Abstract
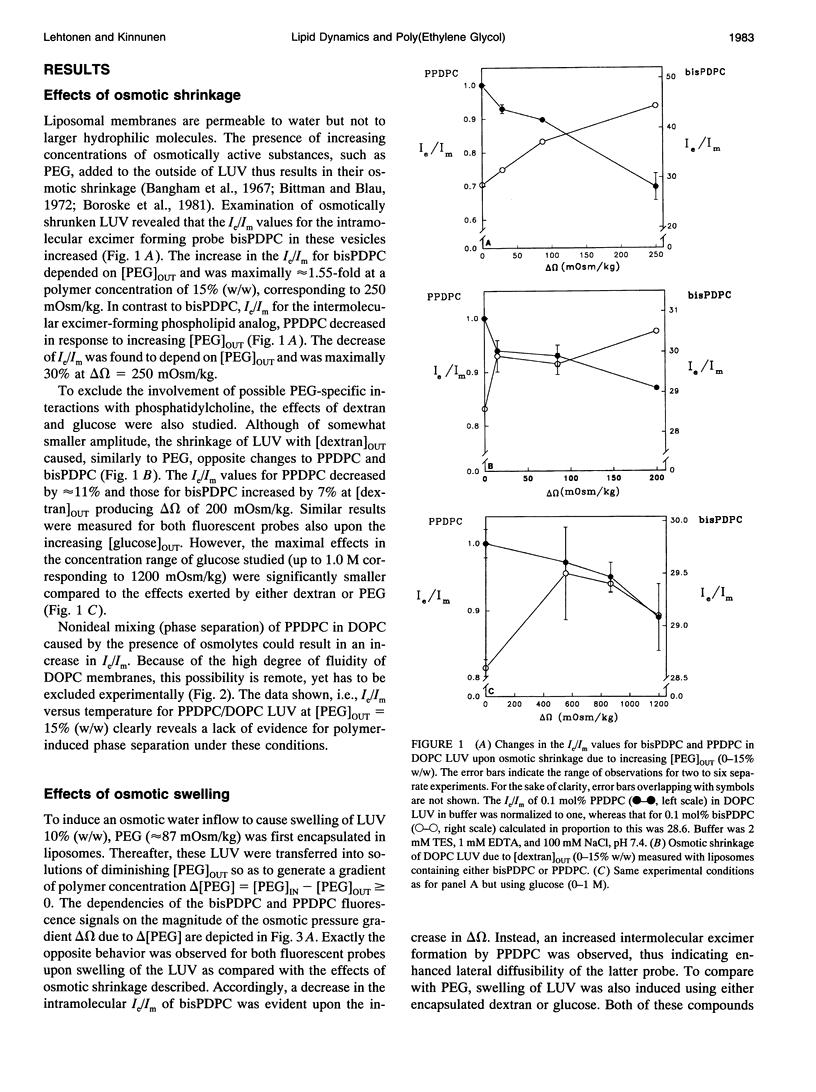
Influence of osmotic shrinkage, swelling, and dehydration on large unilamellar liposomes (LUVs) of 1,2-dioleoylsn-glycero-3-phosphocholine (DOPC) was investigated using the fluorescent lipid probes 1-palmitoyl-2-[10-(pyren-1-yl)]-decanoyl-sn-glycero-3-phosphocholi ne (PPDPC) and 1,2-bis[10-(pyren-1-yl)]decanoyl-sn-glycero-3-phosphocholine (bisPDPC). Increasing concentrations of poly(ethylene glycol) (PEG, average molecular weight of 6000) producing osmotic gradients delta omega up to 250 mOsm/kg were first added to the outside of LUV labeled with 0.1 mol% of either of the above fluorescent phospholipids. The resulting osmotic shrinkage was accompanied by a progressive reduction in the lateral diffusion of the membrane-incorporated PPDPC, evident as a decrease in the rate of its intermolecular excimer formation. In contrast, under the same conditions the rate of intramolecular excimer formation by bisPDPC increased. Notably, signals opposite to those described above were observed for both of the fluorescent probes upon osmotic swelling of DOPC liposomes with encapsulated PEG. The lateral diffusion of PPDPC became progressively reduced upon membrane dehydration due to increasing concentrations of symmetrically distributed PEG (with equal polymer concentrations inside and outside of the liposomes) when neither shrinkage nor swelling occurs while enhanced excimer formation by bisPDPC was evident. The later results were interpreted in terms of osmotically induced changes in the hydration of lipids. In brief, the removal of water from the phospholipid hydration shell diminishes the effective size of the polar headgroup, which subsequently allows for an enhanced lateral packing of the phospholipid acyl chains. Our findings are readily compatible with membrane free volume Vf changes due to osmotic forces under three different kinds of stress (shrinkage, swelling, and dehydration) applied on the lipid bilayers.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams S. B., Yager P. Perturbation of the chain melting transition of DPPC by galactose, agarose and Laurdan as determined by differential scanning calorimetry. Biochim Biophys Acta. 1993 Feb 23;1146(1):127–135. doi: 10.1016/0005-2736(93)90347-3. [DOI] [PubMed] [Google Scholar]
- Ahkong Q. F., Lucy J. A. Osmotic forces in artificially induced cell fusion. Biochim Biophys Acta. 1986 Jun 13;858(1):206–216. doi: 10.1016/0005-2736(86)90308-1. [DOI] [PubMed] [Google Scholar]
- Alexandre J., Lassalles J. P. Hydrostatic and osmotic pressure activated channel in plant vacuole. Biophys J. 1991 Dec;60(6):1326–1336. doi: 10.1016/S0006-3495(91)82170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa T., Timasheff S. N. Mechanism of poly(ethylene glycol) interaction with proteins. Biochemistry. 1985 Nov 19;24(24):6756–6762. doi: 10.1021/bi00345a005. [DOI] [PubMed] [Google Scholar]
- Arnold K., Pratsch L., Gawrisch K. Effect of poly(ethylene glycol) on phospholipid hydration and polarity of the external phase. Biochim Biophys Acta. 1983 Feb 9;728(1):121–128. doi: 10.1016/0005-2736(83)90444-3. [DOI] [PubMed] [Google Scholar]
- Arnold K., Zschoernig O., Barthel D., Herold W. Exclusion of poly(ethylene glycol) from liposome surfaces. Biochim Biophys Acta. 1990 Mar;1022(3):303–310. doi: 10.1016/0005-2736(90)90278-v. [DOI] [PubMed] [Google Scholar]
- Atha D. H., Ingham K. C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J Biol Chem. 1981 Dec 10;256(23):12108–12117. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bessler W. G., Schimmelpfeng L., Peters J. H. Potentiation of mitogen-induced lymphocyte stimulation by polyethylene glycols. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1253–1260. doi: 10.1016/0006-291x(77)90990-1. [DOI] [PubMed] [Google Scholar]
