Abstract
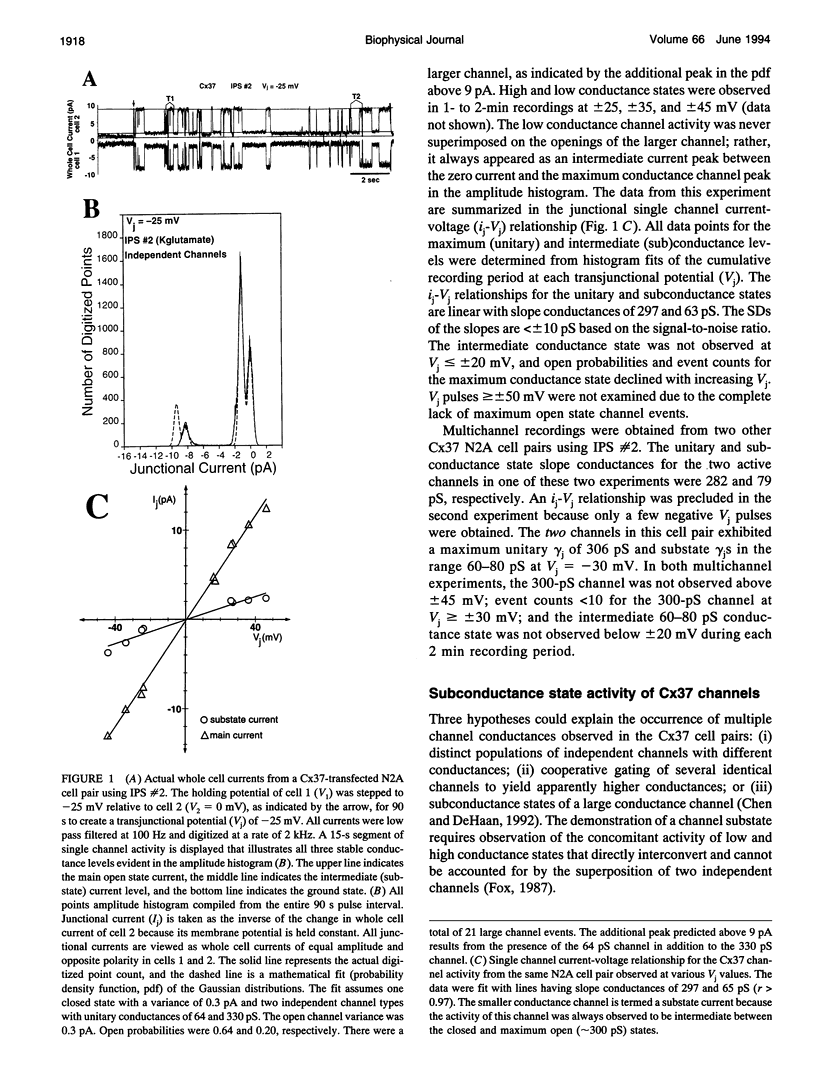
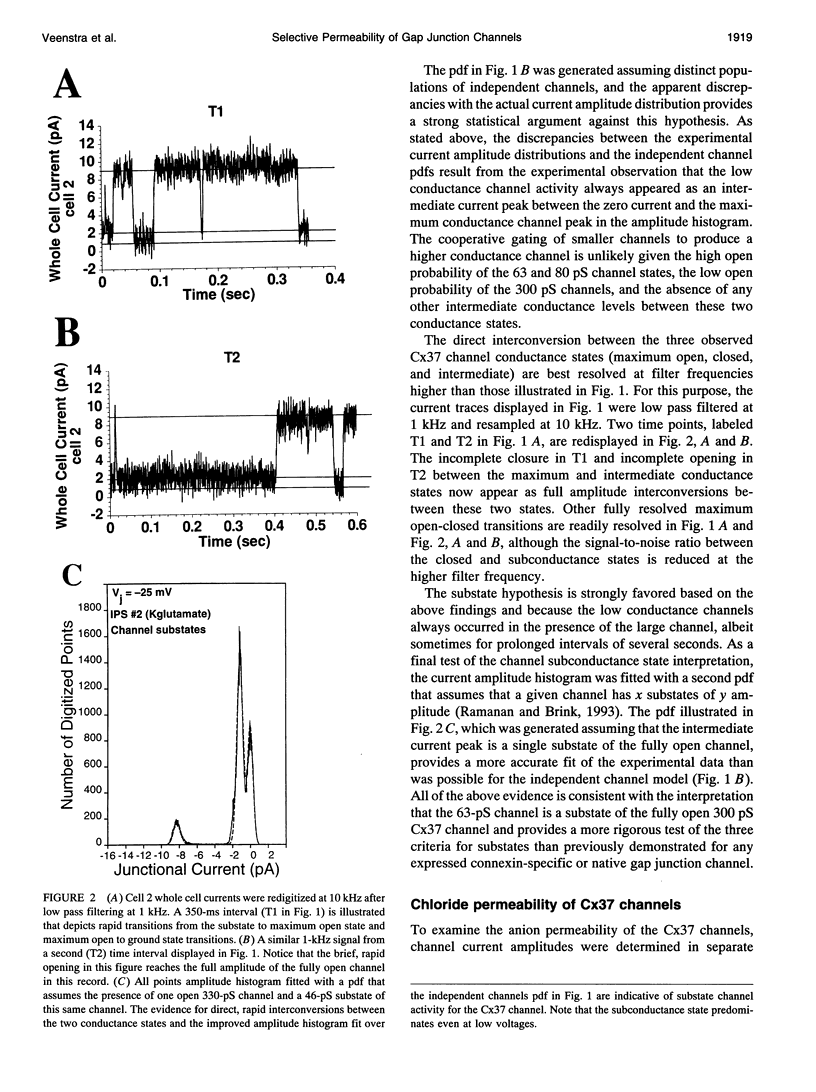
Gap junctions are thought to mediate the direct intercellular coupling of adjacent cells by the open-closed gating of an aqueous pore permeable to ions and molecules of up to 1 kDa or 10-14 A in diameter. We symmetrically altered the ionic composition or asymmetrically added 6-carboxyfluorescein (6-CF, M(r) = 376), a fluorescent tracer, to pairs of connexin37-transfected mouse neuro2A cells to examine the ionic and dye permeability of human connexin37 channels. We demonstrate that the 300-pS channel formed by connexin37 has an effective relative anion/cation permeability ratio of 0.43, directly converts to at least one intermediate (63 pS) subconductance state, and that 6-CF dye transfer is accompanied by a 24% decrease in unitary channel conductance. These observations favor a new interpretation of the gap junction pore consistent with direct ion-channel interactions or electrostatic charge effects common to more conventional multistate ion channels. These results have distinct implications about the different forms of intercellular signaling (cationic, ionic, and/or biochemical) that can occur depending on the expression and conformation of the connexin channel proteins.
Full text
PDF


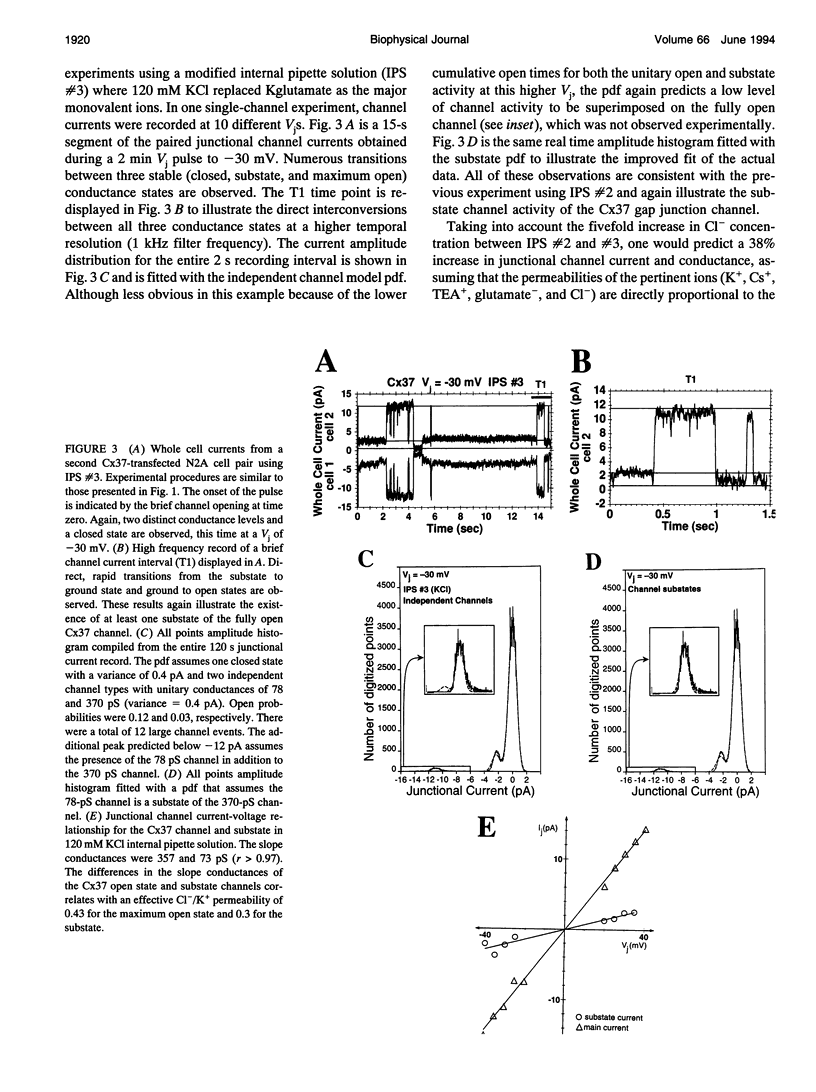
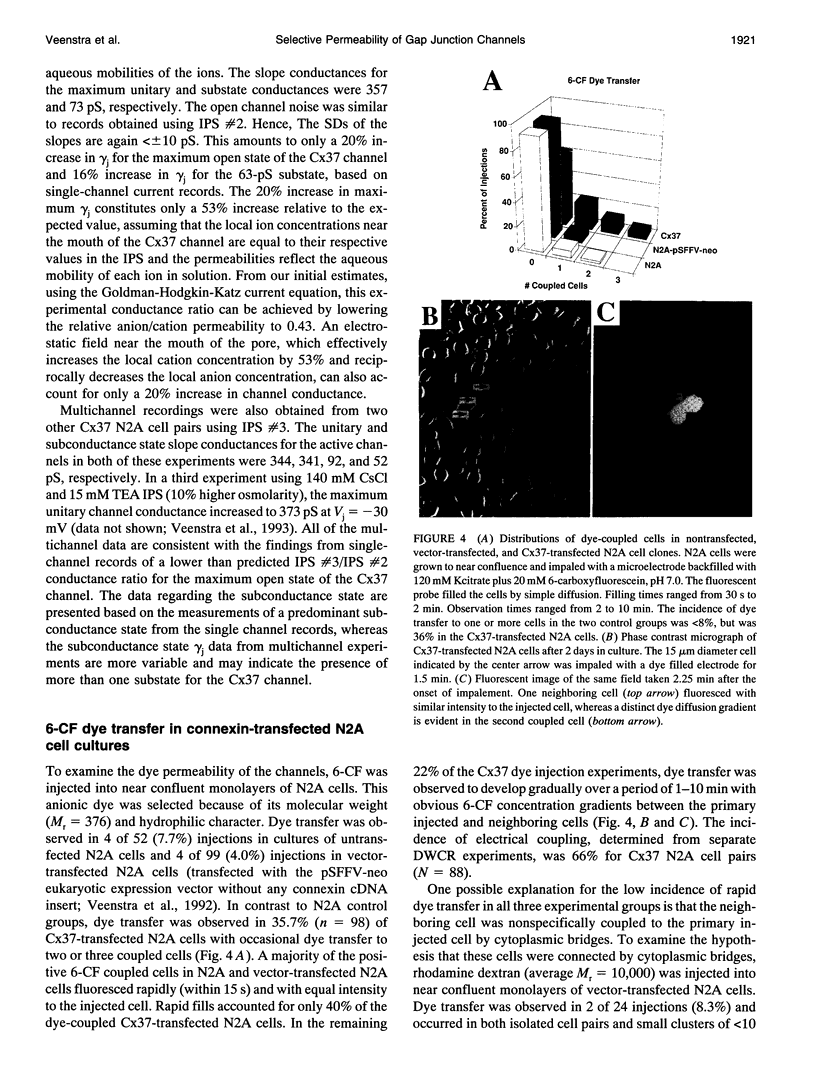
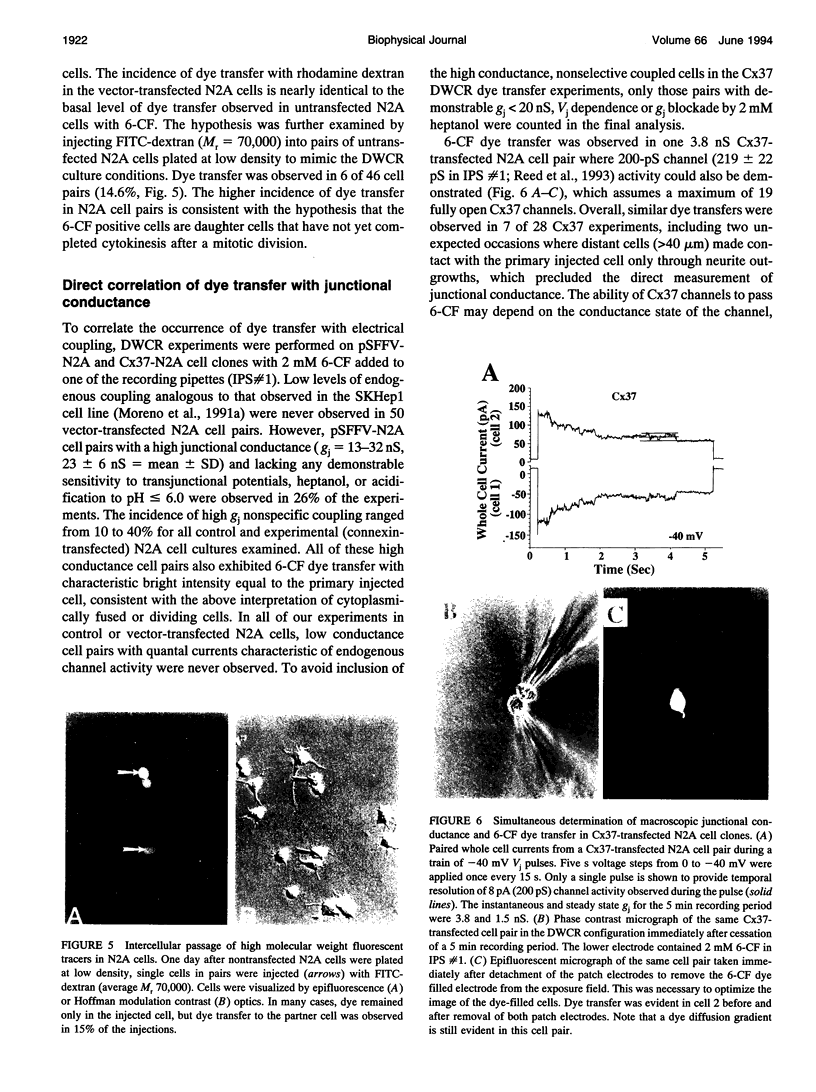

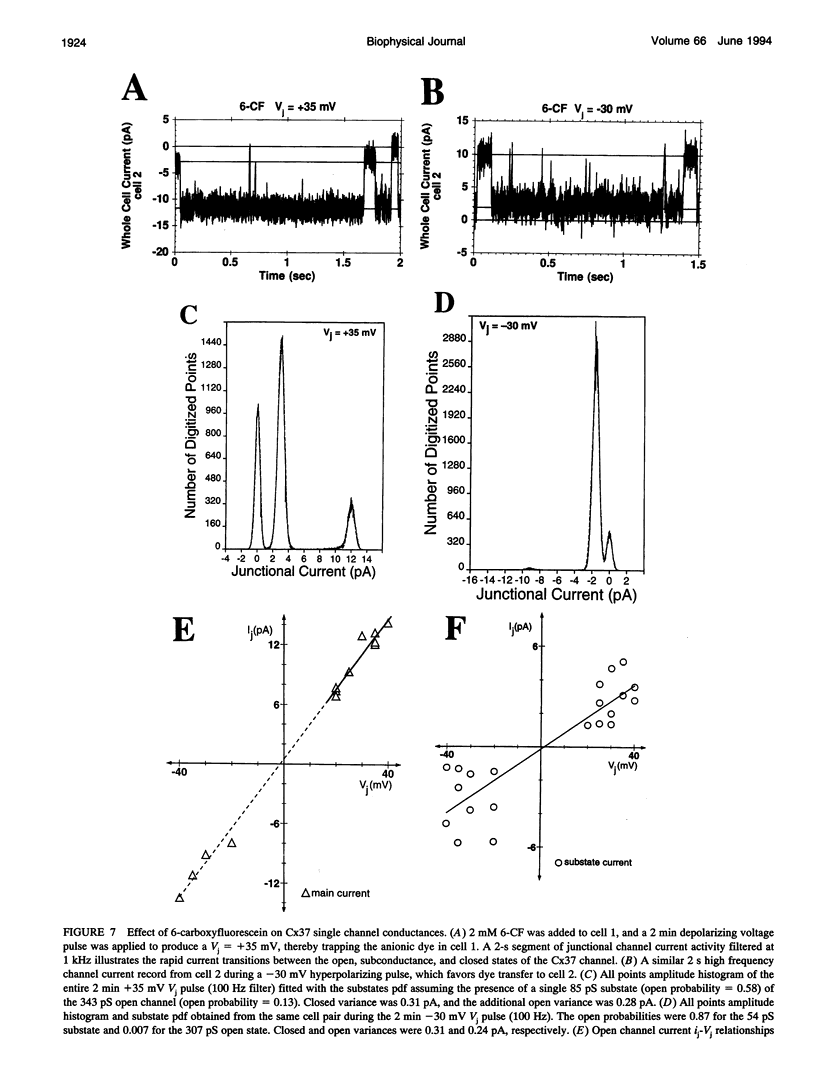




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. C. Molecular cloning and developmental expression of two chick embryo gap junction proteins. J Biol Chem. 1990 Aug 25;265(24):14439–14443. [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin family of gap junction proteins. J Membr Biol. 1990 Jul;116(3):187–194. doi: 10.1007/BF01868459. [DOI] [PubMed] [Google Scholar]
