Abstract
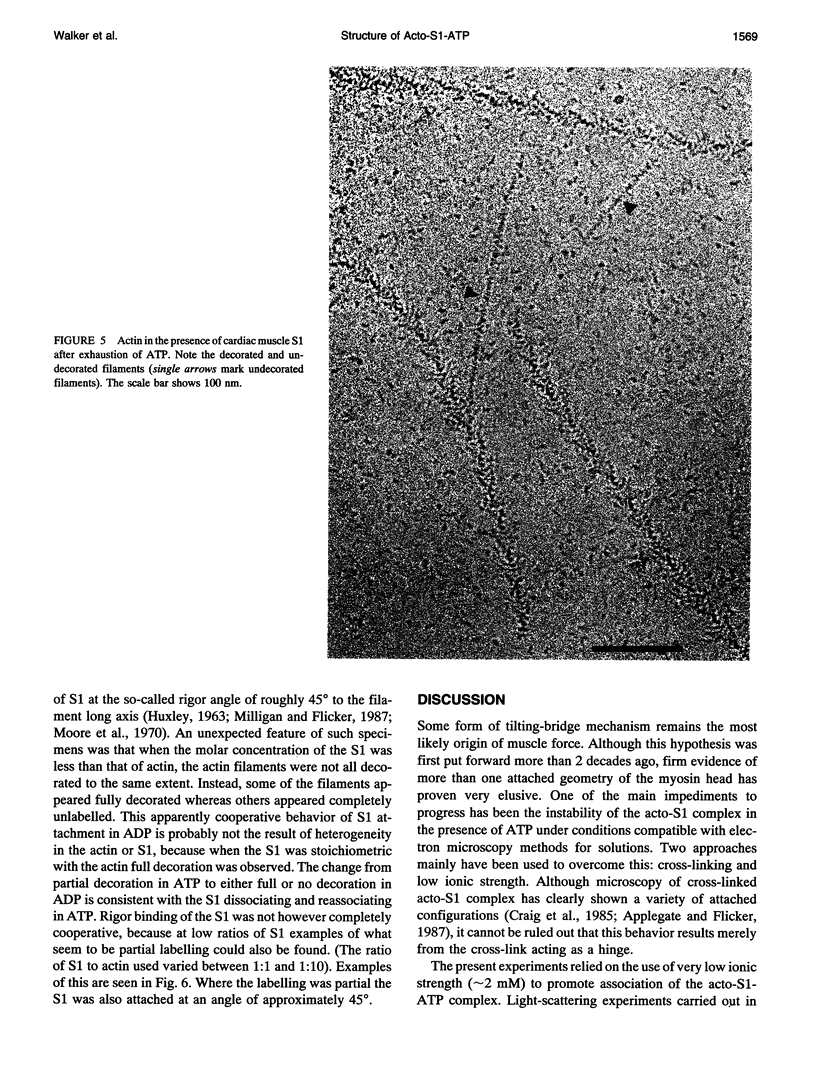
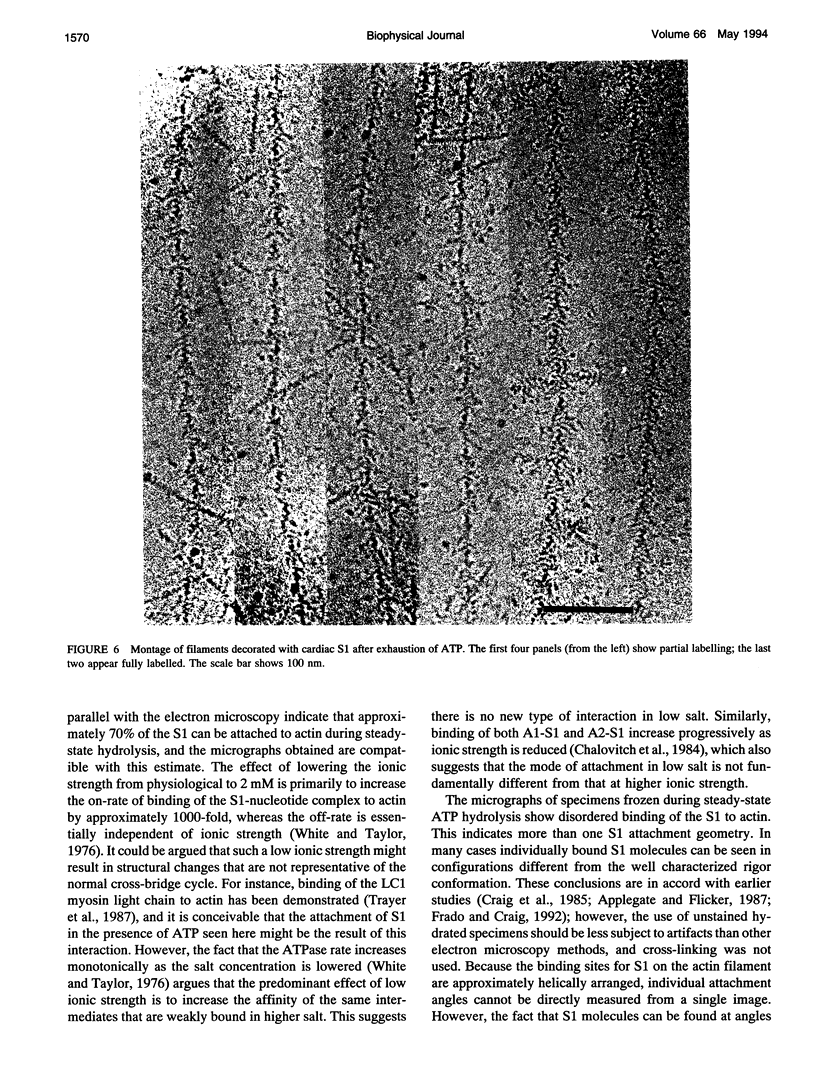
The structure of the complex of actin and myosin subfragment-1 (S1) during steady-state ATP hydrolysis has been examined by electron microscopy. This complex is normally dissociated by ATP in vitro but was stabilized here by low ionic strength. Optimal conditions for attachment were established by light-scattering experiments that showed that approximately 70% of S1 could be bound in the presence of ATP. Micrographs of the unstained complex in vitreous water suggest that S1 attaches to actin in a variety of configurations in ATP; this contrasts with the single attached configuration seen in the presence of ADP. The data are therefore compatible with the idea that a change in attached configuration of the myosin cross-bridge is the origin of muscle force. In control experiments where ATP was allowed to hydrolyze completely the binding of the S1 seemed cooperative.
Full text
PDF

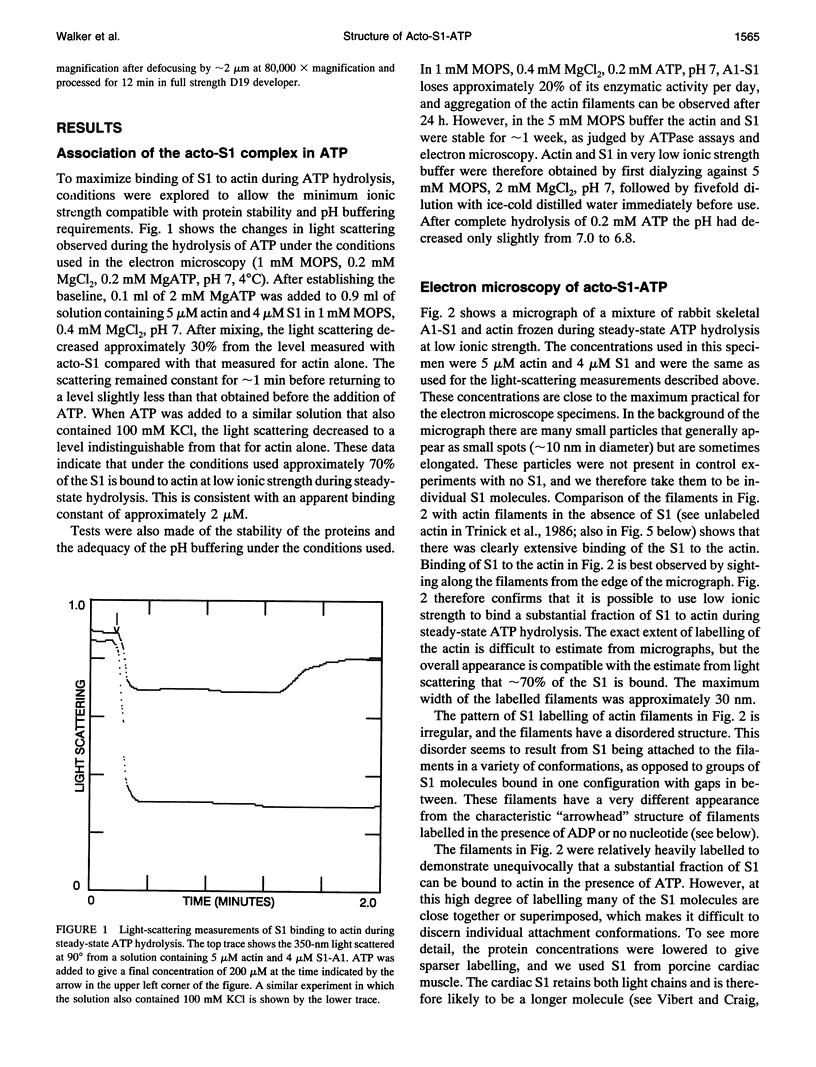
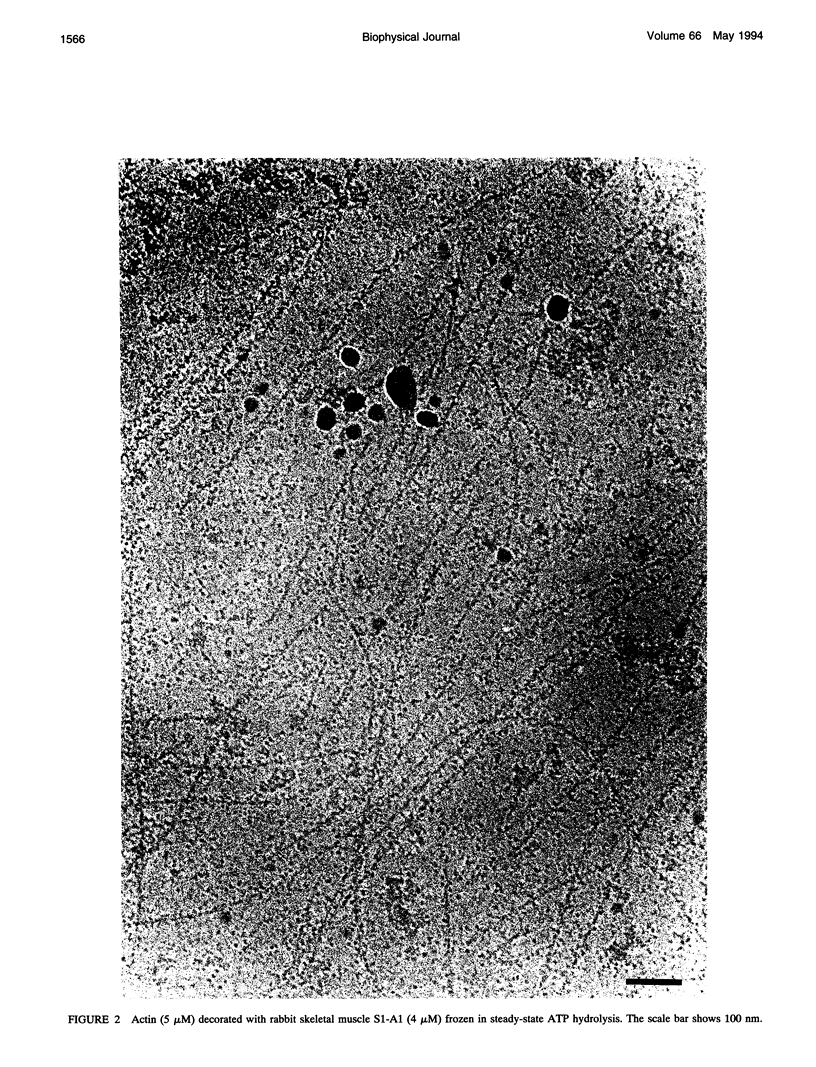

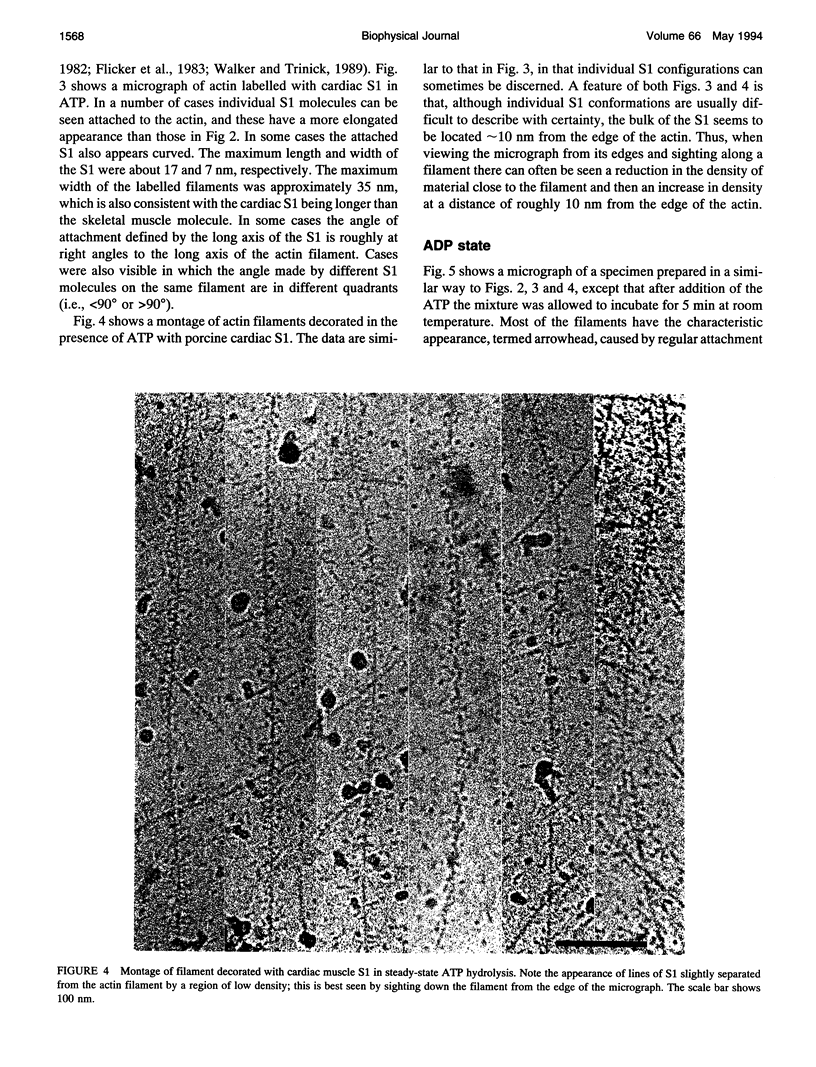


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Applegate D., Flicker P. New states of actomyosin. J Biol Chem. 1987 May 15;262(14):6856–6863. [PubMed] [Google Scholar]