- Bittman R., Blau L. The phospholipid-cholesterol interaction. Kinetics of water permeability in liposomes. Biochemistry. 1972 Dec 5;11(25):4831–4839. doi: 10.1021/bi00775a029. [DOI] [PubMed] [Google Scholar]
- Borochov A., Borochov H. Increase in membrane fluidity in liposomes and plant protoplasts upon osmotic swelling. Biochim Biophys Acta. 1979 Feb 2;550(3):546–549. doi: 10.1016/0005-2736(79)90156-1. [DOI] [PubMed] [Google Scholar]
- Boroske E., Elwenspoek M., Helfrich W. Osmotic shrinkage of giant egg-lecithin vesicles. Biophys J. 1981 Apr;34(1):95–109. doi: 10.1016/S0006-3495(81)84839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Burgess S. W., Massenburg D., Yates J., Lentz B. R. Poly(ethylene glycol)-induced lipid mixing but not fusion between synthetic phosphatidylcholine large unilamellar vesicles. Biochemistry. 1991 Apr 30;30(17):4193–4200. doi: 10.1021/bi00231a013. [DOI] [PubMed] [Google Scholar]
- Burgess S. W., McIntosh T. J., Lentz B. R. Modulation of poly(ethylene glycol)-induced fusion by membrane hydration: importance of interbilayer separation. Biochemistry. 1992 Mar 17;31(10):2653–2661. doi: 10.1021/bi00125a004. [DOI] [PubMed] [Google Scholar]
- Butko P., Cheng K. H. Activation energy and entropy for intramolecular excimer formation in a dipyrenylphosphatidylcholine probe in lamellar and hexagonal lipid phases. Chem Phys Lipids. 1992 Jul;62(1):39–43. doi: 10.1016/0009-3084(92)90052-q. [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Chen S. Y., Butko P., Van der Meer B. W., Somerharju P. Intramolecular excimer formation of pyrene-labeled lipids in lamellar and inverted hexagonal phases of lipid mixtures containing unsaturated phosphatidylethanolamine. Biophys Chem. 1991 Feb;39(2):137–144. [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Finkelstein A. Osmotic swelling of phospholipid vesicles causes them to fuse with a planar phospholipid bilayer membrane. Science. 1982 Jul 30;217(4558):458–460. doi: 10.1126/science.6283637. [DOI] [PubMed] [Google Scholar]
- Cowley A. C., Fuller N. L., Rand R. P., Parsegian V. A. Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry. 1978 Jul 25;17(15):3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Carpenter J. F., Rudolph A. S., Wistrom C. A., Spargo B. J., Anchordoguy T. J. Interactions of sugars with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):367–384. doi: 10.1016/0304-4157(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Whittam M. A., Chapman D., Crowe L. M. Interactions of phospholipid monolayers with carbohydrates. Biochim Biophys Acta. 1984 Jan 11;769(1):151–159. doi: 10.1016/0005-2736(84)90018-x. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuneo P., Magri E., Verzola A., Grazi E. 'Macromolecular crowding' is a primary factor in the organization of the cytoskeleton. Biochem J. 1992 Jan 15;281(Pt 2):507–512. doi: 10.1042/bj2810507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döbereiner H. G., Käs J., Noppl D., Sprenger I., Sackmann E. Budding and fission of vesicles. Biophys J. 1993 Oct;65(4):1396–1403. doi: 10.1016/S0006-3495(93)81203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr K. J., Maeda M., Thompson G. A., Jr Concurrent changes in Dunaliella salina ultrastructure and membrane phospholipid metabolism after hyperosmotic shock. J Cell Biol. 1988 Aug;107(2):529–538. doi: 10.1083/jcb.107.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund K. K., Virtanen J. A., Kinnunen P. K., Kasurinen J., Somerharju P. J. Conformation of phosphatidylcholine in neat and cholesterol-containing liquid-crystalline bilayers. Application of a novel method. Biochemistry. 1992 Sep 15;31(36):8560–8565. doi: 10.1021/bi00151a025. [DOI] [PubMed] [Google Scholar]
