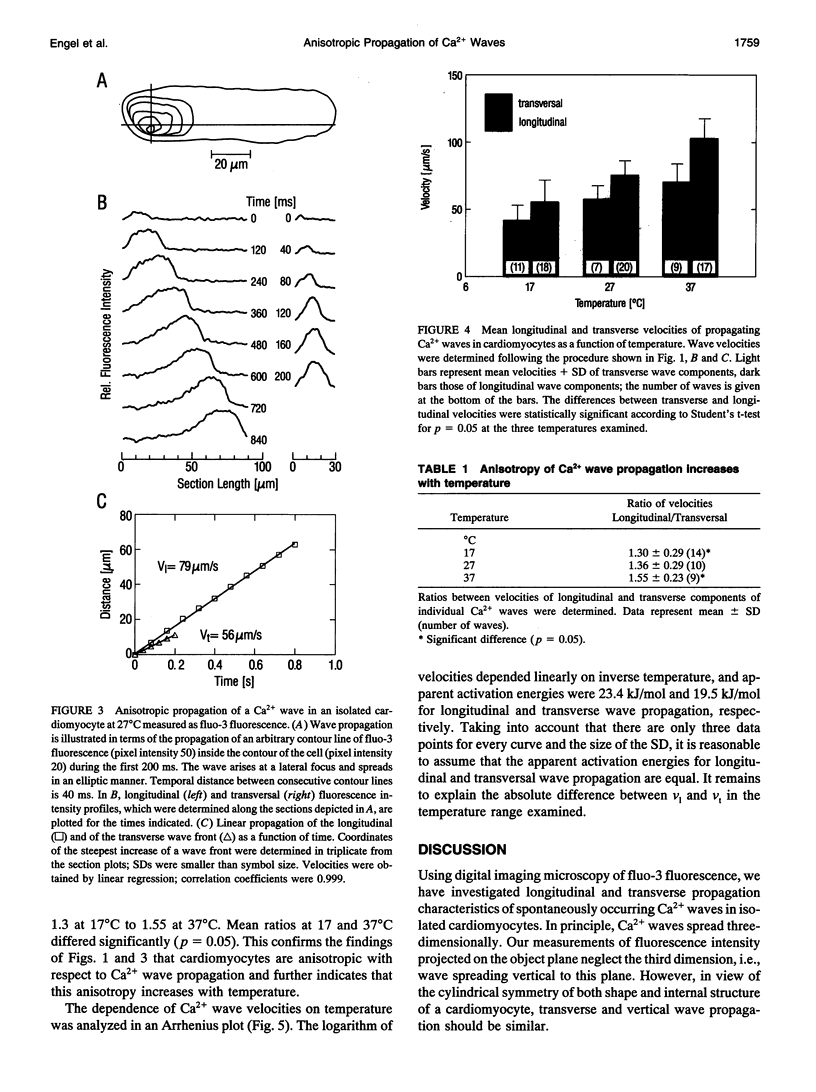
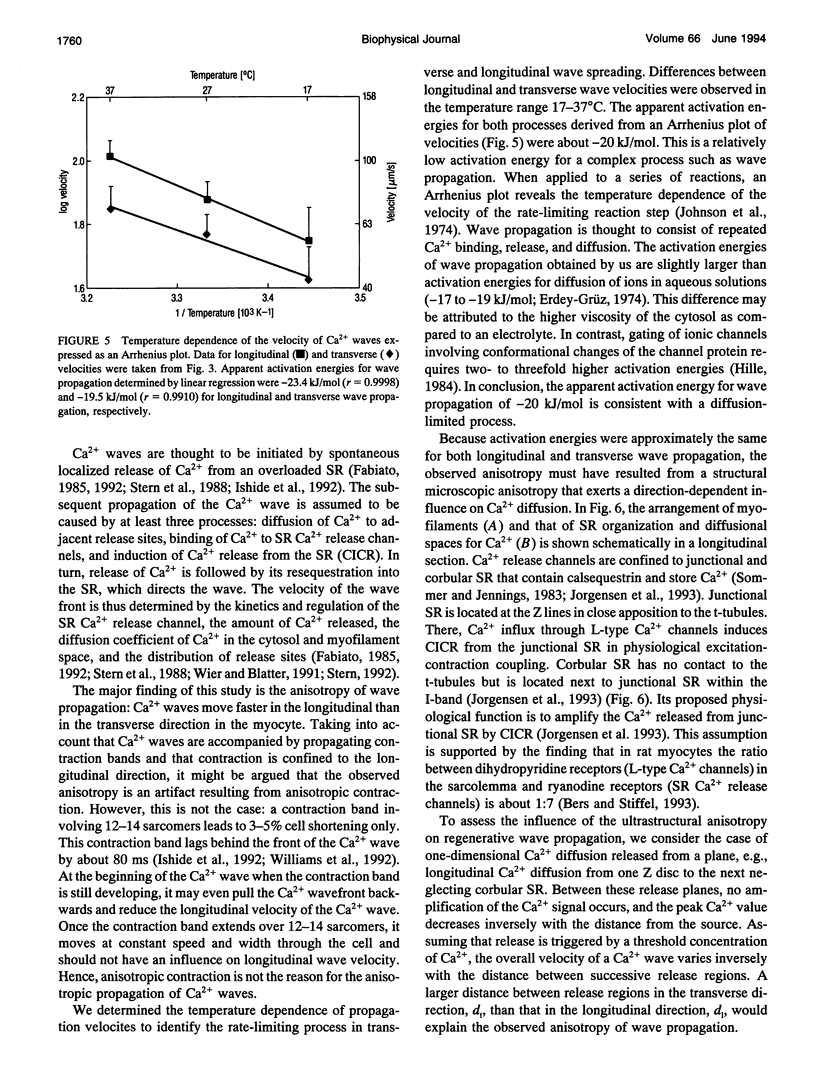
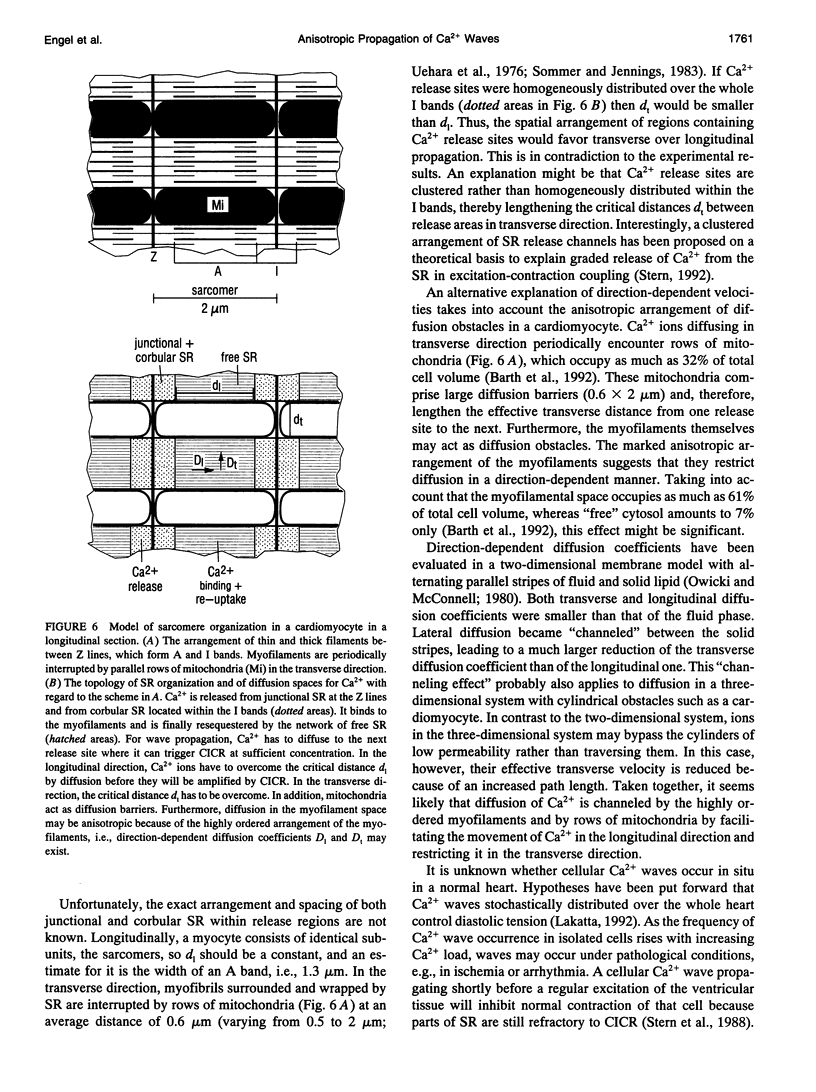
Abstract
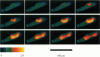
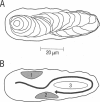
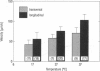
Digital imaging microscopy of fluor-3 fluorescence was used to study the propagation of intracellular Ca2+ waves in isolated adult rat cardiomyocytes from 17 to 37 degrees C. Ca2+ waves spread in both transverse and longitudinal direction of a myocyte. Transverse propagation was pronounced in waves starting from a focus at the edge of a myocyte and in waves following an irregular, curved path (spiral waves). For the former type of waves, propagation velocities were determined. Both transverse and longitudinal wave components propagated at constant velocity ranging from 30 to 125 micron/s. Myocytes were anisotropic with respect to wave propagation: waves propagated faster in the longitudinal than in the transverse direction. The ratio between longitudinal and transverse velocity increased from 1.30 at 17 degrees C to 1.55 at 37 degrees C. Apparent activation energies for transverse and longitudinal wave propagation were estimated to be -20 kJ/mol, suggesting that these processes are limited by diffusion of Ca2+. Direction-dependent propagation velocities are interpreted to result from the highly ordered structure of the myocytes, especially from the anisotropic arrangement of diffusion obstacles such as myofilaments and mitochondria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth E., Stämmler G., Speiser B., Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992 Jul;24(7):669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- Berlin J. R., Cannell M. B., Lederer W. J. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ Res. 1989 Jul;65(1):115–126. doi: 10.1161/01.res.65.1.115. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Stiffel V. M. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am J Physiol. 