- Berger C. L., Svensson E. C., Thomas D. D. Photolysis of a photolabile precursor of ATP (caged ATP) induces microsecond rotational motions of myosin heads bound to actin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8753–8757. doi: 10.1073/pnas.86.22.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. L., Thomas D. D. Rotational dynamics of actin-bound myosin heads in active myofibrils. Biochemistry. 1993 Apr 13;32(14):3812–3821. doi: 10.1021/bi00065a038. [DOI] [PubMed] [Google Scholar]
- Chalovich J. M., Stein L. A., Greene L. E., Eisenberg E. Interaction of isozymes of myosin subfragment 1 with actin: effect of ionic strength and nucleotide. Biochemistry. 1984 Oct 9;23(21):4885–4889. doi: 10.1021/bi00316a011. [DOI] [PubMed] [Google Scholar]
- Craig R., Alamo L., Padrón R. Structure of the myosin filaments of relaxed and rigor vertebrate striated muscle studied by rapid freezing electron microscopy. J Mol Biol. 1992 Nov 20;228(2):474–487. doi: 10.1016/0022-2836(92)90836-9. [DOI] [PubMed] [Google Scholar]
- Craig R., Greene L. E., Eisenberg E. Structure of the actin-myosin complex in the presence of ATP. Proc Natl Acad Sci U S A. 1985 May;82(10):3247–3251. doi: 10.1073/pnas.82.10.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R., Szent-Györgyi A. G., Beese L., Flicker P., Vibert P., Cohen C. Electron microscopy of thin filaments decorated with a Ca2+-regulated myosin. J Mol Biol. 1980 Jun 15;140(1):35–55. doi: 10.1016/0022-2836(80)90355-1. [DOI] [PubMed] [Google Scholar]
- Dantzig J. A., Goldman Y. E., Millar N. C., Lacktis J., Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker P. F., Wallimann T., Vibert P. Electron microscopy of scallop myosin. Location of regulatory light chains. J Mol Biol. 1983 Sep 25;169(3):723–741. doi: 10.1016/s0022-2836(83)80167-3. [DOI] [PubMed] [Google Scholar]