- Eklund K. K., Vuorinen J., Mikkola J., Virtanen J. A., Kinnunen P. K. Ca2+-induced lateral phase separation in phosphatidic acid/phosphatidylcholine monolayers as revealed by fluorescence microscopy. Biochemistry. 1988 May 3;27(9):3433–3437. doi: 10.1021/bi00409a046. [DOI] [PubMed] [Google Scholar]
- Ertel A., Marangoni A. G., Marsh J., Hallett F. R., Wood J. M. Mechanical properties of vesicles. I. Coordinated analysis of osmotic swelling and lysis. Biophys J. 1993 Feb;64(2):426–434. doi: 10.1016/S0006-3495(93)81383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Metcalfe M. Free energy potential for aggregation of mixed phosphatidylcholine/phosphatidylserine lipid vesicles in glucose polymer (dextran) solutions. Biophys J. 1984 Apr;45(4):715–720. doi: 10.1016/S0006-3495(84)84213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W. Excimer-forming lipids in membrane research. Chem Phys Lipids. 1980 Oct;27(3):199–219. doi: 10.1016/0009-3084(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta. 1974 Feb 26;339(1):103–115. doi: 10.1016/0005-2736(74)90336-8. [DOI] [PubMed] [Google Scholar]
- Haines T. H., Li W., Green M., Cummins H. Z. The elasticity of uniform, unilamellar vesicles of acidic phospholipids during osmotic swelling is dominated by the ionic strength of the media. Biochemistry. 1987 Aug 25;26(17):5439–5447. doi: 10.1021/bi00391a034. [DOI] [PubMed] [Google Scholar]
- Hampton R. Y., Holz R. W. Effects of changes in osmolality on the stability and function of cultured chromaffin cells and the possible role of osmotic forces in exocytosis. J Cell Biol. 1983 Apr;96(4):1082–1088. doi: 10.1083/jcb.96.4.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantz E., Cao A., Escaig J., Taillandier E. The osmotic response of large unilamellar vesicles studied by quasielastic light scattering. Biochim Biophys Acta. 1986 Nov 17;862(2):379–386. doi: 10.1016/0005-2736(86)90241-5. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Herrmann A., Clague M. J., Blumenthal R. Enhancement of viral fusion by nonadsorbing polymers. Biophys J. 1993 Jul;65(1):528–534. doi: 10.1016/S0006-3495(93)81054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko R. C., Sugár I. P., Barenholz Y., Thompson T. E. Lateral distribution of a pyrene-labeled phosphatidylcholine in phosphatidylcholine bilayers: fluorescence phase and modulation study. Biochemistry. 1986 Jul 1;25(13):3813–3823. doi: 10.1021/bi00361a012. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Cell volume in the regulation of hepatic function: a mechanism for metabolic control. Biochim Biophys Acta. 1991 Dec 12;1071(4):331–350. doi: 10.1016/0304-4157(91)90001-d. [DOI] [PubMed] [Google Scholar]
- Ito T., Yamazaki M., Ohnishi S. Osmoelastic coupling in biological structures: a comprehensive thermodynamic analysis of the osmotic response of phospholipid vesicles and a reevaluation of the "dehydration force" theory. Biochemistry. 1989 Jun 27;28(13):5626–5630. doi: 10.1021/bi00439a043. [DOI] [PubMed] [Google Scholar]
- Iwasa K. H. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys J. 1993 Jul;65(1):492–498. doi: 10.1016/S0006-3495(93)81053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrasiak G. L., Hasty J. H. The electrical conductivity of hydrated phospholipids. Biochim Biophys Acta. 1974 Apr 26;348(1):45–54. doi: 10.1016/0005-2760(74)90091-5. [DOI] [PubMed] [Google Scholar]
