Abstract
Distinct partitioning has been observed in the composition and diversity of bacterial communities inhabiting the surface and overlying seawater of three coral species infected with black band disease (BBD) on the southern Caribbean island of Curaçao, Netherlands Antilles. PCR amplification and sequencing of bacterial 16S rRNA genes (rDNA) with universally conserved primers have identified over 524 unique bacterial sequences affiliated with 12 bacterial divisions. The molecular sequences exhibited less than 5% similarity in bacterial community composition between seawater and the healthy, black band diseased, and dead coral surfaces. The BBD bacterial mat rapidly migrates across and kills the coral tissue. Clone libraries constructed from the BBD mat were comprised of eight bacterial divisions and 13% unknowns. Several sequences representing bacteria previously found in other marine and terrestrial organisms (including humans) were isolated from the infected coral surfaces, including Clostridium spp., Arcobacter spp., Campylobacter spp., Cytophaga fermentans, Cytophaga columnaris, and Trichodesmium tenue.
Infectious disease in scleractinian corals has emerged as one of the primary causes of the accelerating global destruction of coral reef ecosystems (22, 25, 27, 56, 74). Black band disease (BBD) is one of the most widespread and destructive of these coral infections (see review in reference 50). The diagnostic symptom of BBD is the development of a narrow 0.1- to 7-cm-wide ring-shaped black to red microbial mat that migrates from top to bottom across massive coral colonies, killing healthy coral tissue at rates of as much as 1 cm per day (47, 53). BBD preferentially affects corals such as Montastrea annularis, Montastrea cavernosa, and Diploria strigosa (6, 15, 53). These species, known as framework building corals, form large structures that become the dominant physical elements of reefs. As a result, coral mortality caused by BBD is a potent force in restructuring coral reef ecosystems (15, 36).
There is considerable controversy as to whether BBD is caused by physical and chemical environmental stresses or is an infectious disease or both (50, 56). However, an impediment to determining the cause of BBD has been the lack of information about the diversity and distribution of microbial populations that inhabit normal healthy coral tissue and the BBD bacterial mat. It is known from studies of infectious disease in marine and terrestrial invertebrates, fish, and mammals (including humans) that pathogens are most effectively studied within an ecological context of interactions among microbes, their hosts, and the environmental conditions in which they live (25, 54). Accurate diagnosis and eventual treatment and prevention of BBD will therefore require a basic knowledge of the composition and distribution of the microbial communities associated with healthy as well as diseased organisms. This type of community-based comparative analysis of the microorganisms associated with infectious diseases in corals has not previously been completed.
The purpose of the present report was to complete the first culture-independent 16S rRNA phylogenetic survey of the bacterial communities inhabiting seawater and healthy, BBD-infected, and dead coral surfaces. The coral reef chosen for analysis was on the leeward reef tract of Curaçao, Netherlands Antilles (Fig. 1). A factor that may contribute to BBD is the daily dumping of sewage and other pollutants directly onto the reef from the major commercial, municipal, and military harbor of St. Annabaai (Fig. 1). The following three fundamental questions have been addressed: (i) is there a coral-specific microbial population that is different from that of the bacterioplankton community in the overlying seawater column; (ii) are the microbial communities comprising the BBD mat and the dead coral surfaces distinct from each other; and (iii) have sewage microbes colonized the diseased coral, as might be expected if sewage microbes were contributing to the disease process? Results are presented that indicate the healthy coral tissue is colonized by a microbial population that is unique and distinct from that found in the water column and the BBD mat and dead coral surfaces. Also, bacteria associated with sewage were detected only in the BBD bacterial mat.
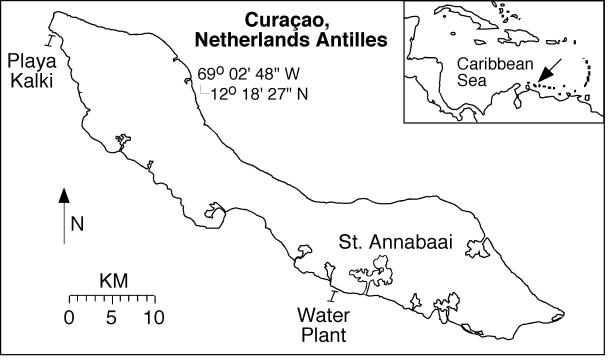
FIG. 1.
Map of Curaçao, Netherlands Antilles, in the southern Caribbean Sea. The regional location of the island is shown with an arrow on the inset map. Study sites were on the leeward reef tract at Water Plant and Playa Kalki. Also shown is the location of the St. Annabaai seaport.
MATERIALS AND METHODS
Field work and sample collection.
Field sampling using standard scuba techniques was conducted in August 2000 on the leeward reef tract of Curaçao, Netherlands Antilles (Fig. 1). Diseased corals were studied at the Playa Kalki and Water Plant sites in back reef environments approximately 0.5 km offshore (33). These included BBD-infected colonies of M. annularis (Fig. 2A and B) and M. cavernosa (Fig. 2C and D) at Playa Kalki at a 5-m water depth, as well as a colony of D. strigosa (Fig. 2E and F) at Water Plant at a 4-m water depth. Three individual BBD-infected coral heads, one each of M. annularis, M. cavernosa, and D. strigosa, were analyzed. From each coral head, four individual samples were collected in situ from the following environments: the overlying seawater, the healthy coral surface, the black band diseased coral surface, and the dead coral surface. Seawater was sampled by collecting 2 liters of seawater in cleaned 1-liter Nalgene high-density polyethylene bottles from directly above the coral colonies. The water was pumped through a sterile 0.45-μm-filter-loaded cup (Pall/Gelman) when brought on shore. All filters and centrifuge tubes were then immediately frozen at −20oC, transported to Illinois on dry ice, and stored at −40 to −80°C. The coral surface samples were collected by removing a 2-cm by 1-cm portion of the uppermost 1 cm of the coral colonies with a chisel and placing the sample in a sterile disposable 50-ml polypropylene centrifuge tube. Furthermore, portions of some BBD microbial mats were physically peeled off the coral surface with forceps and placed in sterile 15-ml polypropylene centrifuge tubes. Immediately upon the return to shore, the seawater within each tube was decanted and coral samples were immersed in either of the following solutions: (i) 80% ethanol for molecular analyses or (ii) Karnovsky's aldehyde fixative (2.0% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer [pH 7.2]) for scanning electron microscopy (SEM) analyses. Coral samples preserved in 80% ethanol were crushed and homogenized in the tube, creating a slurry of coral tissue, zooxanthellae, mucus, microorganisms, and skeletal material.
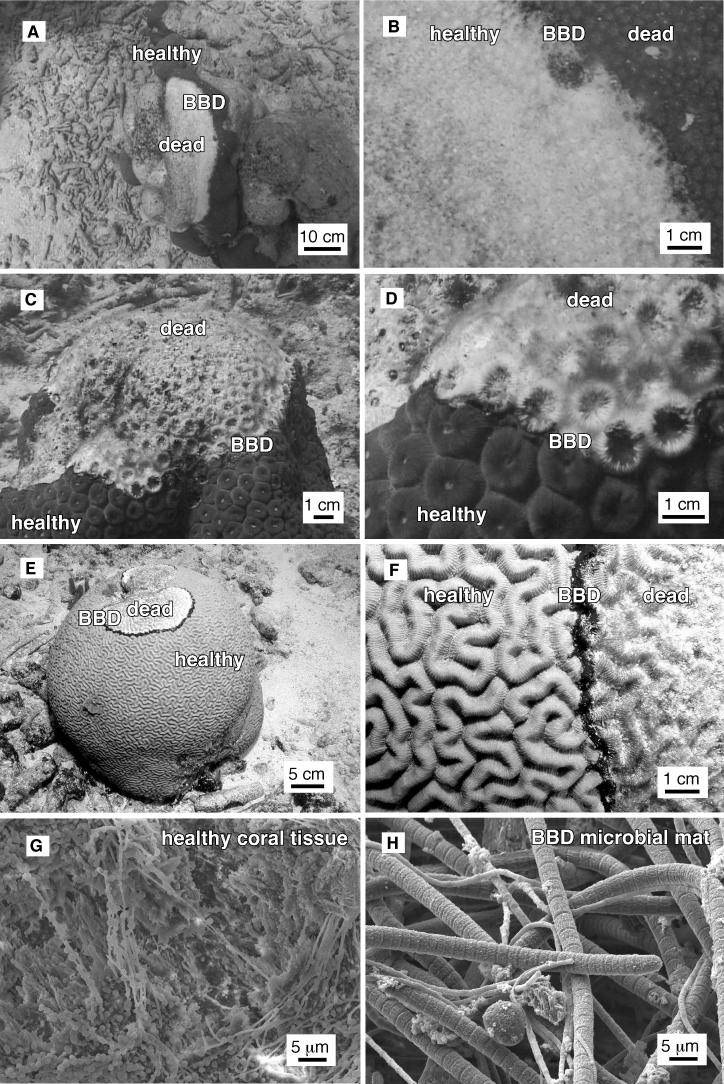
FIG. 2.
Underwater photographs of BBD infection in colonies of M. annularis (panels A and B), M. cavernosa (panels C and D), and D. strigosa (panels E and F). (G) SEM image of healthy tissue from the infected colony of D. strigosa shown in panels E and F. (H) SEM image of the BBD mat from the infected colony of D. strigosa shown in panels E and F.
SEM analyses.
The Karnovsky's aldehyde fixative was removed and replaced with buffered 0.1 M sodium phosphate (pH 7.2). The samples were dehydrated by stepwise immersion in 33, 67, and 95% ethanol for 1 h each and in 100% ethanol for 3 h. The ethanol was then removed and the samples were soaked in hexamethyldisilazane for 2 h, after which they were air dried in a laminar flow hood and placed in a desiccator. Small 0.25-cm-diameter pieces of each specimen were mounted on a 2-cm-diameter aluminum stub using double-stick carbon tabs. Samples were grounded with colloidal silver paint and covered with a 90- to 100-nm-thick gold-palladium coating using a Denton Desk II Turbo sputter coater. Imaging was done on a Phillips XL30 ESEM-FEG apparatus, which is housed at the University of Illinois Urbana-Champaign Beckman Institute, in high-vacuum mode with an accelerating voltage of 15 kV.
DNA extraction.
Several methods of DNA extraction were utilized with each sample to increase the likelihood that no microorganism would escape detection. Filters were sectioned into quarters with flame-sterilized scissors and forceps and placed in a sterile disposable 50-ml polypropylene centrifuge tube with 3 ml of sterile ultrapure water. Cells were then washed off the filter during 3 min of vigorous agitation on a vortexing apparatus and stored frozen at −80°C.
Bead beating (32), freeze-thaw cycling, and chemical lysis protocols (55) were used to extract genomic DNA from the cells collected on the filters, the crushed coral slurries, and the BBD mat samples. For bead beating, 300 μl of sample material was added to a 2-ml O-ring-equipped screw-cap microcentrifuge tube containing 600 μl of sterile ultrapure water and 800 μl of 0.1-mm zirconia-silica beads (BioSpec Products, Bartlesville, Okla.). The beads were cleaned and sterilized beforehand with a series of HCl acid and bleach washes. The tube was then filled to capacity with sterile ultrapure water and shaken on a reciprocating Mini-BeadBeater-8 (BioSpec Products) for 2.5 min at the homogenize (highest) speed setting. This protocol has been optimized by using several samples and Escherichia coli-positive controls. For the freeze-thaw procedure, samples were added to sterile 2-ml O-ring-equipped screw-cap microcentrifuge tubes containing 1 ml of sterile ultrapure water. The tubes were frozen at −80oC and rapidly thawed by plunging the tubes into a 65°C water bath. The freeze-thaw cycle was repeated three times, with the tubes vigorously agitated on a vortex apparatus for approximately 1 min after each thaw cycle. In some instances, samples were treated with an alkaline lysis step by using NaOH (0.13 M final concentration), sodium dodecyl sulfate (SDS; 0.3%) and incubation at 25°C (55).
