Abstract
Solid state deuterium (2H) NMR inversion-recovery and Jeener-Broekaert relaxation experiments were performed on oriented multilamellar dispersions consisting of 1,2-dilauroyl-sn-glycero-3-phosphatidylcholine and 2H exchange-labeled gramicidin D, at a lipid to protein molar ratio (L/P) of 15:1, in order to study the dynamics of the channel conformation of the peptide in a liquid crystalline phase. Our dynamic model for the whole body motions of the peptide includes diffusion of the peptide around its helix axis and a wobbling diffusion around a second axis perpendicular to the local bilayer normal in a simple Maier-Saupe mean field potential. This anisotropic diffusion is characterized by the correlation times, tau R parallel and tau R perpendicular. Aligning the bilayer normal perpendicular to the magnetic field and graphing the relaxation rate, 1/T1Z, as a function of (1-S2N-2H), where S2N-2H represents the orientational order parameter, wer were able to estimate the correlation time, tau R parallel, for rotational diffusion. Although in the quadrupolar splitting, which varies as (3 cos2 theta D-1), has in general two possible solutions to theta D in the range 0 < or = theta D < or = 90 degrees, the 1/T1Z vs. (1-S2N-2H) curve can be used to determine a single value of theta D in this range. Thus, the 1/T1Z vs. (1-S2N-2H) profile can be used both to define the axial diffusion rate and to remove potential structural ambiguities in the splittings. The T1Z anisotropy permits us to solve for the two correlation times (tau R parallel = 6.8 x 10(-9) s and tau R perpendicular = 6 x 10(-6) s). The simulated parameters were corroborated by a Jeener-Broekaert experiment where the bilayer normal was parallel to the principal magnetic field. At this orientation the ratio, J2(2 omega 0)/J1(omega 0) was obtained in order to estimate the strength of the restoring potential in a model-independent fashion. This measurement yields the rms angle, <theta 2>1/2 (= 16 +/- 2 degrees at 34 degrees C), formed by the peptide helix axis and the average bilayer normal.
