intercellular recognition: quantitation of initial binding events. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4975–4979. doi: 10.1073/pnas.78.8.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K., Holowka D., Webb W. W., Baird B. Clustering, mobility, and triggering activity of small oligomers of immunoglobulin E on rat basophilic leukemia cells. J Cell Biol. 1986 Feb;102(2):534–540. doi: 10.1083/jcb.102.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter D. G., Patton J. T., Updyke T. V., Kerbel R. S., Maamer M., McIntire L. V., Nicolson G. L. Transglutaminase stabilizes melanoma adhesion under laminar flow. Cell Biophys. 1991 Apr;18(2):123–143. doi: 10.1007/BF02989810. [DOI] [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Seagrave J., Stump R. F., Pfeiffer J. R., Deanin G. G. Signal transduction and cellular response in RBL-2H3 mast cells. Prog Allergy. 1988;42:185–245. [PubMed] [Google Scholar]
- Pless D. D., Lee Y. C., Roseman S., Schnaar R. L. Specific cell adhesion to immobilized glycoproteins demonstrated using new reagents for protein and glycoprotein immobilization. J Biol Chem. 1983 Feb 25;258(4):2340–2349. [PubMed] [Google Scholar]
- Schnaar R. L., Brandley B. K., Needham L. K., Swank-Hill P., Blackburn C. C. Adhesion of eukaryotic cells to immobilized carbohydrates. Methods Enzymol. 1989;179:542–558. doi: 10.1016/0076-6879(89)79153-9. [DOI] [PubMed] [Google Scholar]
- Schnaar R. L., Lee Y. C. Polyacrylamide gels copolymerized with active esters. A new medium for affinity systems. Biochemistry. 1975 Apr 8;14(7):1535–1541. doi: 10.1021/bi00678a030. [DOI] [PubMed] [Google Scholar]
- Shoup D., Szabo A. Role of diffusion in ligand binding to macromolecules and cell-bound receptors. Biophys J. 1982 Oct;40(1):33–39. doi: 10.1016/S0006-3495(82)84455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurog J. D., Fewtrell C., Becker E. L. IgE mediated triggering of rat basophil leukemia cells: lack of evidence for serine esterase activation. J Immunol. 1979 Jun;122(6):2150–2153. [PubMed] [Google Scholar]
- Tissot O., Pierres A., Foa C., Delaage M., Bongrand P. Motion of cells sedimenting on a solid surface in a laminar shear flow. Biophys J. 1992 Jan;61(1):204–215. doi: 10.1016/S0006-3495(92)81827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenbarger M. R., Graves D. J., Lauffenburger D. A. Specific adhesion of glycophorin liposomes to a lectin surface in shear flow. Biophys J. 1990 Apr;57(4):765–777. doi: 10.1016/S0006-3495(90)82597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]