For both the bead beating and freeze-thaw techniques, 400 μl of the lysate was used for DNA extraction. Standard phenol DNA extraction procedures were used (55). The ethanol-precipitated lysate, phenol-extracted lysate, and untreated lysate were used in subsequent PCRs. This entire protocol was tested with 300 μl of the sterile ultrapure water as both a negative control and contamination screen and with 50 μl of an overnight Luria broth E. coli culture as a positive control. The control preparations were included with their simultaneously prepared environmental samples each time a PCR was performed. In no case were nucleic acids detected in the negative controls.
PCR amplification.
Total DNA was amplified with a Mastercycler gradient thermocycler (Eppendorf, Westbury, N.Y.) by PCR using specific 16S rRNA primers for bacteria. Primers used in the PCR amplifications were obtained from Operon Technologies, Inc. (Alameda, Calif.). B. Paster (personal communication) provided the sequence of each primer as follows: forward primer, 28F (5′-GAGTTTGATYMTGGCTC); reverse primer, 1492R (5′-GYTACCTTGTTACGACTT). Reaction mixtures included a final concentration of 1× TaqMaster buffer (Eppendorf), 1.5 mM MgCl2 (Eppendorf), a 0.2 mM concentration of each deoxynucleoside triphosphate (Gibco/BRL, Rockville, Md.), 200 ng each of the forward and reverse primers, 5 to 30 μl of the sample preparation, and water to bring the total volume to 49.5 or 99.5 μl. Reaction mixtures were layered beneath 50 μl of mineral oil (Sigma, St. Louis, Mo.). An initial denaturation-hot start of 5 min at 95°C was followed by the addition of 0.5 μl (approximately 2 U) of MasterTaq polymerase (Eppendorf) or Taq DNA polymerase (Gibco/BRL). The hot start was followed by 30 cycles of the following incubation pattern: 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min. A final soak at 72°C for 5 min concluded the reaction.
Cloning and sequencing.
PCR products were purified by electrophoresis through a 1.0% low-melting-point agarose gel (SeaPlaque GTG; BioWhittaker Molecular Applications, Rockland, Maine), stained with ethidium bromide, and visualized on a UV transilluminator. The approximately 1,500-bp heterologous ribosomal DNA (rDNA) product was excised from the gel, and the DNA was purified from the gel slice by using the Wizard PCR Prep kit (Promega, Madison, Wis.). The gel-purified PCR product was cloned into the pGEM-T Easy vector (Promega) and transformed into calcium chloride-competent DH5αMCR E. coli cells according to manufacturers' instructions and standard techniques (55). Plasmid DNA was isolated from individual clones (QIAprep Spin Miniprep kit; Qiagen, Inc., Valencia, Calif.). Restriction fragment length polymorphism (RFLP) analysis was used in some samples to verify the presence of an appropriately sized insert and to select unique clones for sequencing. Aliquots from a subset of the samples of purified plasmid DNA were digested with 1 U of the restriction enzyme EcoRI in 1× REact 3 buffer (Gibco/BRL) for more than 3 h at 37°C, and the digested product was separated by electrophoresis on a 0.8% agarose gel (SeaKem LE; BioWhittaker Molecular Applications). After staining with ethidium bromide, the bands were visualized on a UV transilluminator and the RFLP patterns were analyzed to select clones containing the appropriately sized insert. Plasmid DNA from these clones was then digested with the four-base recognition site enzymes MspI and HinP1I in 1× NEB buffer 2 (New England Biolabs, Beverly, Mass.) under the conditions described above. The digest products were then separated by electrophoresis on a 3.0% agarose gel (MetaPhor; BioWhittaker Molecular Applications) and stained with ethidium bromide, and the RFLP patterns were used to identify unique clones to be submitted for sequence analysis. Multiple samples with seemingly identical RFLP patterns were selected for sequence analysis in an effort to capture different sequences with similar RFLP patterns. Clones selected for sequence analysis were patched onto Luria broth agar petri dishes supplemented with 100 μg of ampicillin/ml (Roche Molecular Biochemicals, Indianapolis, Ind.) and incubated overnight at 37°C.
Inoculation, cell culturing, template preparation, and sequencing were performed in the high-throughput laboratory of the W. M. Keck Center for Comparative and Functional Genomics of the University of Illinois at Urbana-Champaign. The plates were used to inoculate 2-ml 96-well culture blocks containing Circle Grow medium (Bio100) supplemented with ampicillin (100 μm/ml). Plasmid template DNA was purified from the cultures by using an automated system and a QIAwell 96 Turbo Prep BioRobot kit (Qiagen). Sequencing was completed by using T7(−26) primer, which was synthesized in house (63). Sequence reactions were performed on the plasmid templates by using a Qiagen Bio Robot 9600 and Big Dye Terminator chemistry apparatus (v.2.0) from ABI. Sequencing was performed on an ABI 3700 capillary sequencer, and then the results were processed in the Bioinformatics Unit of the W. M. Keck Center.
Sequence analyses.
The rDNA sequences were first compared with those in the GenBank database with the basic local alignment search tool (BLAST) network service (4). From the alignments created by this search, the orientation of each cloned 16S rRNA gene could be determined and a rough phylogenetic association was established. Each sequence was analyzed using the CHIMERA_CHECK program (version 2.7) available at the Ribosomal Database Project web site (39). Those sequences deemed to be chimeric were culled from the data set. A 97 to 100% match of the unknown clone with the GenBank dataset was considered an accurate identification to the species level, 93 to 96% similarity was accepted as a genus-level identification, and a 86 to 92% match was considered an accurate identification of a related organism (21).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the 16S rRNA sequences generated in this study are AY037873 through AY038581 and AF441866 through AF442078.
RESULTS
Optical and SEM analyses.
Visual assessment of the surface of infected M. annularis, M. cavernosa, and D. strigosa colonies indicate that healthy coral tissues contain microbes with different morphologies than those of the microbes inhabiting the BBD microbial mats. Bacteria inhabiting healthy coral tissue occurred within a stringy exopolymer matrix. In contrast, the BBD mat is dominated by large (2- to 5-μm by 0.5- to 2-mm) filamentous nonheterocystous cyanobacteria that have previously been optically identified as Phormidium corallyticum (52). A smaller filamentous bacterium that has previously been optically identified as a member of the Beggiatoa spp. (14, 53) occurs intertwined with P. corallyticum. Rare 0.5-mm-diameter bundles of P. corallyticum and Beggiatoa spp. were sometimes observed on healthy coral tissue within a few centimeters of the leading edge of the migrating BBD mat, which is consistent with previous observations (48).
16S rRNA clone library diversity.
High-diversity assemblages of bacterial sequences were detected in seawater and healthy, BBD-infected, and dead coral surfaces in M. annularis, M. cavernosa, and D. strigosa colonies (Tables 1, 2, and 3, respectively). A total 65 bacterial sequences in seawater directly overlying the coral colonies and a total of 230 sequences from healthy (64 sequences), BBD-infected (64 sequences), and dead (102 sequences) coral surfaces. Microorganism identifications were based on comparison of these sequences with the GenBank database. Division-level microbial diversity in each sample was estimated by dividing the number of clones representing each division by the total number of clones in the seawater and healthy, BBD diseased, and dead coral surface libraries associated with M. annularis, M. cavernosa, and D. strigosa (shown as pie diagrams in Fig. 3, 4, and 5, respectively).
TABLE 1.
16S rRNA sequencing results for Montastrea annularis from the back reef at Playa Kalkia
| ID% | No. of similar clones | No. of base pairs sequenced | Best-matched organism | Accession no. | Division | Location of cloneb
|
|||
|---|---|---|---|---|---|---|---|---|---|
| SW | HC | BBD | DC | ||||||
| 98-99 | 3 | 389 | Uncultured marine bacterium ZD0409 | AJ400350 | α-Proteobacteria | × | |||
| 93-97 | 3 | 450 | Uncultured bacterium OCS 126 | AF001638 | α-Proteobacteria | × | |||
| 99 | 1 | 594 | Marine bacterioplankton clone SAR102 | L35460 | α-Proteobacteria | × | |||
| 91 | 1 | 568 | Magnetospirillum sp. strain MSM-6 | Y17391 | α-Proteobacteria | × | |||
| 90 | 1 | 571 | Uncultured bacterium MND8 | AF292999 | α-Proteobacteria | × | |||