- Frado L. L., Craig R. Electron microscopy of the actin-myosin head complex in the presence of ATP. J Mol Biol. 1992 Jan 20;223(2):391–397. doi: 10.1016/0022-2836(92)90659-8. [DOI] [PubMed] [Google Scholar]
- Funatsu T., Kono E., Tsukita S. Time-resolved electron microscopic analysis of the behavior of myosin heads on actin filaments after photolysis of caged ATP. J Cell Biol. 1993 Jun;121(5):1053–1064. doi: 10.1083/jcb.121.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd M. G., Trentham D. R. Relationships between chemical and mechanical events during muscular contraction. Annu Rev Biophys Biophys Chem. 1986;15:119–161. doi: 10.1146/annurev.bb.15.060186.001003. [DOI] [PubMed] [Google Scholar]
- Hirose K., Lenart T. D., Murray J. M., Franzini-Armstrong C., Goldman Y. E. Flash and smash: rapid freezing of muscle fibers activated by photolysis of caged ATP. Biophys J. 1993 Jul;65(1):397–408. doi: 10.1016/S0006-3495(93)81061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Katayama E. The effects of various nucleotides on the structure of actin-attached myosin subfragment-1 studied by quick-freeze deep-etch electron microscopy. J Biochem. 1989 Nov;106(5):751–770. doi: 10.1093/oxfordjournals.jbchem.a122928. [DOI] [PubMed] [Google Scholar]