- Jordan C. F., Lerman L. S., Venable J. H. Structure and circular dichroism of DNA in concentrated polymer solutions. Nat New Biol. 1972 Mar 22;236(64):67–70. doi: 10.1038/newbio236067a0. [DOI] [PubMed] [Google Scholar]
- Kaihovaara P., Raulo E., Kinnunen P. K. Changes in lipid distribution and dynamics in degranulated rat liver rough endoplasmic reticulum due to the membrane attachment of polyribosomes. Biochemistry. 1991 Aug 27;30(34):8380–8386. doi: 10.1021/bi00098a015. [DOI] [PubMed] [Google Scholar]
- Keith A. D., Snipes W., Chapman D. Spin-label studies on the aqueous regions of phospholipid multilayers. Biochemistry. 1977 Feb 22;16(4):634–641. doi: 10.1021/bi00623a013. [DOI] [PubMed] [Google Scholar]
- King M. D., Marsh D. Free volume model for lipid lateral diffusion coefficients. Assessment of the temperature dependence in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim Biophys Acta. 1986 Nov 6;862(1):231–234. doi: 10.1016/0005-2736(86)90489-x. [DOI] [PubMed] [Google Scholar]
- Kinnunen P. K., Rytömaa M., Kõiv A., Lehtonen J., Mustonen P., Aro A. Sphingosine-mediated membrane association of DNA and its reversal by phosphatidic acid. Chem Phys Lipids. 1993 Nov;66(1-2):75–85. doi: 10.1016/0009-3084(93)90033-y. [DOI] [PubMed] [Google Scholar]
- Klose G., Stelzner F. NMR investigations of the interaction of water with lecithin in benzene solutions. Biochim Biophys Acta. 1974 Aug 21;363(1):1–8. doi: 10.1016/0005-2736(74)90002-9. [DOI] [PubMed] [Google Scholar]
- Komatsu H., Rowe E. S. Effect of cholesterol on the ethanol-induced interdigitated gel phase in phosphatidylcholine: use of fluorophore pyrene-labeled phosphatidylcholine. Biochemistry. 1991 Mar 5;30(9):2463–2470. doi: 10.1021/bi00223a024. [DOI] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P. Measurement and modification of forces between lecithin bilayers. Biophys J. 1977 May;18(2):209–230. doi: 10.1016/S0006-3495(77)85608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Lee L. L. Preferential solvent interactions between proteins and polyethylene glycols. J Biol Chem. 1981 Jan 25;256(2):625–631. [PubMed] [Google Scholar]
- Lentz B. R., McIntyre G. F., Parks D. J., Yates J. C., Massenburg D. Bilayer curvature and certain amphipaths promote poly(ethylene glycol)-induced fusion of dipalmitoylphosphatidylcholine unilamellar vesicles. Biochemistry. 1992 Mar 17;31(10):2643–2653. doi: 10.1021/bi00125a003. [DOI] [PubMed] [Google Scholar]
- Lerebours B., Wehrli E., Hauser H. Thermodynamic stability and osmotic sensitivity of small unilamellar phosphatidylcholine vesicles. Biochim Biophys Acta. 1993 Oct 10;1152(1):49–60. doi: 10.1016/0005-2736(93)90230-w. [DOI] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Interactions between neutral phospholipid bilayer membranes. Biophys J. 1982 Mar;37(3):657–665. [PMC free article] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Measurement of the lateral compressibility of several phospholipid bilayers. Biophys J. 1982 Mar;37(3):667–672. [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. C., MacDonald R. I., Menco B. P., Takeshita K., Subbarao N. K., Hu L. R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta. 1991 Jan 30;1061(2):297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- MacDonald R. I. Membrane fusion due to dehydration by polyethylene glycol, dextran, or sucrose. Biochemistry. 1985 Jul 16;24(15):4058–4066. doi: 10.1021/bi00336a039. [DOI] [PubMed] [Google Scholar]