| 99 | 1 | 622 | Uncultured Roseobacter sp. strain NAC11-1 | AF245630 | α-Proteobacteria | × | |||
| 98 | 1 | 283 | Unidentified alpha proteobacterium | U78945 | α-Proteobacteria | × | |||
| 99 | 3 | 483 | Pseudoalteromonas sp. strain A28 | AF227238 | γ-Proteobacteria | × | |||
| 94-96 | 3 | 601 | Uncultured marine eubacterium HstpL49 | AF159675 | γ-Proteobacteria | × | |||
| 99 | 2 | 567 | Pseudoalteromonas sp. strain PRLIST2 | Y15323 | γ-Proteobacteria | × | |||
| 97 | 1 | 567 | Alteromonas macleodii strain CH-518 | Y18232 | γ-Proteobacteria | × | |||
| 95 | 1 | 640 | Uncultured bacterium CHAB-I-7 | AJ240911 | γ-Proteobacteria | × | |||
| 99 | 6 | 589 | Prochlorococcus sp. strain MIT9202 | AF115269 | Cyanobacteria | × | |||
| 99 | 2 | 597 | Prochlorococcus sp. strain MIT9312 | AF053398 | Cyanobacteria | × | |||
| 98 | 2 | 605 | Marine bacterioplankton clone SAR7 | X52171 | Cyanobacteria | × | |||
| 99 | 1 | 480 | Prochlorococcus marinus subsp. pastoris | AF180967 | Cyanobacteria | × | |||
| 97 | 1 | 406 | Synechococcus sp. strain WR8101 | AJ292905 | Cyanobacteria | × | |||
| 91 | 1 | 621 | Cytophaga sp. strain JTB250 | AB015264 | CFB | × | |||
| 96 | 1 | 619 | Unidentified eukaryote OM8 1 | U70717 | Chloroplasts | × | |||
| 94 | 1 | 542 | Magnetite-containing magnetic Vibrio sp. | L06455 | α-Proteobacteria | × | |||
| 94 | 1 | 432 | Magnetospirillum sp. strain MSM-6 | Y17391 | α-Proteobacteria | × | |||
| 95 | 2 | 302 | Rhodothalassium salexigens | M59070 | α-Proteobacteria | × | |||
| 95 | 2 | 285 | Unknown proteobacterium clone JAP553 | U09830 | α-Proteobacteria | × | × | ||
| 98-99 | 3 | 337 | Uncultured bacterium SUR-FREE-32 | AF114653 | α-Proteobacteria | × | × | ||
| 98 | 1 | 340 | Uncultured bacterium BURTON-9 | AF142829 | α-Proteobacteria | × | |||
| 96 | 1 | 594 | Unidentified bacterium isolate HNSS21 | Z88572 | α-Proteobacteria | × | |||
| 92-95 | 2 | 516 | Unidentified alpha proteobacterium OM65 | U70682 | α-Proteobacteria | × | × | ||
| 98 | 1 | 443 | Unidentified beta proteobacterium | AB015567 | β-Proteobacteria | × | |||
| 94 | 1 | 609 | Uncultured bacterium S2551 | AF177428 | δ-Proteobacteria | × | |||
| 90-92 | 3 | 382 | Agricultural soil bacterium clone SC-I-15 | AJ252618 | δ-Proteobacteria | × | |||
| 94 | 1 | 587 | Legionella feeleii sgp2 (ATCC 35849) | X73406 | γ-Proteobacteria | × | |||
| 94 | 1 | 595 | Legionella londiniensis | AF129525 | γ-Proteobacteria | × | |||
| 90 | 1 | 321 | Unidentified eubacterium, clone LRE18 | AJ232879 | γ-Proteobacteria | × | |||
| 92 | 1 | 310 | Ornithodoros moubata symbiote A | AB001521 | γ-Proteobacteria | × | |||
| 89 | 1 | 563 | Uncultured bacterium strain JTB256 | AB015255 | γ-Proteobacteria | × | |||
| 94-95 | 11 | 548 | Chlorobium vibrioforme | Y10648 | Green sulfur bacteria | × | |||
| 91 | 1 | 609 | Planctomyces brasiliensis | X85247 | Planctomycetales | × | |||
| 86 | 2 | 648 | Uncultured bacterium ACE-29 | AF142804 | Planctomycetales | × | × | ||
| 94 | 1 | 566 | Uncultured marine eubacterium HstpL64 | AF159640 | Planctomycetales | × | |||
| 90 | 1 | 297 | Bacterium 2-400 C2.5 | Z77532 | Planctomycetales | × | |||
| 95 | 1 | 448 | Uncultured bacterium 03 19-7F4 | AF234144 | Planctomycetales | × | |||
| 88 | 1 | 509 | Unidentified bacterium, strain BD2-16 | AB015544 | Unknown | × | |||
| 89 | 2 | 607 | Uncultured bacterium ACE-29 | AF142804 | Planctomycetales | × | × | ||
| 91 | 1 | 446 | Thermophilic bacterium MV 1087 | AJ272422 | Firmicutes | × | |||
| 90 | 1 | 610 | Clostridium aldrichii | X71846 | Firmicutes | × | |||
| 87 | 2 | 497 | Moorella thermoacetica | AJ242494 | Firmicutes | × | × | ||
| 91 | 1 | 325 | Unidentified eubacterium clone BSV72 | AJ229216 | Firmicutes | × | |||
| 89-91 | 3 | 559-613 | Unidentified soil bacterium clone BSV73 | AJ229217 | Firmicutes | × | |||
| 91 | 1 | 223 | Dietzia sp. strain CIP104293 | Y08313 | Firmicutes | × | |||
| 90 | 1 | 564 | Cytophaga diffluens | M58765 | CFB | × | |||
| 96 | 1 | 365 | Leptolyngbya sp. strain PCC7375 | AB039011 | Cyanobacteria | × | |||
| 89-92 | 2 | 398 | Mesostigma viride | L49152 | Chloroplasts | × | |||
| 86 | 1 | 500 | Astasia longa | AJ294725 | Chloroplasts | × | |||
| 92 | 1 | 326 | Codium fragile | U08345 | Chloroplasts | × | |||
| 84 | 1 | 307 | Prototheca wickerhamii | AJ245645 | Chloroplasts | × | |||
| 99 | 1 | 417 | Rape rhizosphere bacterium tsb088 | AJ295458 | Unknown | × | |||
| 96 | 1 | 462 | Uncultured sponge symbiont PAWS51 | AF186441 | Unknown | × | |||
| 86 | 1 | 453 | Unidentified bacterium, strain BD2-3 | AB015533 | Unknown | × | |||
| 96-97 | 3 | 501 | Desulfovibrio salexigens | M34401 | δ-Proteobacteria | × | |||
| 97 | 1 | 617 | Unidentified bacterium isolate Aspo3 | X95231 | δ-Proteobacteria | × | |||
| 93 | 1 | 502 | Uncultured epsilon proteobacterium KTc1160 | AF235116 | ɛ-Proteobacteria | × | |||
| 92 | 1 | 644 | Uncultured epsilon proteobacterium 1065 | AB030598 | ɛ-Proteobacteria | × | |||
| 92 | 1 | 589 | Uncultured epsilon proteobacterium | AF246706 | ɛ-Proteobacteria | × | |||
| 92 | 1 | 604 | Arcobacter sp. clone D1a1 | AJ271654 | ɛ-Proteobacteria | × | |||
| 91-94 | 7 | 551 | Cytophaga fermentans | M58766 | CFB | × | × | ||
| 92-93 | 3 | 430 | Uncultured Cytophaga kps30 | AF195441 | CFB | × | |||
| 92 | 1 | 601 | Cytophaga sp. strain BD1-16 | AB015525 | CFB | × | |||
| 93 | 1 | 595 | Trichodesmium tenue | AF013029 | Cyanobacteria | × | |||
| 92 | 3 | 441 | Bacterium strain 77003 | AF227847 | Unknown | × | |||
| 91 | 1 | 594 | Clostridium fimetarium | AF126687 | Firmicutes | × | |||
| 91 | 1 | 637 | Clostridium paradoxum clone para99 | Z69939 | Firmicutes | × | |||
| 89 | 1 | 349 | Uncultured marine bacterium 90d10 | AF295117 | Firmicutes | × | |||
| 90-91 | 2 | 487 | Guillardia theta | AF041468 | Chloroplasts | × | |||
| 87 | 1 | 318 | Unidentified eubacterium clone BSV85 | AJ229229 | Unknown | × | |||
| 97 | 1 | 496 | Roseobacter sp. (P. decipiens symbiont) | AF107210 | α-Proteobacteria | × | |||
| 96 | 1 | 568 | Roseobacter sp. (P. filiformis symbiont) | AF107209 | α-Proteobacteria | × | |||
| 95 | 1 | 626 | Sulfitobacter pontiacus | AF182018 | α-Proteobacteria | × | |||
| 96 | 1 | 507 | Uncultured Agrobacterium sp. strain kpc102rc | AF194391 | α-Proteobacteria | × | |||
| 99 | 1 | 515 | Uncultured marine eubacterium HstpL78 | AF159652 | α-Proteobacteria | × | |||
| 86-93 | 9 | 454 | Desulfocella halophila | AF022936 | δ-Proteobacteria | × | |||
| 96-98 | 4 | 487 | Desulforhopalus singaporensis | AF118453 | δ-Proteobacteria | × | |||
| 97-98 | 4 | 549 | Desulfobotulus sp. strain BG14 | U85470 | δ-Proteobacteria | × | |||
| 91 | 2 | 483 | Desulfofrigus fragile | AF099065 | δ-Proteobacteria | × | |||
| 98 | 1 | 524 | Desulfobacterium catecholicum | AJ237602 | δ-Proteobacteria | × | |||
| 94 | 1 | 218 | Uncultured bacterium ODPB-B3 | AF121088 | δ-Proteobacteria | × | |||
| 94 | 1 | 612 | Unidentified bacterium clone NB1-k | AB013832 | δ-Proteobacteria | × | |||
| 91 | 1 | 587 | Plesiomonas shigelloides | X60418 | γ-Proteobacteria | × | |||
| 93 | 1 | 583 | Uncultured bacterium strain BD1-7 | AB015519 | γ-Proteobacteria | × | |||
| 92 | 1 | 564 | Pseudoalteromonas sp. strain A25 | AF227237 | γ-Proteobacteria | × | |||
| 98 | 1 | 652 | Vibrio alginolyticus (ATCC 17749T) | X56576 | γ-Proteobacteria | × | |||
| 89 | 1 | 582 | Unidentified bacterium, clone NB1-o | AB013836 | γ-Proteobacteria | × | |||
| 94 | 1 | 398 | Uncultured bacterium adhufec29.25 | AF153855 | γ-Proteobacteria | × | |||
| 91 | 1 | 571 | Cytophaga sp. strain JTB244 | AB015262 | CFB | × | |||
| 86 | 1 | 325 | Flexibacter tractuosus | M58789 | CFB | × | |||
| 94 | 1 | 487 | Microscilla furvescens | M58792 | CFB | × | |||
| 91 | 1 | 331 | Psychroserpens burtonensis ACAM188 | U62913 | CFB | × | |||
| 96 | 1 | 503 | Uncultured bacterium KC305 | AB022513 | CFB | × | |||
| 92 | 1 | 589 | Uncultured cytophagales QSSC9L-1 | AF170779 | CFB | × | |||
| 96 | 1 | 602 | Clostridium halophilum DSM 5387 | X77837 | Firmicutes | × | |||
| 89 | 1 | 411 | Uncultured soil bacterium clone K20-71 | AF145861 | Firmicutes | × | |||
| 87 | 1 | 415 | Uncultured bacterium Sva0855 | AJ240981 | Firmicutes | × | |||
| 87 | 1 | 566 | Uncultured eubacterium clone vadinBB35 | U81761 | Unknown | × | |||
Listed are the percent identities (ID%) to previously identified sequences, the numbers of similar clones, the numbers of base pairs sequenced (bp), and the accession numbers and divisions of the best s-matched organism in GenBank.
× signs indicate whether the clone occurred in the overlying seawater (SW), healthy coral tissues (HC), black band diseased coral tissue (BBD), or dead coral skeleton surfaces (DC).
TABLE 2.