Full text
PDF



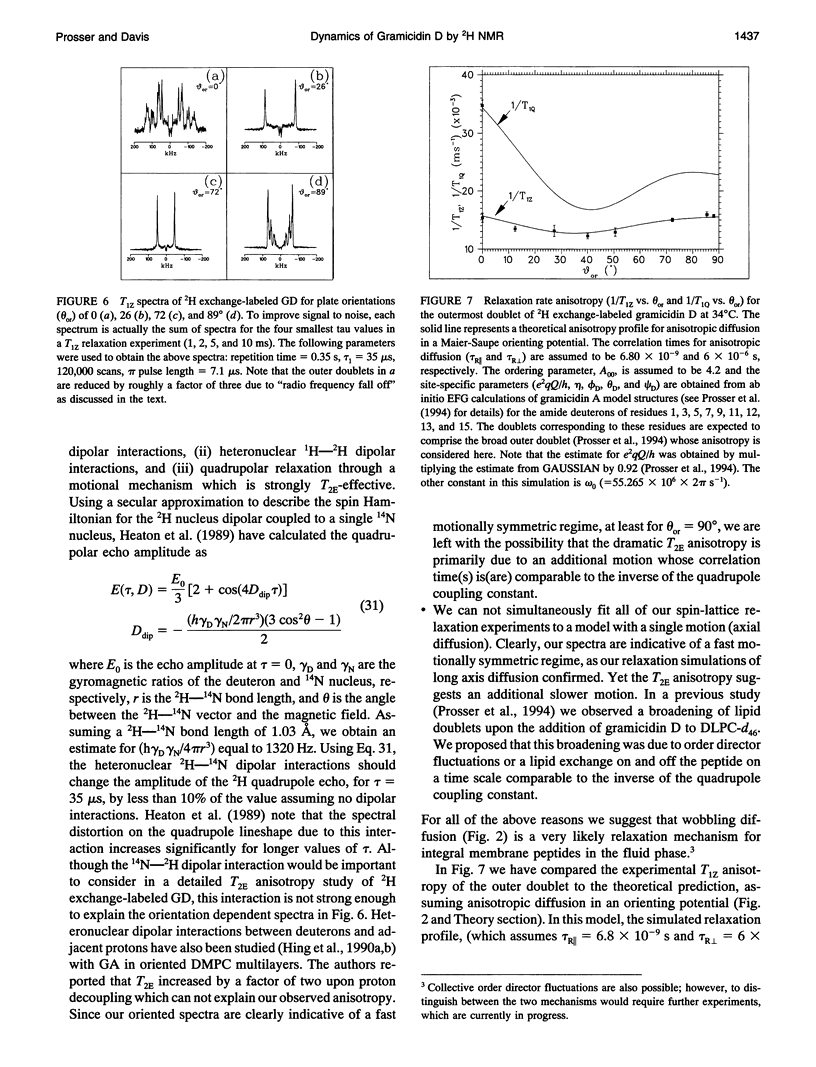
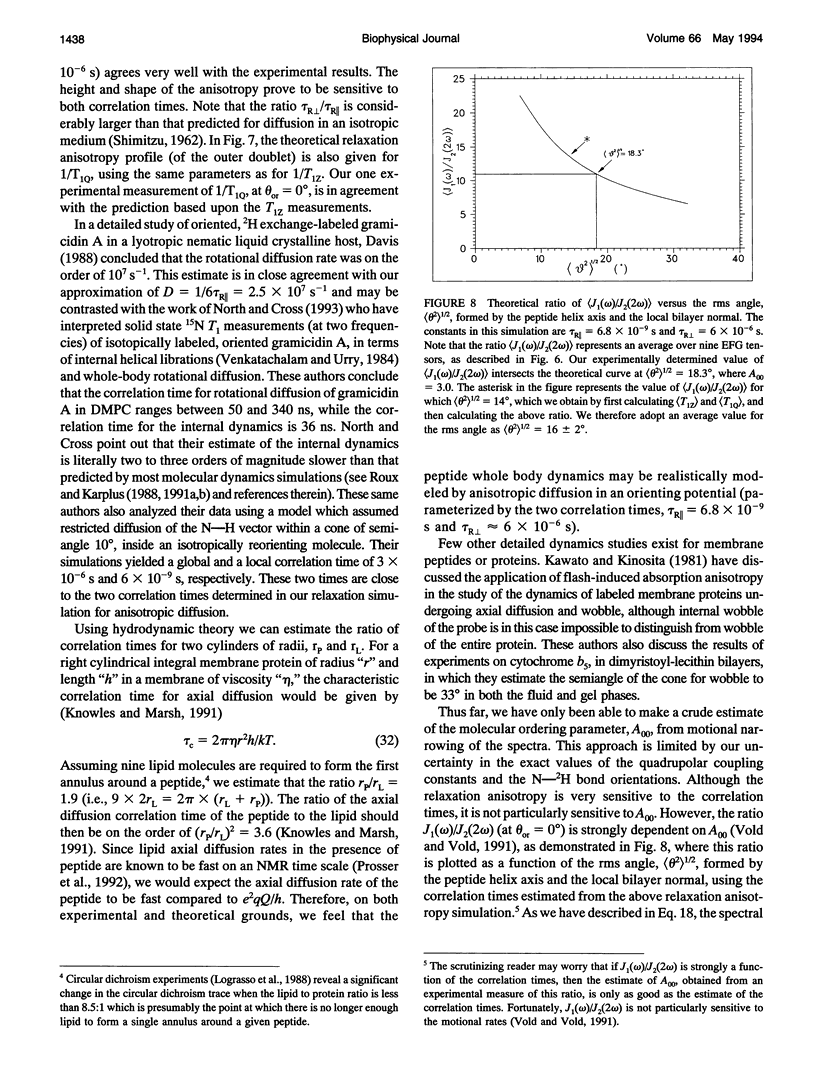


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis J. H. 2H nuclear magnetic resonance of exchange-labeled gramicidin in an oriented lyotropic nematic phase. Biochemistry. 1988 Jan 12;27(1):428–436. doi: 10.1021/bi00401a064. [DOI] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- De Loof H., Harvey S. C., Segrest J. P., Pastor R. W. Mean field stochastic boundary molecular dynamics simulation of a phospholipid in a membrane. Biochemistry. 1991 Feb 26;30(8):2099–2113. doi: 10.1021/bi00222a015. [DOI] [PubMed] [Google Scholar]
- Hing A. W., Adams S. P., Silbert D. F., Norberg R. E. Deuterium NMR of 2HCO-Val1...gramicidin A and 2HCO-Val1-D-Leu2...gramicidin A in oriented DMPC bilayers. Biochemistry. 1990 May 1;29(17):4156–4166. doi: 10.1021/bi00469a019. [DOI] [PubMed] [Google Scholar]
- Hing A. W., Adams S. P., Silbert D. F., Norberg R. E. Deuterium NMR of Val1...(2-2H)Ala3...gramicidin A in oriented DMPC bilayers. Biochemistry. 1990 May 1;29(17):4144–4156. doi: 10.1021/bi00469a018. [DOI] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr Time-dependent absorption anisotropy and rotational diffusion of proteins in membranes. Biophys J. 1981 Oct;36(1):277–296. doi: 10.1016/S0006-3495(81)84728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles P. F., Marsh D. Magnetic resonance of membranes. Biochem J. 1991 Mar 15;274(Pt 3):625–641. doi: 10.1042/bj2740625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGrasso P. V., Moll F., 3rd, Cross T. A. Solvent history dependence of gramicidin A conformations in hydrated lipid bilayers. Biophys J. 1988 Aug;54(2):259–267. doi: 10.1016/S0006-3495(88)82955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald P. M., Seelig J. Dynamic properties of gramicidin A in phospholipid membranes. Biochemistry. 1988 Apr 5;27(7):2357–2364. doi: 10.1021/bi00407a017. [DOI] [PubMed] [Google Scholar]