- Maeda M., Thompson G. A., Jr On the mechanism of rapid plasma membrane and chloroplast envelope expansion in Dunaliella salina exposed to hypoosmotic shock. J Cell Biol. 1986 Jan;102(1):289–297. doi: 10.1083/jcb.102.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Lucy J. A. Interactions of water-soluble fusogens with phospholipids in monolayers. FEBS Lett. 1978 Oct 15;94(2):301–304. doi: 10.1016/0014-5793(78)80962-4. [DOI] [PubMed] [Google Scholar]
- Martinac B., Adler J., Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990 Nov 15;348(6298):261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- Martinac B., Buechner M., Delcour A. H., Adler J., Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massenburg D., Lentz B. R. Poly(ethylene glycol)-induced fusion and rupture of dipalmitoylphosphatidylcholine large, unilamellar extruded vesicles. Biochemistry. 1993 Sep 7;32(35):9172–9180. doi: 10.1021/bi00086a024. [DOI] [PubMed] [Google Scholar]
- Mathai J. C., Sauna Z. E., John O., Sitaramam V. Rate-limiting step in electron transport. Osmotically sensitive diffusion of quinones through voids in the bilayer. J Biol Chem. 1993 Jul 25;268(21):15442–15454. [PubMed] [Google Scholar]
- McCammon J. R., Fan V. S. Release of membrane constituents following polyethylene glycol treatment of HEp-2 cells. Biochim Biophys Acta. 1979 Feb 20;551(1):67–73. doi: 10.1016/0005-2736(79)90353-5. [DOI] [PubMed] [Google Scholar]
- McCown J. T., Evans E., Diehl S., Wiles H. C. Degree of hydration and lateral diffusion in phospholipid multibilayers. Biochemistry. 1981 May 26;20(11):3134–3138. doi: 10.1021/bi00514a023. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- Minetti M., Aducci P., Viti V. Interaction of neutral polysaccharides with phosphatidylcholine multilamellar liposomes. Phase transitions studied by the binding of fluorescein-conjugated dextrans. Biochemistry. 1979 Jun 12;18(12):2541–2548. doi: 10.1021/bi00579a017. [DOI] [PubMed] [Google Scholar]
- Mui B. L., Cullis P. R., Evans E. A., Madden T. D. Osmotic properties of large unilamellar vesicles prepared by extrusion. Biophys J. 1993 Feb;64(2):443–453. doi: 10.1016/S0006-3495(93)81385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustonen P., Kinnunen P. K. Activation of phospholipase A2 by adriamycin in vitro. Role of drug-lipid interactions. J Biol Chem. 1991 Apr 5;266(10):6302–6307. [PubMed] [Google Scholar]
- Mustonen P., Kinnunen P. K. On the reversal by deoxyribonucleic acid of the binding of adriamycin to cardiolipin-containing liposomes. J Biol Chem. 1993 Jan 15;268(2):1074–1080. [PubMed] [Google Scholar]
- Mustonen P., Lehtonen J., Kõiv A., Kinnunen P. K. Effects of sphingosine on peripheral membrane interactions: comparison of adriamycin, cytochrome c, and phospholipase A2. Biochemistry. 1993 May 25;32(20):5373–5380. doi: 10.1021/bi00071a012. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Virtanen J. A., Somerharju P. J., Kinnunen P. K. Binding of cytochrome c to liposomes as revealed by the quenching of fluorescence from pyrene-labeled phospholipids. Biochemistry. 1987 Jun 2;26(11):2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- Müller H. J., Galla H. J. Pressure variation of the lateral diffusion in lipid bilayer membranes. Biochim Biophys Acta. 1983 Sep 7;733(2):291–294. doi: 10.1016/0005-2736(83)90535-7. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Wilkinson D. A. Lecithin bilayers. Density measurement and molecular interactions. Biophys J. 1978 Aug;23(2):159–175. doi: 10.1016/S0006-3495(78)85441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman G. C., Huang C. Structural studies on phophatidylcholine-cholesterol mixed vesicles. Biochemistry. 1975 Jul 29;14(15):3363–3370. doi: 10.1021/bi00686a012. [DOI] [PubMed] [Google Scholar]