1993 Jun;264(6 Pt 1):C1587–C1593. doi: 10.1152/ajpcell.1993.264.6.C1587. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Two kinds of calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cardiac cells. Adv Exp Med Biol. 1992;311:245–262. doi: 10.1007/978-1-4615-3362-7_18. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem. 1989;18:333–364. doi: 10.1146/annurev.bb.18.060189.002001. [DOI] [PubMed] [Google Scholar]
- Grouselle M., Stuyvers B., Bonoron-Adele S., Besse P., Georgescauld D. Digital-imaging microscopy analysis of calcium release from sarcoplasmic reticulum in single rat cardiac myocytes. Pflugers Arch. 1991 Mar;418(1-2):109–119. doi: 10.1007/BF00370459. [DOI] [PubMed] [Google Scholar]
- Ishide N., Miura M., Sakurai M., Takishima T. Initiation and development of calcium waves in rat myocytes. Am J Physiol. 1992 Aug;263(2 Pt 2):H327–H332. doi: 10.1152/ajpheart.1992.263.2.H327. [DOI] [PubMed] [Google Scholar]
- Ishide N., Urayama T., Inoue K., Komaru T., Takishima T. Propagation and collision characteristics of calcium waves in rat myocytes. Am J Physiol. 1990 Sep;259(3 Pt 2):H940–H950. doi: 10.1152/ajpheart.1990.259.3.H940. [DOI] [PubMed] [Google Scholar]
- Jorgensen A. O., Shen A. C., Arnold W., McPherson P. S., Campbell K. P. The Ca2+-release channel/ryanodine receptor is localized in junctional and corbular sarcoplasmic reticulum in cardiac muscle. J Cell Biol. 1993 Feb;120(4):969–980. doi: 10.1083/jcb.120.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta E. G. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovasc Res. 1992 Mar;26(3):193–214. doi: 10.1093/cvr/26.3.193. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Microscopic spiral waves reveal positive feedback in subcellular calcium signaling. Biophys J. 1993 Dec;65(6):2272–2276. doi: 10.1016/S0006-3495(93)81316-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- O'Rourke B., Reibel D. K., Thomas A. P. High-speed digital imaging of cytosolic Ca2+ and contraction in single cardiomyocytes. Am J Physiol. 1990 Jul;259(1 Pt 2):H230–H242. doi: 10.1152/ajpheart.1990.259.1.H230. [DOI] [PubMed] [Google Scholar]