16S rRNA sequencing results for a Montastrea cavernosa colony from the back reef at Playa Kalkia
| ID% | No. of similar clones | No. of base pairs sequenced | Best-matched organism | Accession no. | Division | Location of Cloneb
|
|||
|---|---|---|---|---|---|---|---|---|---|
| SW | HC | BBD | DC | ||||||
| 98 | 2 | 594 | Uncultured bacterium SAR102 | L35460 | α-Proteobacteria | × | |||
| 94-98 | 2 | 534 | Uncultured bacterium OCS126 | AF001638 | α-Proteobacteria | × | |||
| 91 | 1 | 543 | Caedibacter caryophilus | AJ238683 | α-Proteobacteria | × | |||
| 92 | 1 | 573 | Olavius loisae endosymbiont 2 | AF104473 | α-Proteobacteria | × | |||
| 99 | 1 | 596 | Uncultured Roseobacter NAC11-1 | AF245630 | α-Proteobacteria | × | |||
| 98 | 1 | 610 | Uncultured bacterium EBAC31A08 | AF268219 | δ-Proteobacteria | × | |||
| 92 | 1 | 334 | Uncultured bacterium CHAB-II-49 | AJ240909 | δ-Proteobacteria | × | |||
| 98-99 | 2 | 546 | Unknown marine bacterioplankton SAR7 | X52171 | δ-Proteobacteria | × | |||
| 94 | 2 | 587 | Unidentified marine bacterium OM60 | U70696 | γ-Proteobacteria | × | |||
| 98-99 | 9 | 587 | Prochlorococcus sp. strain MIT9202 | AF115269 | Cyanobacteria | × | |||
| 98 | 3 | 546 | Uncultured Synechococcus sp. strain NAC1-5 | AF245618 | Cyanobacteria | × | |||
| 96 | 1 | 454 | Prochlorococcus marinus pastori | AF180967 | Cyanobacteria | × | |||
| 99 | 1 | 603 | Prochlorococcus sp. strain MIT9312 | AF053398 | Cyanobacteria | × | |||
| 99 | 1 | 602 | Synechococcus WH8101 | AF001480 | Cyanobacteria | × | |||
| 92 | 2 | 675 | Uncultured Cytophagales CRE-FL75 | AF141488 | CFB | × | |||
| 93 | 1 | 688 | Marine psychrophile sp. SW17 | AF001368 | CFB | × | |||
| 93 | 1 | 645 | Uncultured marine bacterium ZD0403 | AJ400347 | CFB | × | |||
| 99 | 1 | 588 | Unidentified cytophagales OM188 | U70687 | CFB | × | |||
| 94 | 1 | 601 | Unidentified planctomycete OM190 | U70712 | Planctomycetales | × | |||
| 98 | 1 | 567 | Unidentified bacterium isolate HRV16 | Z88588 | Unknown | × | |||
| 95 | 12 | 564 | Unidentified beta proteobacterium OPB30 | AF026979 | β-Proteobacteria | × | |||
| 94 | 6 | 558 | Hydrogenophilus thermoluteolus TH-4 | AB009829 | β-Proteobacteria | × | |||
| 95 | 1 | 478 | Unidentified beta proteobacterium OPS140 | AF026983 | β-Proteobacteria | × | |||
| 95-96 | 2 | 664 | Uncultured epsilon proteobacterium KTc1160 | AF235116 | ɛ-Proteobacteria | × | |||
| 98-99 | 34 | 539 | Chromatium sp. RW | AF384210 | γ-Proteobacteria | × | |||
| 99 | 1 | 406 | Escherichia coli K12 MG1655 | AE000345 | γ-Proteobacteria | × | |||
| 95 | 1 | 527 | Uncultured alpha proteobacterium SIC.926 | AF277517 | α-Proteobacteria | × | |||
| 99 | 1 | 583 | Uncultured proteobacterium clone CD5H5 | AY038412 | α-Proteobacteria | × | |||
| 93 | 1 | 588 | Maricaulis sp. strain MCS18 | AJ227806 | α-Proteobacteria | × | |||
| 94 | 1 | 607 | Uncultured alpha proteobacterium KTc0993 | AF235129 | α-Proteobacteria | × | |||
| 95 | 1 | 583 | Uncultured proteobacterium clone CD4D6 | AY038529 | δ-Proteobacteria | × | |||
| 99 | 2 | 515 | Desulfovibrio sp. strain TBP-1 | AF090830 | δ-Proteobacteria | × | × | ||
| 98 | 1 | 582 | Uncultured proteobacterium clone CD5B11 | AY038410 | ɛ-Proteobacteria | × | |||
| 96 | 1 | 321 | Shewanella sp. clone NB65-G | AB013842 | γ-Proteobacteria | × | |||
| 93 | 1 | 606 | Marinobacter hydrocarbonoclasticus | Y18240 | γ-Proteobacteria | × | |||
| 99 | 1 | 566 | Pseudomonas stutzeri | AY017341 | γ-Proteobacteria | × | |||
| 93-94 | 2 | 443 | Uncultured gamma proteobacterium clone 26 | AF369718 | γ-Proteobacteria | × | |||
| 96 | 1 | 542 | Unidentified proteobacterium strain NKB4 | AB013256 | γ-Proteobacteria | × | |||
| 92 | 2 | 616 | Oceanospirillum linum | M22365 | γ-Proteobacteria | × | |||
| 88 | 1 | 530 | Marine bacterium BBFL7 | AY028207 | CFB | × | |||
| 99 | 1 | 630 | Uncultured Cytophagales clone CD4B12 | AY038511 | CFB | × | |||
| 92 | 1 | 597 | Parasporobacterium paucivorans sp. SYR1 | AJ272036 | Firmicutes | × | |||
| 92-95 | 2 | 478 | Spirochaeta litoralis | M88723 | Spirochaetales | × | × | ||
| 91 | 1 | 220 | Uncultured rumen bacterium 4C3d-12 | AB034093 | Unknown | × | |||
| 91 | 1 | 612 | Unidentified butyrate-producing L1-92 | AJ270487 | Unknown | × | |||
| 97 | 1 | 613 | Uncultured Crater Lake bacterium CL0-45 | AF316686 | Unknown | × | |||
| 98 | 2 | 435 | Silicibacter lacuscaerulensis | U77644 | α-Proteobacteria | × | |||
| 97 | 1 | 489 | Alpha proteobacterium MBIc1876 | AB026194 | α-Proteobacteria | × | |||
| 88 | 1 | 489 | Uncultured alpha proteobacterium MB13E08 | AY033327 | α-Proteobacteria | × | |||
| 95 | 2 | 497 | Caulobacter sp. strain MCS33 | AJ227811 | α-Proteobacteria | × | |||
| 96 | 1 | 341 | Uncultured Rhodobacter CtaxPhil-16 | AF259624 | α-Proteobacteria | × | |||
| 97 | 1 | 477 | Agrobacterium gelatinovorum | D88523 | α-Proteobacteria | × | |||
| 95 | 1 | 342 | Uncultured alpha proteobacterium CHAB-I-5 | AJ240910 | α-Proteobacteria | × | |||
| 97 | 1 | 373 | Sulfitobacter pontiacus | AF182018 | α-Proteobacteria | × | |||
| 90 | 1 | 399 | Uncultured ferromanganous bacterium MND | AF292999 | α-Proteobacteria | × | |||
| 99 | 1 | 333 | Beta proteobacterium OS-ac-15 | U46749 | β-Proteobacteria | × | |||
| 87 | 1 | 296 | Desulfovibrio zosterae | Y18049 | δ-Proteobacteria | × | |||
| 90-91 | 2 | 555 | Desulfocella halophila | AF022936 | δ-Proteobacteria | × | |||
| 91 | 1 | 425 | Delta proteobacterium S2551 | AF177428 | δ-Proteobacteria | × | |||
| 89 | 1 | 302 | Desulfovibrio alaskensis NCIMB13491 | Y11984 | δ-Proteobacteria | × | |||
| 87 | 1 | 462 | Uncultured delta proteobacterium Sva0447 | AJ240999 | δ-Proteobacteria | × | |||
| 92 | 1 | 535 | Desulfofrigus oceanense strain ASv26 | AF099064 | δ-Proteobacteria | × | |||
| 96 | 1 | 371 | Desulfobotulus sp. strain BG14 | U85470 | δ-Proteobacteria | × | |||
| 94 | 2 | 525-567 | Desulfovibrio aespoeensis (isolate Aspo2) | X95230 | δ-Proteobacteria | × | × | ||
| 95 | 1 | 546 | North Sea bacterium H120 | AF069667 | Unknown | × | |||
| 88 | 1 | 626 | Unidentified bacterium strain BD2-14 | AB015542 | Unknown | × | |||
| 93 | 1 | 544 | Bacterium strain 77003 | AF227847 | Unknown | × | |||
| 90 | 1 | 356 | Marine snow bacterium Adriatic87 | AF030773 | Unknown | × | |||
| 94 | 1 | 568 | Uncultured bacterium PENDANT-24 | AF142936 | α-Proteobacteria | × | |||
| 99 | 1 | 338 | Escherichia coli K12 MG1655 | AE000345 | γ-Proteobacteria | × | |||
| 91 | 1 | 768 | Uncultured gamma proteobacterium CHAB-IV-34 | AJ240917 | γ-Proteobacteria | × | |||
| 93 | 1 | 297 | Uncultured gamma proteobacterium DSS65 | AJ401386 | γ-Proteobacteria | × | |||
| 94 | 1 | 355 | Uncultured gamma proteobacterium KTc119 | AF235120 | γ-Proteobacteria | × | |||
| 94 | 4 | 612 | Chlorobium vibrioforme | Y08103 | Green Sulfur Bacteria | × | |||
| 96 | 1 | 622 | Prosthecochloris aestuarii | AJ291826 | Green Sulfur Bacteria | × | |||
| 96 | 1 | 505 | Uncultured bacillariophyte AWS98-19b | AF327029 | Chloroplasts | × | |||
| 91 | 1 | 614 | Epulopiscium sp. morphotype B | M99574 | Firmicutes | × | |||
| 97-98 | 2 | 576 | Uncultured marine eubacterium HstpL78 | AF159652 | Unknown | × | |||
Abbreviations are as defined in footnote a to Table 1.
× signs indicate whether the clone occurred in the overlying seawater (SW), healthy coral tissue (HC), black band diseased coral tissue (BBD), or dead coral skeleton surfaces (DC).
TABLE 3.