- Ohno H., Maeda Y., Tsuchida E. 1H-NMR study of the effect of synthetic polymers on the fluidity, transition temperature and fusion of dipalmitoyl phosphatidylcholine small vesicles. Biochim Biophys Acta. 1981 Mar 20;642(1):27–36. doi: 10.1016/0005-2736(81)90134-6. [DOI] [PubMed] [Google Scholar]
- Oliet S. H., Bourque C. W. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993 Jul 22;364(6435):341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Signal transduction schemes of bacteria. Cell. 1993 Jun 4;73(5):857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- Perry J. W., Oka T. Regulation of ornithine decarboxylase in cultured mouse mammary gland by the osmolarity in the cellular environment. Biochim Biophys Acta. 1980 Apr 17;629(1):24–35. doi: 10.1016/0304-4165(80)90261-5. [DOI] [PubMed] [Google Scholar]
- Pollard H. B., Pazoles C. J., Creutz C. E., Scott J. H., Zinder O., Hotchkiss A. An osmotic mechanism for exocytosis from dissociated chromaffin cells. J Biol Chem. 1984 Jan 25;259(2):1114–1121. [PubMed] [Google Scholar]
- Poulin R., Pegg A. E. Regulation of ornithine decarboxylase expression by anisosmotic shock in alpha-difluoromethylornithine-resistant L1210 cells. J Biol Chem. 1990 Mar 5;265(7):4025–4032. [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Butko P., Francis G., Nicholls P. Measured change in protein solvation with substrate binding and turnover. Biochemistry. 1993 Jun 15;32(23):5925–5929. doi: 10.1021/bi00074a001. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Gruner S. M., Parsegian V. A. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 1990 Jan 9;29(1):76–87. doi: 10.1021/bi00453a010. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N., Parsegian V. A., Rau D. C. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 1988 Oct 4;27(20):7711–7722. doi: 10.1021/bi00420a021. [DOI] [PubMed] [Google Scholar]
- Rand R. P. Raising water to new heights. Science. 1992 May 1;256(5057):618–618. doi: 10.1126/science.256.5057.618. [DOI] [PubMed] [Google Scholar]
- Rayner M. D., Starkus J. G., Ruben P. C., Alicata D. A. Voltage-sensitive and solvent-sensitive processes in ion channel gating. Kinetic effects of hyperosmolar media on activation and deactivation of sodium channels. Biophys J. 1992 Jan;61(1):96–108. doi: 10.1016/S0006-3495(92)81819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytömaa M., Mustonen P., Kinnunen P. K. Reversible, nonionic, and pH-dependent association of cytochrome c with cardiolipin-phosphatidylcholine liposomes. J Biol Chem. 1992 Nov 5;267(31):22243–22248. [PubMed] [Google Scholar]
- Sackin H. A stretch-activated K+ channel sensitive to cell volume. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1731–1735. doi: 10.1073/pnas.86.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Hui S. W. Direct measurement of headgroup hydration of polar lipids in inverted micelles. Chem Phys Lipids. 1988 Dec;49(3):179–184. doi: 10.1016/0009-3084(88)90005-9. [DOI] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Yamazaki M., Ito T. Osmoelastic coupling in biological structures: formation of parallel bundles of actin filaments in a crystalline-like structure caused by osmotic stress. Biochemistry. 1989 Jul 25;28(15):6513–6518. doi: 10.1021/bi00441a052. [DOI] [PubMed] [Google Scholar]
- Tang D., Chong P. L. E/M dips. Evidence for lipids regularly distributed into hexagonal super-lattices in pyrene-PC/DMPC binary mixtures at specific concentrations. Biophys J. 1992 Oct;63(4):903–910. doi: 10.1016/S0006-3495(92)81672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. P., Huang C. H., Broccoli A. V., Leake L. Nuclear magnetic resonance studies of amphiphile hydration. Effects of cholesterol on phosphatidyl choline hydration. Arch Biochem Biophys. 1977 Sep;183(1):83–89. doi: 10.1016/0003-9861(77)90421-0. [DOI] [PubMed] [Google Scholar]