- Mayer E., Astl G. Limits of cryofixation as seen by Fourier transform infrared spectra of metmyoglobin azide and carbonyl hemoglobin in vitrified and freeze-concentrated aqueous solution. Ultramicroscopy. 1992 Sep;45(2):185–197. doi: 10.1016/0304-3991(92)90508-h. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Flicker P. F. Structural relationships of actin, myosin, and tropomyosin revealed by cryo-electron microscopy. J Cell Biol. 1987 Jul;105(1):29–39. doi: 10.1083/jcb.105.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. B., Huxley H. E., DeRosier D. J. Three-dimensional reconstruction of F-actin, thin filaments and decorated thin filaments. J Mol Biol. 1970 Jun 14;50(2):279–295. doi: 10.1016/0022-2836(70)90192-0. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Bhandari D., Maupin P., Wachsstock D., Weeds A. G., Zot H. G. Direct visualization by electron microscopy of the weakly bound intermediates in the actomyosin adenosine triphosphatase cycle. Biophys J. 1993 Feb;64(2):454–471. doi: 10.1016/S0006-3495(93)81387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Stein L. A., Greene L. E., Chock P. B., Eisenberg E. Rate-limiting step in the actomyosin adenosinetriphosphatase cycle: studies with myosin subfragment 1 cross-linked to actin. Biochemistry. 1985 Mar 12;24(6):1357–1363. doi: 10.1021/bi00327a013. [DOI] [PubMed] [Google Scholar]
- Trayer I. P., Trayer H. R., Levine B. A. Evidence that the N-terminal region of A1-light chain of myosin interacts directly with the C-terminal region of actin. A proton magnetic resonance study. Eur J Biochem. 1987 Apr 1;164(1):259–266. doi: 10.1111/j.1432-1033.1987.tb11019.x. [DOI] [PubMed] [Google Scholar]