16S rRNA sequencing results for a Diploria strigosa colony from the back reef at Water Planta
| ID% | No. of similar clones | No. of base pairs sequenced | Best-matched organism | Accession no. | Division | Location of cloneb
|
|||
|---|---|---|---|---|---|---|---|---|---|
| SW | HC | BBD | DC | ||||||
| 98 | 1 | 567 | Uncultured marine bacterium ZD0409 | AJ400350 | α-Proteobacteria | × | |||
| 96 | 1 | 568 | Uncultured marine eubacterium HstpL28 | AF159650 | α-Proteobacteria | × | |||
| 99 | 3 | 604 | Pseudoalteromonas sp. isolate PRLIST2 | Y15323 | γ-Proteobacteria | × | |||
| 99 | 2 | 610 | Pseudomonas sp. strain MBIC2027 | AB030085 | γ-Proteobacteria | × | |||
| 97 | 1 | 622 | Alteromonas macleodii strain CH-518 | Y18232 | γ-Proteobacteria | × | |||
| 99 | 1 | 423 | Alteromonas macleodii strain DSM 6062 | Y18228 | γ-Proteobacteria | × | |||
| 99 | 1 | 444 | Pseudoalteromonas sp. strain A28 | AF227238 | γ-Proteobacteria | × | |||
| 99 | 1 | 572 | Uncultured bacterium Car164 | AF285610 | γ-Proteobacteria | × | |||
| 98 | 1 | 560 | Uncultured CHAB-I-7 | AJ240911 | γ-Proteobacteria | × | |||
| 91 | 1 | 619 | Uncultured KTc0924 | AF235121 | γ-Proteobacteria | × | |||
| 97 | 1 | 511 | Uncultured marine eubacterium HstpL66 | AF159670 | γ-Proteobacteria | × | |||
| 97 | 1 | 598 | Uncultured OCS44 | AF001650 | γ-Proteobacteria | × | |||
| 99 | 1 | 611 | Unidentified bacterium | Z93992 | γ-Proteobacteria | × | |||
| 98-99 | 6 | 593 | Prochlorococcus sp. strain MIT9202 | AF115269 | Cyanobacteria | × | |||
| 99 | 3 | 531 | Prochlorococcus marinus subsp. pastoris | AF180967 | Cyanobacteria | × | |||
| 99 | 1 | 603 | Prochlorococcus sp. strain MIT9312 | AF053398 | Cyanobacteria | × | |||
| 98 | 1 | 603 | Synechococcus WH8101 | AF397728 | Cyanobacteria | × | |||
| 98-99 | 3 | 513 | Cyanophora paradoxa cyanelle | M19493 | Chloroplasts | × | × | ||
| 97 | 1 | 603 | Guillardia theta | AF041468 | Chloroplasts | × | |||
| 91 | 2 | 638 | Cyanophora paradoxa cyanelle | M19493 | Chloroplasts | × | × | ||
| 92 | 1 | 659 | Marine psychrophile SW17 | AF001368 | CFB | × | |||
| 92 | 1 | 419 | Fusibacter paucivorans | AF050099 | Firmicutes | × | |||
| 98 | 1 | 594 | Unidentified eubacterium clone SAR276 | U65915 | Planctomycetales | × | |||
| 98 | 1 | 532 | Unidentified planctomycete OM55 | U70681 | Planctomycetales | × | |||
| 96 | 1 | 548 | Uncultured eubacterium TRA2-10 | AF047642 | Unknown | × | |||
| 100 | 1 | 554 | Unidentified bacterium isolate HRV39 | Z88591 | Unknown | × | |||
| 95-96 | 3 | 347 | Uncultured marine eubacterium HstpL93 | AF159684 | γ-Proteobacteria | × | |||
| 96 | 7 | 389 | Uncultured marine eubacterium HstpL43 | AF159674 | γ-Proteobacteria | × | |||
| 93-96 | 4 | 466 | Unidentified strain NKB4 | AB013256 | γ-Proteobacteria | × | |||
| 93 | 13 | 542 | Uncultured gamma proteobacterium clone 26 | AF369718 | γ-Proteobacteria | × | |||
| 92 | 13 | 698 | Oceanospirillum linum | AF260752 | γ-Proteobacteria | × | |||
| 92 | 1 | 567 | Pseudomonas sp. strain IMT40 | AF302796 | γ-Proteobacteria | × | |||
| 92 | 1 | 471 | Pseudomonas denitrificans IAM 12023 | AB021419 | γ-Proteobacteria | × | |||
| 93 | 1 | 541 | Uncultured proteobacterium MBIC3958 | AB020600 | γ-Proteobacteria | × | |||
| 97 | 1 | 385 | Haemophilus paraphrophilus | M75042 | γ-Proteobacteria | × | |||
| 92 | 1 | 613 | Oceanospirillum maris williamsae IFO15468 | AB006763 | γ-Proteobacteria | × | |||
| 99-100 | 4 | 604 | Propionibacterium acnes | AF154832 | Firmicutes | × | |||
| 97 | 1 | 373 | Uncultured Cytophagales clone CRE-PA10 | AF141499 | CFB | × | |||
| 91 | 1 | 576 | Flexibacter aggregans | M64628 | CFB | × | |||
| 98 | 1 | 590 | Uncultured Cytophagales clone CD4E12 | AY038534 | CFB | × | |||
| 91 | 1 | 635 | Planktothrix sp. strain FPI | AF212922 | Cyanobacteria | × | |||
| 93 | 2 | 588 | Xenococcus PCC7305 | AF132783 | Cyanobacteria | × | |||
| 92 | 1 | 546 | Arctic seawater bacterium R7366 | AJ293826 | Unknown | × | |||
| 89-98 | 8 | 471 | Clostridium halophilum DSM 5387 | X77837 | Firmicutes | × | × | ||
| 88 | 1 | 370 | Eubacterium oxidoreducens strain G2-2 | AF202259 | Firmicutes | × | |||
| 96 | 1 | 605 | Uncultured Cytophaga kps30 | AF195441 | Firmicutes | × | |||
| 89-94 | 5 | 470 | Cytophaga sp. strain JTB132 | AB015260 | CFB | × | |||
| 87-93 | 3 | 535 | Cytophaga fermentans | M58766 | CFB | × | |||
| 89 | 1 | 433 | Flavobacterium columnare | AB023660 | CFB | × | |||
| 90 | 1 | 348 | Flavobacterium sp. strain A43 | AB008043 | CFB | × | |||
| 97 | 1 | 480 | Roseobacter sp. (Prionitis symbiont) | AF107210 | α-Proteobacteria | × | |||
| 91 | 1 | 596 | Unidentified proteobacterium strain BD7-3 | AB015579 | α-Proteobacteria | × | |||
| 96 | 1 | 440 | Desulfobotulus sp. strain BG14 | U85470 | δ-Proteobacteria | × | |||
| 96 | 3 | 512-607 | Uncultured epsilon proteobacterium KTc1160 | AF235116 | ɛ-Proteobacteria | × | × | ||
| 91-92 | 2 | 624 | Uncultured proteobacterium strain BD1-29 | AB015529 | ɛ-Proteobacteria | × | |||
| 94-99 | 3 | 557 | Alteromonas macleodii | AF025957 | γ-Proteobacteria | × | |||
| 94-95 | 2 | 462 | Oceanospirillum sp. strain ME113 | AJ302700 | γ-Proteobacteria | × | |||
| 92 | 2 | 499 | Neptunomonas naphthovorans | AF053734 | γ-Proteobacteria | × | |||
| 95 | 1 | 489 | Unclassified Pseudomonas group | AF102866 | γ-Proteobacteria | × | |||
| 99 | 1 | 565 | Vibrio campbelli ATCC 25920T | X74692 | γ-Proteobacteria | × | |||
| 82 | 1 | 547 | Spinacia oleracea | M21453 | Chloroplasts | × | |||
| 89 | 1 | 473 | Nephroselmis olivacea | X74754 | Chloroplasts | × | |||
| 93 | 1 | 577 | Spirochaeta litoralis | M88723 | Spirochaetales | × | |||
| 93 | 1 | 532 | Trichodesmium tenue | AF013029 | Cyanobacteria | × | |||
| 89 | 1 | 491 | Uncultured bacterium TIHP302-14 | AB031600 | Unknown | × | |||
| 94 | 2 | 525-567 | Desulfovibrio aespoeensis (isolate Aspo2) | X95230 | δ-Proteobacteria | × | × | ||
| 95 | 1 | 546 | North Sea bacterium H120 | AF069667 | Unknown | × | |||
| 88 | 1 | 626 | Unidentified bacterium strain BD2-14 | AB015542 | Unknown | × | |||
| 93 | 1 | 544 | Bacterium strain 77003 | AF227847 | Unknown | × | |||
| 90 | 1 | 356 | Marine snow bacterium Adriatic87 | AF030773 | Unknown | × | |||
| 94 | 1 | 568 | Uncultured bacterium PENDANT-24 | AF142936 | α-Proteobacteria | × | |||
| 99 | 1 | 338 | Escherichia coli K12 MG1655 | AE000345 | γ-Proteobacteria | × | |||
| 91 | 1 | 768 | Uncult. gamma proteobact. CHAB-IV-34 | AJ240917 | γ-Proteobacteria | × | |||
| 93 | 1 | 297 | Uncultured gamma proteobacterium DSS65 | AJ401386 | γ-Proteobacteria | × | |||
| 94 | 1 | 355 | Uncultured gamma proteobacterium KTc119 | AF235120 | γ-Proteobacteria | × | |||
| 94 | 4 | 612 | Chlorobium vibrioforme | Y08103 | Green Sulfur Bacteria | × | |||
| 96 | 1 | 622 | Prosthecochloris aestuarii | AJ291826 | Green Sulfur Bacteria | × | |||
| 96 | 1 | 505 | Uncultured bacillariophyte AWS98-19b | AF327029 | Chloroplasts | × | |||
| 91 | 1 | 614 | Epulopiscium sp. morphotype B | M99574 | Firmicutes | × | |||
| 97-98 | 2 | 576 | Uncultured marine eubacterium HstpL78 | AF159652 | Unknown | × | |||
Abbreviations are as defined in footnote a to Table 1.
× signs indicate whether the clone occurred in the overlying seawater (SW), healthy coral tissue (HC), black bound diseased coral tissue (BBD), or dead coral skeleton surfaces (DC).
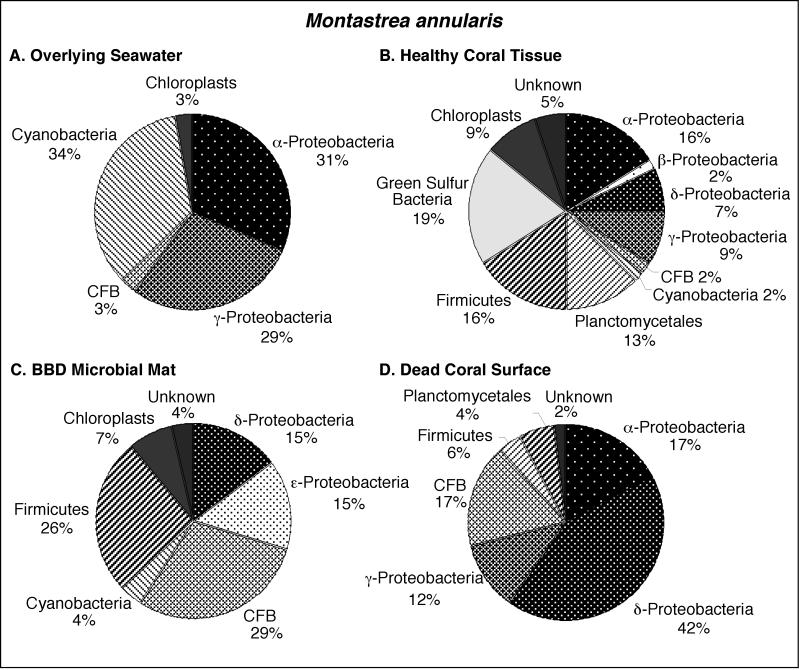
FIG. 3.
Pie diagrams illustrating the division-level diversity of the partial 16S rRNA bacterial sequences comprising the clone libraries associated with M. annularis. The colony was growing in the back reef environment at Playa Kalki at a 5-m water depth. The seawater at the time and location of sampling in August 2000 was at a temperature of 27.5°C.
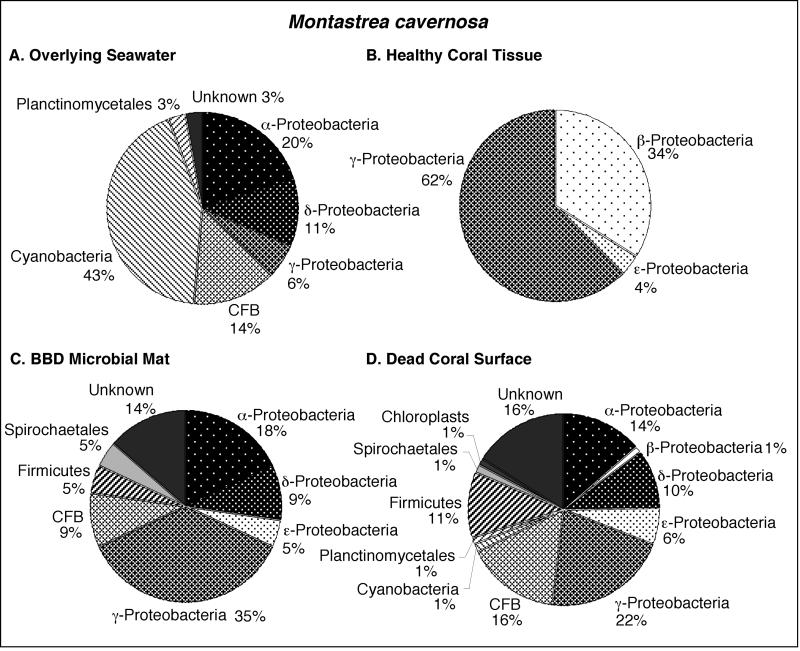
FIG. 4.
Pie diagrams illustrating the division-level diversity of the partial 16S rRNA bacterial sequences comprising the clone libraries associated with M. cavernosa. The colony was growing in the back reef environment at Playa Kalki at a 5-m water depth. The seawater at the time and location of sampling in August 2000 was at a temperature of 27.5°C.
FIG. 5.