- Brink P. R., Dewey M. M. Evidence for fixed charge in the nexus. Nature. 1980 May 8;285(5760):101–102. doi: 10.1038/285101a0. [DOI] [PubMed] [Google Scholar]
- Brink P. R., Fan S. F. Patch clamp recordings from membranes which contain gap junction channels. Biophys J. 1989 Sep;56(3):579–593. doi: 10.1016/S0006-3495(89)82705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas F., Kempf C., Weingart R. Electrical coupling between cells of the insect Aedes albopictus. J Physiol. 1992 Mar;448:321–337. doi: 10.1113/jphysiol.1992.sp019044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., DeHaan R. L. Multiple-channel conductance states and voltage regulation of embryonic chick cardiac gap junctions. J Membr Biol. 1992 Apr;127(2):95–111. doi: 10.1007/BF00233282. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., DeHaan R. L. Temperature dependence of embryonic cardiac gap junction conductance and channel kinetics. J Membr Biol. 1993 Nov;136(2):125–134. doi: 10.1007/BF02505757. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Takeda K., Brehm P. Activation of a calcium-dependent photoprotein by chemical signalling through gap junctions. Nature. 1987 Jan 1;325(6099):60–62. doi: 10.1038/325060a0. [DOI] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Beyer E. C., Swenson K. I., Paul D. L., Goodenough D. A. Cloning and expression of a Xenopus embryonic gap junction protein. Science. 1989 Mar 3;243(4895):1194–1195. doi: 10.1126/science.2466337. [DOI] [PubMed] [Google Scholar]