- Trinick J., Cooper J. Concentration of solutes during preparation of aqueous suspensions for cryo-electron microscopy. J Microsc. 1990 Aug;159(Pt 2):215–222. doi: 10.1111/j.1365-2818.1990.tb04778.x. [DOI] [PubMed] [Google Scholar]
- Trinick J., Cooper J., Seymour J., Egelman E. H. Cryo-electron microscopy and three-dimensional reconstruction of actin filaments. J Microsc. 1986 Mar;141(Pt 3):349–360. doi: 10.1111/j.1365-2818.1986.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Yano M. Actomyosin structure in contracting muscle detected by rapid freezing. Nature. 1985 Sep 12;317(6033):182–184. doi: 10.1038/317182a0. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Oosawa F. Protein motors and Maxwell's demons: does mechanochemical transduction involve a thermal ratchet? Adv Biophys. 1990;26:97–134. doi: 10.1016/0065-227x(90)90009-i. [DOI] [PubMed] [Google Scholar]
- Vibert P., Craig R. Three-dimensional reconstruction of thin filaments decorated with a Ca2+-regulated myosin. J Mol Biol. 1982 May 15;157(2):299–319. doi: 10.1016/0022-2836(82)90236-4. [DOI] [PubMed] [Google Scholar]
- Walker M., Trinick J. Electron microscopy of negatively stained scallop myosin molecules. Effect of regulatory light chain removal on head structure. J Mol Biol. 1989 Aug 5;208(3):469–475. doi: 10.1016/0022-2836(89)90510-x. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]