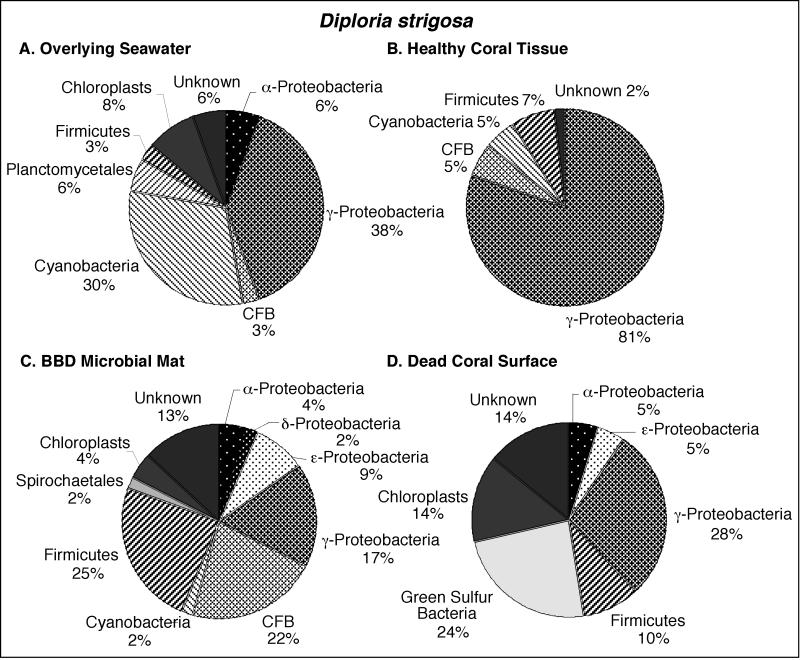
Pie diagrams illustrating the division-level diversity of the partial 16S rRNA bacterial sequences comprising the clone libraries associated with D. strigosa. The colony was growing in the back reef environment at Water Plant at a 4-m water depth. The seawater at the time and location of sampling in August 2000 was at a temperature of 27.5°C.
Microbial communities inhabiting overlying seawater.
Bacterioplankton clone libraries were constructed from samples of seawater collected at 10 cm immediately above the surfaces of BBD-infected M. annularis, M. cavernosa, and D. strigosa colonies (Fig. 3A, 4A, and 5A, respectively). The most abundant sequences in the seawater clone libraries at both locations represented cyanobacteria (30 to 43%). The next most abundant clones in the seawater libraries represented the γ-proteobacteria (6 to 38%) and α-proteobacteria (6 to 31%) divisions. Chloroplast sequences, which comprised 3 to 8% of the libraries, were likely derived from the rDNA of chlorophyll-containing organelles in planktonic algae and free-living zooxanthellae just above the coral colony surfaces (45). Only two sequences were common to all three seawater clone libraries: those which represented the genera Prochlorococcus and Synechococcus (Tables 1, 2, and 3).
Microbial communities inhabiting healthy coral tissue.
Clone libraries from healthy tissues indicated that each of the three coral species contained a significantly different assemblage of bacterial sequences (Fig. 3B, 4B, and 5B). A maximum of only 5% of the sequences were representative of cyanobacteria, in contrast to the dominance of cyanobacteria sequences in the overlying seawater clone libraries (Fig. 3A and B, 4A and B, and 5A and B). Chloroplasts in these libraries were most likely derived from zooxanthellae inhabiting the coral tissues. M. annularis sequences (Fig. 3B) exhibited significantly more microbial diversity than those of either M. cavernosa or D. strigosa. (Fig. 4B and 5B, respectively) and were dominated by green sulfur bacteria (19%), α-proteobacteria (16%), firmicutes (16%), and planctomycetales (13%). Healthy tissue libraries from M. cavernosa and D. strigosa contained sequences representing only three and five divisions, respectively, both of which were dominated by γ-proteobacteria (62 to 81%). None of the same bacterial sequences were detected in all three of the healthy coral species (Tables 1, 2, and 3).
Microbial communities inhabiting the BBD microbial mat.
Clone libraries constructed from the BBD microbial mat on M. annularis, M. cavernosa, and D. strigosa colonies consistently exhibit high division-level diversity (Fig. 3C, 4C, and 5C, respectively). Sequences were dominated by firmicutes (5 to 26%), Cytophaga-Flavobacterium-Bacteroides group (CFB) (9 to 29%), γ-proteobacteria (17 to 35%), and δ-proteobacteria (2 to 15%). The chloroplasts (4 to 7%) were most likely derived from coral zooxanthellae. These results indicate that the BBD microbial communities are completely distinct from those inhabiting healthy coral tissue, sharing no common 16S rRNA sequences on any individual coral colony (Tables 1, 2, and 3). These clone libraries also confirm that the BBD mats contain significantly more microbial diversity than that suggested by optical analyses (Fig. 2D). Sequences representing Desulfovibrio and the associated genus Desulfobotulus were detected in clone libraries from all three coral species (Tables 1, 2, and 3). Sequences associated with Trichodesmium and Clostridium occurred in BBD mat clone libraries from M. annularis and D. strigosa (Tables 1 and 3, respectively).
Microbial communities inhabiting dead coral surfaces.
Clone libraries from dead surfaces of the M. annularis, M. cavernosa, and D. strigosa colonies also contained a high diversity of sequences, representing 6 to 9 microbial divisions (Fig. 3D, 4D, and 5D, respectively). However, the relative proportions of sequences representing each division varied significantly between species. In general, clone libraries were dominated by δ-proteobacteria (0 to 42%), α-proteobacteria (5 to 17%), γ-proteobacteria (0 to 28%), and CFB (0 to 17%). Sequences detected on dead coral surfaces were 95% distinct from those detected in the overlying seawater, healthy coral tissues, and the BBD microbial mats.
DISCUSSION
The optical and molecular analyses completed in this study of corals infected with BBD on Curaçao indicate that bacterial communities are distinctly partitioned between overlying seawater and healthy, diseased, and dead coral surfaces. The following discussion evaluates the implications of this partitioning with respect to the proportion of cyanobacterium-related sequences in reef tract seawater clone libraries, the possibility of coral species-specific microbial communities, and the microbial ecology of BBD in M. annularis, M. cavernosa, and D. strigosa colonies.
Reef tract bacterioplankton.
Sequences affiliated with two globally distributed bacterioplankton were detected in all seawater clone libraries collected from the Curaçao reef tract. The most common was Prochlorococcus, which is the smallest yet most abundant oxygenic photoautotroph in tropical and subtropical seas (16, 44). The second cyanobacterium was Synechococcus, a genus which has been combined with that of Prochlorococcus into a single clade called the picophytoplankton (69). The phenomenon of the large proportion of cyanobacterium sequences detected in the Curaçao seawater clone library is somewhat unique with respect to bacterioplankton found in open ocean surface seawater. Sequences affiliated with cyanobacteria from the Mediterranean Sea comprised only 6 to 8% of the clone libraries from offshore surface waters (1, 2, 3). Similarly low proportions of cyanobacteria have also been detected in surface waters of the Pacific and North Atlantic oceans and the North Sea (12, 19, 20, 24, 41, 44, 46, 64, 65, 66).
The relatively large proportion of sequences affiliated with cyanobacteria in Curaçao seawater relative to those reported in rDNA surveys from other locations may reflect the ecological differences between near-shore and offshore marine environments. The near-shore reef tract setting on Curaçao is strongly influenced by the physical and chemical effects of terrestrial runoff, which include coastal pollution and organic as well as inorganic sedimentation (37). It is therefore not unexpected that the composition of bacterioplankton communities in near-shore and offshore environments would track these environmental differences (66). Another possibility is that this discrepancy in the proportions of cyanobacteria may result from methodological differences. The bead beating used in the present study may have more effectively lysed the tough cyanobacterial cell walls than the techniques used for the offshore studies, none of which applied bead beating. Furthermore, the consistently high proportion of cyanobacteria in all three of the seawater samples analyzed from Curaçao (Fig. 3A, 4A, and 5A) suggests that this is an accurate representation of the reef tract bacterioplankton clone library.
Healthy coral microbial communities.
Sequencing results in the present study indicate that the microbial communities inhabiting healthy coral tissue have the following characteristics: (i) they are unique in that they are distinct from the bacterioplankton in overlying seawater, especially with respect to cyanobacteria sequences; and (ii) they are markedly different among the three coral species. Significant reductions in the proportion of cyanobacterium-affiliated sequences from the seawater clone libraries (30 to 43%) to those from the healthy coral tissue clone libraries (0 to 5%) (Fig. 3A and B, 4A and B, and 5A and B, respectively) are likely to be the result of chemical defense mechanisms that inhibit coral tissue colonization by cyanobacteria (35). This partitioning is also consistent with the results of previous optical and culture-based studies of the mucus-rich biofilm covering coral tissue, which is called the coral surface microlayer (CSM) (13, 14, 38, and 45). Expelled and free-living zooxanthellae are prominent components of the CSM, which protects coral from light, exposure, and sedimentation and is the first line of defense against disease infection (11, 38, 45, 56).
Inferred metabolisms from the M. annularis clone library sequences suggest that mixtures of aerobic and anaerobic microbes inhabit the healthy coral tissue (Tables 1, 2, and 3). This type of mixed metabolic assemblage is similar to those of the diverse microbial communities detected in the tissues of marine sponges, where low-oxygen microniches occur in the pores of well-oxygenated sponge surfaces (73). In addition, several other bacteria inhabiting healthy M. annularis tissue have not previously been observed in marine environments. Specifically, sequences of several microbial strains, which comprise 3% of the M. annularis healthy tissue clone library, were previously isolated exclusively from terrestrial soils (e.g., agricultural soil bacterium SC-I-iS and unidentified soil bacterium clones BSV72 and BSV73; Tables 1, 2, and 3).
Sequences from clone libraries constructed from healthy M. annularis tissue exhibit significantly higher bacterial diversity (10 divisions) than those from clone libraries constructed from healthy M. cavernosa and D. strigosa tissue (3 and 4 divisions, respectively; Fig. 3B, 4B, and 5B, respectively). These preliminary results imply that scleractinian corals may contain species-specific microbial communities. The abundance of γ-proteobacteria and β-proteobacteria and the low overall sequence diversity levels observed in M. cavernosa and D. strigosa (Fig. 4B and 5B) are somewhat similar to those of the microbial communities detected with molecular screening of healthy tissues from a single Montastrea franksi colony in Panama (4 divisions) (51). Sequences affiliated with Silicibacter lacuscaerulensis were also detected in several other healthy M. franksi colonies by using denaturing gradient gel electrophoresis techniques (51). However, S. lacuscaerulensis sequences were only detected in the present study on Curaçao in the dead coral surface clone library of M. cavernosa (Table 2). This further supports the possibility that individual coral species contain unique microbial consortia. An important difference from the Curaçao corals is that M. franksi healthy tissue clone libraries from Panama were dominated by cyanobacteria (51), which is surprising given the chemical mechanisms inhibiting cyanobacterial settlement on healthy coral tissues (35). In addition, M. franski did not contain chloroplast-affiliated sequences in the healthy tissue clone libraries (51), which was unexpected given the abundance of zooxanthellae in the coral tissue and the CSM (45) and their use of primers similar to those applied in the present study.
Microbes associated with BBD.
The BBD microbial mat is dominated by large filamentous cyanobacteria originally identified as Oscillatoria submembranaceae (A. Antonius, Abstr. 10th Meet. Assoc. Isl. Mar. Lab. Caribb., p. 3, 1973 [abstr.]) andlater systematically reclassified as Phormidium corallyticum (52). Other bacteria previously identified in the BBD mat include motile, nonphotosynthetic sulfide-oxidizing Beggiatoa spp. (optically identified) (14, 18, and 52) and the sulfate-reducing Desulfovibrio spp. (identified optically and with 16S rRNA oligonucleotide probes) (18 and 53 and S. Schnell, B. Assmus, and L. L. Richardson, abstract from the Annual Meeting of the VAAM [Vereinigung fuer Allgemeine und Angewandte Mikrobiologie] and GBCH [Gesellschaft fuer Biologische Chemie], Biospecktrum, p. 116, 1996 [abstr.]). Although SEM imaging for the present report indicated that the BBD microbial mat was predominantly composed of filamentous cyanobacteria (Fig. 2G and H), cyanobacterial sequences represented only 0 to 4% of the BBD mat clone libraries (Table 1). Furthermore, neither P. corallyticum or Beggiatoa sp. sequences were detected in the BBD mat (Tables 1, 2, and 3).