- Fishman G. I., Moreno A. P., Spray D. C., Leinwand L. A. Functional analysis of human cardiac gap junction channel mutants. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3525–3529. doi: 10.1073/pnas.88.9.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman G. I., Spray D. C., Leinwand L. A. Molecular characterization and functional expression of the human cardiac gap junction channel. J Cell Biol. 1990 Aug;111(2):589–598. doi: 10.1083/jcb.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg-Newton J. L., Dahl G., Loewenstein W. R. Cell junction and cyclic AMP: 1. Upregulation of junctional membrane permeability and junctional membrane particles by administration of cyclic nucleotide or phosphodiesterase inhibitor. J Membr Biol. 1981;63(1-2):105–121. doi: 10.1007/BF01969452. [DOI] [PubMed] [Google Scholar]
- Flagg-Newton J., Simpson I., Loewenstein W. R. Permeability of the cell-to-cell membrane channels in mammalian cell juncton. Science. 1979 Jul 27;205(4404):404–407. doi: 10.1126/science.377490. [DOI] [PubMed] [Google Scholar]
- Fox J. A. Ion channel subconductance states. J Membr Biol. 1987;97(1):1–8. doi: 10.1007/BF01869609. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Fine S. M., Unanue E. R., Chaplin D. D. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5649–5653. doi: 10.1073/pnas.85.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennemann H., Schwarz H. J., Willecke K. Characterization of gap junction genes expressed in F9 embryonic carcinoma cells: molecular cloning of mouse connexin31 and -45 cDNAs. Eur J Cell Biol. 1992 Feb;57(1):51–58. [PubMed] [Google Scholar]