- Pastor R. W., Venable R. M., Karplus M. Model for the structure of the lipid bilayer. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):892–896. doi: 10.1073/pnas.88.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls K. P., MacKay A. L., Söderman O., Bloom M., Tanjea A. K., Hodges R. S. Dynamic properties of the backbone of an integral membrane polypeptide measured by 2H-NMR. Eur Biophys J. 1985;12(1):1–11. doi: 10.1007/BF00254089. [DOI] [PubMed] [Google Scholar]
- Prosser R. S., Daleman S. I., Davis J. H. The structure of an integral membrane peptide: a deuterium NMR study of gramicidin. Biophys J. 1994 May;66(5):1415–1428. doi: 10.1016/S0006-3495(94)80932-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser R. S., Davis J. H., Dahlquist F. W., Lindorfer M. A. 2H nuclear magnetic resonance of the gramicidin A backbone in a phospholipid bilayer. Biochemistry. 1991 May 14;30(19):4687–4696. doi: 10.1021/bi00233a008. [DOI] [PubMed] [Google Scholar]
- Prosser R. S., Davis J. H., Mayer C., Weisz K., Kothe G. Deuterium NMR relaxation studies of peptide-lipid interactions. Biochemistry. 1992 Oct 6;31(39):9355–9363. doi: 10.1021/bi00154a005. [DOI] [PubMed] [Google Scholar]
- Roux B., Karplus M. Ion transport in a model gramicidin channel. Structure and thermodynamics. Biophys J. 1991 May;59(5):961–981. doi: 10.1016/S0006-3495(91)82311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., Karplus M. The normal modes of the gramicidin-A dimer channel. Biophys J. 1988 Mar;53(3):297–309. doi: 10.1016/S0006-3495(88)83107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz K., Gröbner G., Mayer C., Stohrer J., Kothe G. Deuteron nuclear magnetic resonance study of the dynamic organization of phospholipid/cholesterol bilayer membranes: molecular properties and viscoelastic behavior. Biochemistry. 1992 Feb 4;31(4):1100–1112. doi: 10.1021/bi00119a019. [DOI] [PubMed] [Google Scholar]