One explanation for these discrepancies may be that P. corallyticum has not been previously sequenced. While several species of terrestrial Phormidium have been detected in desert soil crusts (17), no marine species have previously been reported in GenBank. Similarly, multiple Beggiatoa species have been sequenced from other marine environments (3) but their sequences were not similar to those in the BBD mat clone library. Another explanation could be that P. corallyticum and Beggiatoa spp. were represented by one of the unknown bacterial sequences in the BBD mat clone library (Fig. 3, 4, and 5). Other possibilities are that they are present in the BBD mat but their rDNA was not extracted, amplified, and sequenced and they are not present in the BBD mat. It is important to note that sequences affiliated with the filamentous cyanobacterium Trichodesmium tenue were found in the BBD mats (Tables 1, 2, and 3). Members of the family Trichodesmium (formerly Oscillatoria) are nonheterocystous nitrogen-fixing (diazotrophic) cyanobacteria that are a globally abundant component of bacterioplankton in tropical and subtropical oceans (9, 34).
Implications for the microbial ecology of BBD.
Several of the microbial sequences detected in the seawater and healthy, BBD-infected, and dead coral surface clone libraries (Tables 1, 2, and 3) are affiliated with microbes with one or more of the following characteristics: (i) they have previously been reported in humans, some commonly occurring in human sewage; (ii) they are known pathogens in other marine and terrestrial organisms; or (iii) they were derived from terrestrial soils. The following discussion summarizes what is known of the ecologies and pathogenicities of the microorganisms whose affiliated sequences have been found in the BBD mat.
Several of the sequences detected on the coral surfaces are affiliated with microbes that are known pathogens in humans. Sequences affiliated with the genus Clostridium, which occur in the BBD mat clone library of M. annularis (Table 1), are commonly part of mixed-pathogen infections in a variety of terrestrial organisms, including humans and birds (61, 62). The presence of clostridial spores is commonly used as an indication of fecal contamination in drinking water. Pathogenic clostridia, which are gram-positive obligatory anaerobes incapable of dissimilatory reduction of sulfate, produce toxins that necrotize body tissues or interfere with nerve transmission (31, 60). Sequences affiliated with the genera Campylobacter and Arcobacter were also detected in the BBD and dead coral surface clone libraries (Tables 1, 2, and 3). These ɛ-proteobacteria have been grouped into the bacterial family Campylobacteraceae, a diverse group of gram-negative commensal and pathogenic bacteria that colonize the mucosal surfaces of the intestinal tracts, oral cavities, or urogenital tracts in humans and other animal hosts (68, 70). Arcobacter spp. are known enteropathogens that cause abdominal cramps in children (71, 72). Campylobacter spp. are a major cause of bacterial enteritis in humans and are commonly found in polluted seawater contaminated with sewage (28, 29). The absence of these bacteria on the dead coral surfaces suggests that none of the BBD bacteria remain behind as the BBD mat migrates. Thus the mat itself could be a requirement for the survival of these bacteria. Therefore, the BBD bacterial community has specificity for the living and dying coral tissues but not for the dead skeletal surface of the coral. One possible source for these bacteria could be human sewage.
It is also possible that BBD has been transmitted by infected fish that bite, eat, defecate upon, or otherwise come in contact with healthy corals. Cytophaga fermentans, detected in the BBD mats of M. annularis and D. strigosa, is thought to be responsible for a number of illnesses in freshwater and saltwater fish, including eroded mouth, columnaris, cotton wool, cold water, hemorrhagic gill, saddle back, and branchionephritis diseases and ulcers, tail and fin rot, and black patch necrosis (5, 7, 30). Although the precise etiology of each of these diseases is not well understood, environmental factors correlated with the onset of infection include extremely high or low temperatures, low oxygen levels, and reduced availability of dissolved Fe3+ (8, 40). Another possibility is that T. tenue may have carried out the pathogenic functions previously ascribed to P. corallyticum (49, 53, 67). T. tenue produces intracellular biotoxins that have proven to be toxic to zooplankton and other higher animals, such as the copepod Acartia tonsa (23, 26, 58). If present in the BBD mat, T. tenue would primarily utilize nitrogenase linked to O2-depleted microzones within aggregated filaments to carry out highly efficient nitrogen fixation during oxygenic photosynthesis (42, 43). These nutrient and oxygen regimes are similar to those of the microenvironments in the BBD mat (10, 49).
An additional possibility raised by the results in this report is that bacteria derived from terrestrial soils may have played a role in the development of BBD infections on the Curaçao reef tract. This conjecture is based on the detection of a large number of sequences affiliated with terrestrial soil bacteria on the healthy, BBD-infected, and dead coral surfaces (Tables 1, 2, and 3). It is possible that these soil bacteria were attached to soil-derived particles that were transported via terrestrial runoff onto the reef tract, settled onto coral colonies, and inoculated healthy coral tissues. However, it is unknown if these bacteria were alive or dead at the time that they were collected. The feasibility of this mechanism is exemplified by the results of studies of the terrestrial fungus Aspergillus sydowii, an organism that was derived from terrestrial runoff, which has been identified as a pathogen in marine sea fans (59; G. W. Smith, L. D. Ives, I. A. Nagelkerken, and K. B. Ritchie, Letter, Nature 383:487, 1996). It has been further hypothesized that A. sydowii can be attached to dust particles and can thus cause coral disease, as aerosols settle on the sea surface and become suspended sediment (57).
In summary, the culture-independent molecular methods applied in this study indicate that microbiota are distinctly partitioned between seawater and the surface of BBD-infected corals. Sequences affiliated with known pathogens in humans and other organisms detected in the BBD mat suggest that human sewage, infection from other marine organisms, and terrestrial runoff may all have contributed to the development of the disease. Further advances in understanding BBD etiology will require tracking variations in these microbial communities, as the host corals and zooxanthellae respond to environmental changes in seawater temperature and pollution chemistry.
Acknowledgments
This work was supported by research grants from the Office of Naval Research (N00014-00-1-0609), the Petroleum Research Fund of the American Chemical Society (34549-G2), and the Geological Society of America (7058-01).
Ongoing discussions with A. Salyers and C. Woese throughout the project significantly added to all aspects of the data collection and interpretation. Reviews by A. Salyers and A. Murray significantly improved the manuscript. S. Schaffers and M. Fortwengler are thanked for taking the underwater photographs.
REFERENCES
- 1.Acinas, S. G., F. Rodriguez-Valera, and C. Pedros-Alio. 1997. Spatial and temporal variation in marine bacterioplankton diversity as shown by RFLP fingerprinting of PCR amplified 16S rRNA. FEMS Microb. Ecol. 24:27-40. [Google Scholar]
- 2.Acinas, S. G., J. Anton, and F. Rodriguez-Valera. 1999. Diversity of free-living and attached bacteria in offshore western Mediterranean waters as depicted by analysis of genes encoding 16S rRNA. Appl. Environ. Microbiol. 65:514-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad, A., J. P. Barry, and D. C. Nelson. 1999. Phylogenetic affinity of a wide, vacuolate, nitrate-accumulating Beggiatoa sp. from Monterey Canyon, California, with Thioploca spp. Appl. Environ. Microbiol. 65:270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul, S. F., W. Gish, W. Miller, E. W. Meyers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 59:143-169. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, J. I. W., and D. A. Conroy. 1969. The pathogenic myxobacteria with special reference to fish diseases. J. Appl. Bacteriol. 32:30-39. [DOI] [PubMed] [Google Scholar]
- 6.Antonius, A. 1981. The “band” diseases in coral reefs, p. 7-14. Fourth International Coral Reef Symposium, vol. 2, Manila, Philippines.
- 7.Becker, C. D., and M. P. Fujihara. 1978. The bacterial pathogen Flexibacter columnaris and its epizootiology among Columbia River fish. Monograph No. 2. American Fishery Society, Washington, D.C.
- 8.Campbell, A. C., and J. A. Buswell. 1982. An investigation into the bacterial etiology of “black patch necrosis” in Dover sole, Solea solea. J. Fish Dis. 5:495-508. [Google Scholar]
- 9.Capone, D. G., J. P. Zehr, H. W. Paerl, B. Bergman, and E. J. Carpenter. 1997. Trichodesmium, a globally significant marine cyanobacterium. Science 276:1221-1229. [Google Scholar]
- 10.Carlton, R. G., and L. L. Richardson. 1995. Oxygen and sulfide dynamics in a horizontally migrating cyanobacterial mat: black band disease of corals. FEMS Microbiol. Ecol. 18:155-162. [Google Scholar]
- 11.Coffroth, M. A. 1990. Mucous sheet formation on poritid corals: an evaluation of coral mucous as an energy source on reefs. Mar. Biol. 105:39-49. [Google Scholar]
- 12.Cottrell, M. T., and D. L. Kirchman. 2000. Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl. Environ. Microbiol. 66:1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducklow, H. W., and D. Mitchell. 1979. Composition of mucus released by coral coelenterates. Limnol. Oceanogr. 24:706-714. [Google Scholar]
- 14.Ducklow, H. W., and R. Mitchell. 1979. Bacterial populations and adaptations in the mucus layers on living corals. Limnol. Oceanogr. 24:715-725. [Google Scholar]
- 15.Edmunds, P. J. 1991. Extent and effect of black band disease on Caribbean reefs. Coral Reefs 10:161-165. [Google Scholar]
- 16.Fuhrman, J. A., and L. Campbell. 1998. Marine ecology: microbial diversity. Nature 393:410-411. [Google Scholar]
- 17.Garcia-Pichel, F., and O. Pringault. 2001. Cyanobacteria track water in desert soils. Nature 413:380-381. [DOI] [PubMed] [Google Scholar]
- 18.Garrett, P., and P. Ducklow. 1975. Coral disease in Bermuda. Nature 253:349-350. [Google Scholar]
- 19.Giovannoni, S., and M. S. Rappé. 2000. Evolution, diversity, and molecular ecology of marine prokaryotes. p. 47-84. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, Inc., New York, N.Y.