- Imanaga I., Kameyama M., Irisawa H. Cell-to-cell diffusion of fluorescent dyes in paired ventricular cells. Am J Physiol. 1987 Jan;252(1 Pt 2):H223–H232. doi: 10.1152/ajpheart.1987.252.1.H223. [DOI] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Kanter H. L., Saffitz J. E., Beyer E. C. Cardiac myocytes express multiple gap junction proteins. Circ Res. 1992 Feb;70(2):438–444. doi: 10.1161/01.res.70.2.438. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Somogyi R. Biochemical and biophysical analysis of cell-to-cell channels and regulation of gap junctional permeability. Rev Physiol Biochem Pharmacol. 1991;118:1–47. doi: 10.1007/BFb0031480. [DOI] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Phillips W. C., Goodenough D. A. Gap junction structures. V. Structural chemistry inferred from X-ray diffraction measurements on sucrose accessibility and trypsin susceptibility. J Mol Biol. 1984 Apr 15;174(3):449–481. doi: 10.1016/0022-2836(84)90331-0. [DOI] [PubMed] [Google Scholar]
- Manivannan K., Ramanan S. V., Mathias R. T., Brink P. R. Multichannel recordings from membranes which contain gap junctions. Biophys J. 1992 Jan;61(1):216–227. doi: 10.1016/S0006-3495(92)81828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantz J., Cordier J., Giaume C. Effects of general anesthetics on intercellular communications mediated by gap junctions between astrocytes in primary culture. Anesthesiology. 1993 May;78(5):892–901. doi: 10.1097/00000542-199305000-00014. [DOI] [PubMed] [Google Scholar]