- Ter-Minassian-Saraga L., Madelmont G. Cholesterol-induced modulation of membrane hydration studies by thermal analysis. FEBS Lett. 1982 Jan 11;137(1):137–140. doi: 10.1016/0014-5793(82)80332-3. [DOI] [PubMed] [Google Scholar]
- Thuren T., Virtanen J. A., Kinnunen P. K. Estimation of the equilibrium lateral pressure in 1-palmitoyl-2-[6(pyren-1-yl)]hexanoyl-glycerophospholipid liposomes. Chem Phys Lipids. 1986 Oct-Nov;41(3-4):329–334. doi: 10.1016/0009-3084(86)90030-7. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Fisher D. Interaction of phospholipid membranes with poly(ethylene glycol)s. Biochim Biophys Acta. 1979 Oct 19;557(1):53–61. doi: 10.1016/0005-2736(79)90089-0. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Fisher D. The interaction of phospholipid membranes with poly(ethylene glycol). Vesicle aggregation and lipid exchange. Biochim Biophys Acta. 1982 Jun 14;688(2):645–652. doi: 10.1016/0005-2736(82)90375-3. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., van den Berghe N., Tertoolen L. G., Edixhoven M. J., de Jonge H. R. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J Biol Chem. 1993 Sep 25;268(27):19919–19922. [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. A., Morowitz H. J., Prestegard J. H. Hydration of phosphatidylocholine. Adsorption isotherm and proton nuclear magnetic resonance studies. Biophys J. 1977 Nov;20(2):169–179. doi: 10.1016/S0006-3495(77)85542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. A., Nagle J. F. Dilatometric study of binary mixtures of phosphatidylcholines. Biochemistry. 1979 Sep 18;18(19):4244–4249. doi: 10.1021/bi00586a032. [DOI] [PubMed] [Google Scholar]
- Wollnik B., Kubisch C., Maass A., Vetter H., Neyses L. Hyperosmotic stress induces immediate-early gene expression in ventricular adult cardiomyocytes. Biochem Biophys Res Commun. 1993 Jul 30;194(2):642–646. doi: 10.1006/bbrc.1993.1869. [DOI] [PubMed] [Google Scholar]
- Wu J. R., Lentz B. R. Mechanism of poly(ethylene glycol)-induced lipid transfer between phosphatidylcholine large unilamellar vesicles: a fluorescent probe study. Biochemistry. 1991 Jul 9;30(27):6780–6787. doi: 10.1021/bi00241a022. [DOI] [PubMed] [Google Scholar]
- Xiang T. X. A computer simulation of free-volume distributions and related structural properties in a model lipid bilayer. Biophys J. 1993 Sep;65(3):1108–1120. doi: 10.1016/S0006-3495(93)81156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M., Ohnishi S., Ito T. Osmoelastic coupling in biological structures: decrease in membrane fluidity and osmophobic association of phospholipid vesicles in response to osmotic stress. Biochemistry. 1989 May 2;28(9):3710–3715. doi: 10.1021/bi00435a013. [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Ohshika M., Kashiwagi N., Asano T. Phase transitions of phospholipid vesicles under osmotic stress and in the presence of ethylene glycol. Biophys Chem. 1992 May;43(1):29–37. doi: 10.1016/0301-4622(92)80039-8. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Parsegian V. A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986 Sep 4;323(6083):36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]
- vom Dahl S., Hallbrucker C., Lang F., Häussinger D. Regulation of cell volume in the perfused rat liver by hormones. Biochem J. 1991 Nov 15;280(Pt 1):105–109. doi: 10.1042/bj2800105. [DOI] [PMC free article] [PubMed] [Google Scholar]