- 20.Glöckner, F. O., B. M. Fuchs, and R. Amann. 1999. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl. Environ. Microbiol. 65:3721-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goebel, B. M., and E. Stackebrandt. 1994. Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Appl. Environ. Microbiol. 60:1614-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goreau, T. J., J. Cervino, M. Goreau, R. Hayes, M. Hayes, L. Richardson, G. Smith, K. DeMeyer, I. Nagelkerken, F. J. Garzon, D. Gil, G. Garrison, E. H. Williams, W. L. Bunkley, C. Quirolo, K. Patterson, J. W. Porter, and K. Porter. 1998. Rapid spread of diseases in Caribbean coral reefs. Rev. Biol. Trop. 46:157-171. [Google Scholar]
- 23.Guo, C., and P. A. Tester. 1994. Toxic effect of the bloom-forming Trichodesmium sp. (Cyanophyta) to the copepod Acartia tonsa. Nat. Tox. 2:222-227. [DOI] [PubMed] [Google Scholar]
- 24.Hagström, A., J. Pinhassi, and U. L. Zweifel. 2000. Biogeographical diversity among marine bacterioplankton. Aquat. Microb. Ecol. 21: 231-244. [Google Scholar]
- 25.Harvell, C. D., K. Kim, J. M. Burkholder, R. R. Colwell, P. R. Epstein, D. J. Grimes, E. E. Hofmann, E. K. Lipp, A. D. M. E. Osterhause, R. M. Overstreet, J. W. Porter, G. W. Smith, and G. R. Vasta. 1999. Emerging marine diseases—climate links and anthropomorphic factors. Science 285:1505-1510. [DOI] [PubMed] [Google Scholar]
- 26.Hawser, S. P., J. M. O'Neil, M. R. Roman, and G. A. Codd. 1992. Toxicity of blooms of the cyanobacterium Trichodesmium to zooplankton. J. Appl. Phycol. 4:79-86. [Google Scholar]
- 27.Hayes, R. L., and N. I. Goreau. 1998. The significance of emerging diseases in the tropical coral reef ecosystem. Rev. Biol. Trop. 46:173-185. [Google Scholar]
- 28.Healing, T. D., M. H. Greenwood, and A. D. Pearson. 1992. Campylobacters and enteritis. Rev. Med. Microbiol. 3:159-167. [Google Scholar]
- 29.Hernandez, J., A. Fayos, J. L. Alonso, and R. J. Rowen. 1996. Ribotypes and AP-PCR fingerprints of thermophilic campylobacters from marine recreational waters. J. Appl. Bacteriol. 80:157-164. [DOI] [PubMed] [Google Scholar]
- 30.Hikida, M., H. Wakabayashi, S. Egusa, and S. Masumura. 1979. Flexibacter sp., a gliding bacterium pathogenic to some marine fishes in Japan. Bull. Jpn. Soc. Sci. Fish. 45:421-428. [Google Scholar]
- 31.Hippe, H., J. R. Andreesen, and G. Gottschalk. 2001. The genus Clostridium. In The Prokaryotes. Springer-Verlag, New York, N.Y.
- 32.Huggenholtz, P., C. Pitulle, K. L. Hershberger, and N. R. Pace. 1998. Novel division level bacterial diversity in a Yellowstone hot spring. J. Bacteriol. 180:366-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James, N. P., and P. A. Bourque. 1992. Reefs and Mounds, p. 323-348. In R. G. Walker and N. P. James (ed.), Facies models: response to sea level change. Geological Association of Canada, St. John's, Canada.
- 34.Janson, S., B. Bergman, E. J. Carpenter, S. J. Giovannoni, and K. Vergin. 1999. Genetic analysis of natural populations of the marine diazotrophic cyanobacterium Trichodesmium. FEMS Microbiol. Ecol. 30:57-65. [Google Scholar]
- 35.Koh, E. G. L. 1997. Do scleractinian corals engage in chemical warfare against microbes? J. Chem. Ecol. 23:379-398. [Google Scholar]
- 36.Kuta, K. G., and L. L. Richardson. 1997. Black band disease and the fate of diseased coral colonies in the Florida Keys, p. 601-606. Eighth International Coral Reef Symposium, vol. 1, Balboa, Republic of Panama.
- 37.Laws, E. A. 2000. Aquatic pollution. John Wiley and Sons, New York, N.Y.
- 38.Lyons, M. M., P. Aas, J. D. Pakulski, L. Van Waasbergen, R. V. Miller, D. L. Mitchell, and W. H. Jeffrey. 1998. DNA damage induced by ultraviolet radiation in coral-reef microbial communities. Mar. Biol. 130:537-543. [Google Scholar]
- 39.Maidik, B. L., G. J. Olsen, N. Larsen, R. Overbeek, M. J. McCaughey, and C. R. Woese. 1997. The RDP (Ribosomal Database Project). Nucleic Acids Res. 25:109-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McVicar, A. H., and P. G. White. 1982. The prevention and cure of an infectious disease in cultivated juvenile Dover sole, Solea solea. Aquaculture 26:213-222. [Google Scholar]
- 41.Olson, R. J., S. W. Chisholm, E. R. Zettler, and E. V. Armburst. 1990. Pigments, size and distribution of Synechococcus in the North Atlantic and Pacific Oceans. Limnol. Oceanogr. 35:45-58. [Google Scholar]
- 42.Paerl, H. W., B. M. Bebout, and L. E. Prufert. 1989. Bacterial associations with marine Oscillatoria sp. (Trichodesmium sp.) populations: ecophysiological implications. J. Phycol. 25:773-784. [Google Scholar]
- 43.Paerl, H. W., B. M. Bebout, and L. E. Prufert. 1989. Naturally occurring patterns of oxygenic photosynthesis and N2 fixation in a marine microbial mat, p. 326-341. In Y. Cohen and E. Rosenberg (ed.), Microbial mats: physiological ecology of benthic microbial communities. American Society for Microbiology, Washington, D.C.
- 44.Partensky, F., W. R. Hess, and D. Vaulot. 1999. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol. Mol. Biol. Rev. 63:106-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul, J. H., M. F. DePlaun, and W. H. Jeffrey. 1986. Elevated levels of microbial activity in the coral surface microlayer. Mar. Ecol. Prog. Ser. 33:29-40. [Google Scholar]
- 46.Rappé, M. S., K. Vergin, and S. Giovannoni. 2000. Phylogenetic comparisons of a coastal bacterioplankton community with its counterparts in open ocean and freshwater systems. FEMS Microbiol. Ecol. 33:219-232. [DOI] [PubMed] [Google Scholar]
- 47.Richardson, L. L. 1996. Horizontal and vertical migration patterns of Phormidium corallyticum and Beggiatoa spp. associated with black-band disease of corals. Microb. Ecol. 32:323-335. [DOI] [PubMed] [Google Scholar]
- 48.Richardson, L. L. 1997. Occurrence of the black band disease cyanobacterium on healthy corals of the Florida Keys. Bull. Mar. Sci. 61:485-490. [Google Scholar]
- 49.Richardson, L. L., K. G. Kuta, S. Schnell, and R. G. Carlton. 1997. Ecology of the black band disease microbial consortium, p. 597-600. Eighth International Coral Reef Symposium, vol. 1, Balboa, Panama.
- 50.Richardson, L. L. 1998. Coral disease: what is really known? Trends Ecol. Evol. 13:438-443. [DOI] [PubMed] [Google Scholar]
- 51.Rohwer, F., M. Breitbart, J. Jara, F. Azam, and N. Knowlton. 2001. Diversity of bacteria associated with the Caribbean coral Montastrea franksi. Coral Reefs 20:85-91. [Google Scholar]
- 52.Rützler, K., and D. L. Santavy. 1983. The black band disease of Atlantic reef corals. I. Description of the cyanophyte pathogen. Mar. Ecol. 4:301-319. [Google Scholar]
- 53.Rützler, K., D. L. Santavy, and A. Antonius. 1983. The black band disease of Atlantic reef corals. III. Distribution, ecology, and development. Mar. Ecol. 4:329-358. [Google Scholar]
- 54.Salyers, A. A., and D. D. Whitt. 1994. Regulation of virulence genes. In A. A. Salyers and D. D. Whitt (ed.), Bacterial pathogenesis: a molecular approach. ASM Press, Washington, D.C.
- 55.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 56.Santavy, D. L., and E. C. Peters. 1997. Microbial pests: coral disease in the Western Atlantic, p. 607-612. Eighth International Coral Reef Symposium, vol. 1, Balboa, Panama.
- 57.Shinn, E. A., G. W. Smith, J. M. Prospero, P. Betzer, M. L. Hayes, V. Garrison, and R. T. Barber. 2000. African dust and the demise of Caribbean coral reefs. Geophys. Res. Lett. 27:3029-3032. [Google Scholar]
- 58.Skulberg, O. M., B. Underdal, and H. Utkilen. 1994. Toxic waterblooms with cyanophytes in Norway—current knowledge. Archiv Hydrobiol. Suppl. 105:279-289. [Google Scholar]
- 59.Smith, G. W. 1998. Response of sea fans to Aspergillus sp. Rev. Biol. Mar. 46:205-208. [Google Scholar]
- 60.Smith, L. D. S. 2001. The genus Clostridium—medical. In The Prokaryotes. Springer-Verlag, New York, N.Y.
- 61.Smith, L. D. S., and B. L. Williams. 1984. The pathogenic anaerobic bacteria. Charles C Thomas Publishers, Springfield, Mass.
- 62.Sterne, M., and I. Batty. 1975. Pathogenic clostridia. Butterworths, London, England.
- 63.Studier, W. F., and J. J. Dunn. 1983. Complete nucleotide sequence of bacteriophage T7 DNA and the location of T7 genetic elements. J. Mol. Biol. 166:477-535. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki, M., M. S. Rappé, Z. W. Haimberger, H. Winfield, N. Adair, J. Strobel, and S. Giovannoni. 1997. Bacterial diversity among small-subunit rRNA gene clones and cellular isolates from the same seawater sample. Appl. Environ. Microbiol. 63:983-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki, M., M. S. Rappé, and S. Giovannoni. 1998. Kinetic bias in estimates of coastal picroplankton community structure obtained by measurements of small-subunit rRNA gene PCR amplicon length heterogeneity. Appl. Environ. Microbiol. 64:4522-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki, M. T., O. Beja, L. T. Taylor, and E. F. Delong. 2001. Phylogenetic analysis of ribosomal RNA operons from uncultivated coastal marine bacterioplankton. Environ. Microbiol. 3:323-331. [DOI] [PubMed] [Google Scholar]
- 67.Taylor, D. L. 1983. The black band disease of Atlantic reef corals. II. Isolation, cultivation, and growth of Phormidium corallyticum. Mar. Ecol. 4:320-328. [Google Scholar]
- 68.Trust, T. J., S. M. Logan, C. E. Gustafson, P. J. Romanuik, N. W. Kim, V. L. Chan, M. A. Ragan, P. Guerry, and R. R. Gutell. 1994. Phylogenetic and molecular characterization of a 23S rRNA gene positions the genus Campylobacter in the epsilon subdivision of the Proteobacteria and shows that the presence of transcribed spacers is common in Campylobacter spp. J. Bacteriol. 176:4597-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Urbach, E., D. J. Scanlan, D. L. Distel, J. B. Waterbury, and S. W. Chisholm. 1998. Rapid diversification of marine picophytoplankton with dissimilar light harvesting structures inferred from sequences of Prochlorococcus and Synechococcus. J. Mol. Evol. 46:188-201. [DOI] [PubMed] [Google Scholar]
- 70.VanDamme, P., and J. De Ley. 1991. Proposal for a new family, Campylobacteraceae. Int. J. Syst. Bacteriol. 41:451-455. [Google Scholar]
- 71.Vandamme, P., P. Pugina, G. Benzi, R. Van Etterijck, L. Vlaes, K. Kersters, J. P. Butzler, H. Lior, and S. Lauwers. 1992. Outbreak of recurrent abdominal cramps associated with Arcobacter butzleri in an Italian school. J. Clin. Microbiol. 30:2335-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wassenaar, T. M., and D. G. Newell. 2001. The genus Campylobacter. In The Prokaryotes. Springer-Verlag, New York, N.Y.
- 73.Webster, N. S., K. J. Wilson, L. L. Blackall, and R. T. Hill. 2001. Phylogenetic diversity of bacteria associated with the marine sponge Rhopaloeides odorabile. Appl. Environ. Microbiol. 67:434-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams, E. H., and L. Bunkley-Williams. 1990. The world-wide coral reef bleaching cycle and related sources of coral mortality. Atoll Res. Bull. 335:1-71. [Google Scholar]