- Owicki J. C., McConnell H. M. Lateral diffusion in inhomogeneous membranes. Model membranes containing cholesterol. Biophys J. 1980 Jun;30(3):383–397. doi: 10.1016/S0006-3495(80)85103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper H. M., Probst I., Schwartz P., Hütter F. J., Spieckermann P. G. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982 Jul;14(7):397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- Rieser G., Sabbadini R., Paolini P., Fry M., Inesi G. Sarcomere motion in isolated cardiac cells. Am J Physiol. 1979 Jan;236(1):C70–C77. doi: 10.1152/ajpcell.1979.236.1.C70. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Capogrossi M. C., Lakatta E. G. Spontaneous calcium release from the sarcoplasmic reticulum in myocardial cells: mechanisms and consequences. Cell Calcium. 1988 Dec;9(5-6):247–256. doi: 10.1016/0143-4160(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu T., Minamikawa T., Kawachi H., Fujita S. Imaging of calcium wave propagation in guinea-pig ventricular cell pairs by confocal laser scanning microscopy. Cell Struct Funct. 1991 Aug;16(4):341–346. doi: 10.1247/csf.16.341. [DOI] [PubMed] [Google Scholar]
- Takamatsu T., Wier W. G. Calcium waves in mammalian heart: quantification of origin, magnitude, waveform, and velocity. FASEB J. 1990 Mar;4(5):1519–1525. doi: 10.1096/fasebj.4.5.2307330. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Blatter L. A. Ca(2+)-oscillations and Ca(2+)-waves in mammalian cardiac and vascular smooth muscle cells. Cell Calcium. 1991 Feb-Mar;12(2-3):241–254. doi: 10.1016/0143-4160(91)90024-9. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Delbridge L. M., Cody S. H., Harris P. J., Morgan T. O. Spontaneous and propagated calcium release in isolated cardiac myocytes viewed by confocal microscopy. Am J Physiol. 1992 Mar;262(3 Pt 1):C731–C742. doi: 10.1152/ajpcell.1992.262.3.C731. [DOI] [PubMed] [Google Scholar]