- Moreno A. P., Eghbali B., Spray D. C. Connexin32 gap junction channels in stably transfected cells. Equilibrium and kinetic properties. Biophys J. 1991 Nov;60(5):1267–1277. doi: 10.1016/S0006-3495(91)82160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A. P., Eghbali B., Spray D. C. Connexin32 gap junction channels in stably transfected cells: unitary conductance. Biophys J. 1991 Nov;60(5):1254–1266. doi: 10.1016/S0006-3495(91)82159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A. P., Fishman G. I., Spray D. C. Phosphorylation shifts unitary conductance and modifies voltage dependent kinetics of human connexin43 gap junction channels. Biophys J. 1992 Apr;62(1):51–53. doi: 10.1016/S0006-3495(92)81775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Single-channel currents of an intercellular junction. 1985 Sep 26-Oct 2Nature. 317(6035):331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Nicholson B., Dermietzel R., Teplow D., Traub O., Willecke K., Revel J. P. Two homologous protein components of hepatic gap junctions. Nature. 1987 Oct 22;329(6141):732–734. doi: 10.1038/329732a0. [DOI] [PubMed] [Google Scholar]
- Ramanan S. V., Brink P. R. Multichannel recordings from membranes which contain gap junctions. II. Substates and conductance shifts. Biophys J. 1993 Oct;65(4):1387–1395. doi: 10.1016/S0006-3495(93)81193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. E., Westphale E. M., Larson D. M., Wang H. Z., Veenstra R. D., Beyer E. C. Molecular cloning and functional expression of human connexin37, an endothelial cell gap junction protein. J Clin Invest. 1993 Mar;91(3):997–1004. doi: 10.1172/JCI116321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. B., Verselis V. K., Bennett M. V., Bargiello T. A. Molecular analysis of voltage dependence of heterotypic gap junctions formed by connexins 26 and 32. Biophys J. 1992 Apr;62(1):183–195. doi: 10.1016/S0006-3495(92)81804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rup D. M., Veenstra R. D., Wang H. Z., Brink P. R., Beyer E. C. Chick connexin-56, a novel lens gap junction protein. Molecular cloning and functional expression. J Biol Chem. 1993 Jan 5;268(1):706–712. [PubMed] [Google Scholar]
- Safranyos R. G., Caveney S., Miller J. G., Petersen N. O. Relative roles of gap junction channels and cytoplasm in cell-to-cell diffusion of fluorescent tracers. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2272–2276. doi: 10.1073/pnas.84.8.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann G., Wiegandt H., Rose B., Zimmerman A., Ben-Haim D., Loewenstein W. R. Diameter of the cell-to-cell junctional membrane channels as probed with neutral molecules. Science. 1981 Jul 31;213(4507):551–553. doi: 10.1126/science.7244653. [DOI] [PubMed] [Google Scholar]
- Simpson I., Rose B., Loewenstein W. R. Size limit of molecules permeating the junctional membrane channels. Science. 1977 Jan 21;195(4275):294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Burt J. M. Structure-activity relations of the cardiac gap junction channel. Am J Physiol. 1990 Feb;258(2 Pt 1):C195–C205. doi: 10.1152/ajpcell.1990.258.2.C195. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Cherbas L., Cherbas P., Morales E. A., Carrow G. M. Ionic coupling and mitotic synchrony of siblings in a Drosophila cell line. Exp Cell Res. 1989 Oct;184(2):509–517. doi: 10.1016/0014-4827(89)90348-0. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Jordan J. R., Beyer E. C., Paul D. L. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 1989 Apr 7;57(1):145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Connor J. A., Spray D. C., Bennett M. V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Weingart R. Inotropic effect of cyclic AMP in calf ventricular muscle studied by a cut end method. J Physiol. 1976 Aug;260(1):117–141. doi: 10.1113/jphysiol.1976.sp011507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra R. D., DeHaan R. L. Measurement of single channel currents from cardiac gap junctions. Science. 1986 Aug 29;233(4767):972–974. doi: 10.1126/science.2426781. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D. Developmental changes in regulation of embryonic chick heart gap junctions. J Membr Biol. 1991 Feb;119(3):253–265. doi: 10.1007/BF01868730. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., Wang H. Z., Westphale E. M., Beyer E. C. Multiple connexins confer distinct regulatory and conductance properties of gap junctions in developing heart. Circ Res. 1992 Nov;71(5):1277–1283. doi: 10.1161/01.res.71.5.1277. [DOI] [PubMed] [Google Scholar]
- Verselis V., Brink P. R. The gap junction channel. Its aqueous nature as indicated by deuterium oxide effects. Biophys J. 1986 Nov;50(5):1003–1007. doi: 10.1016/S0006-3495(86)83542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R., Levine E., Rabadan-Diehl C., Dahl G. Formation of hybrid cell-cell channels. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5380–5384. doi: 10.1073/pnas.86.14.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Fouly M. H., Trosko J. E., Chang C. C. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987 Feb;168(2):422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]