Abstract
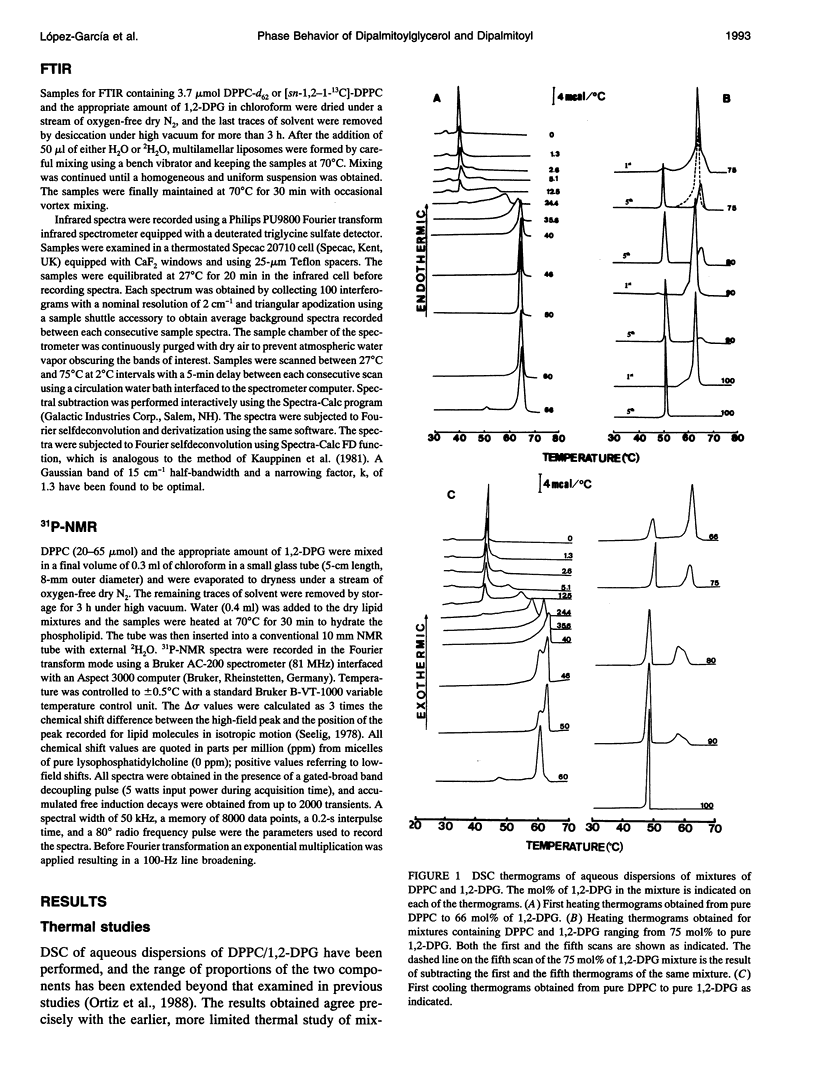
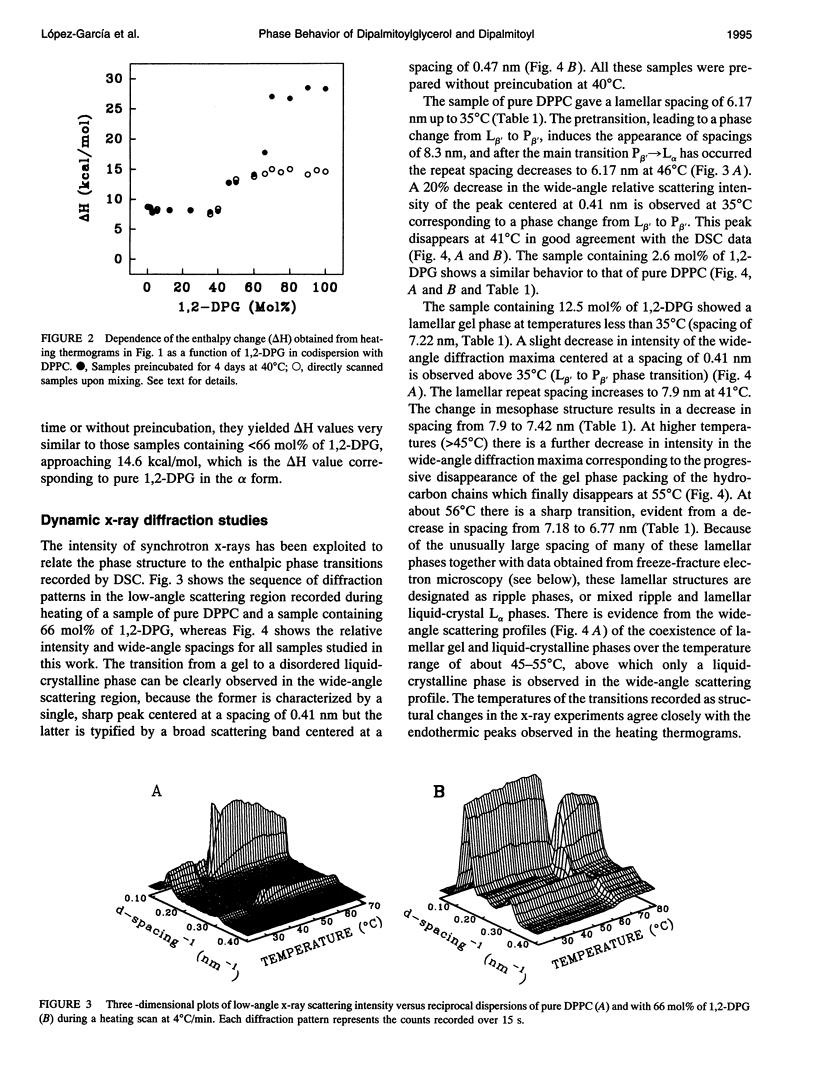
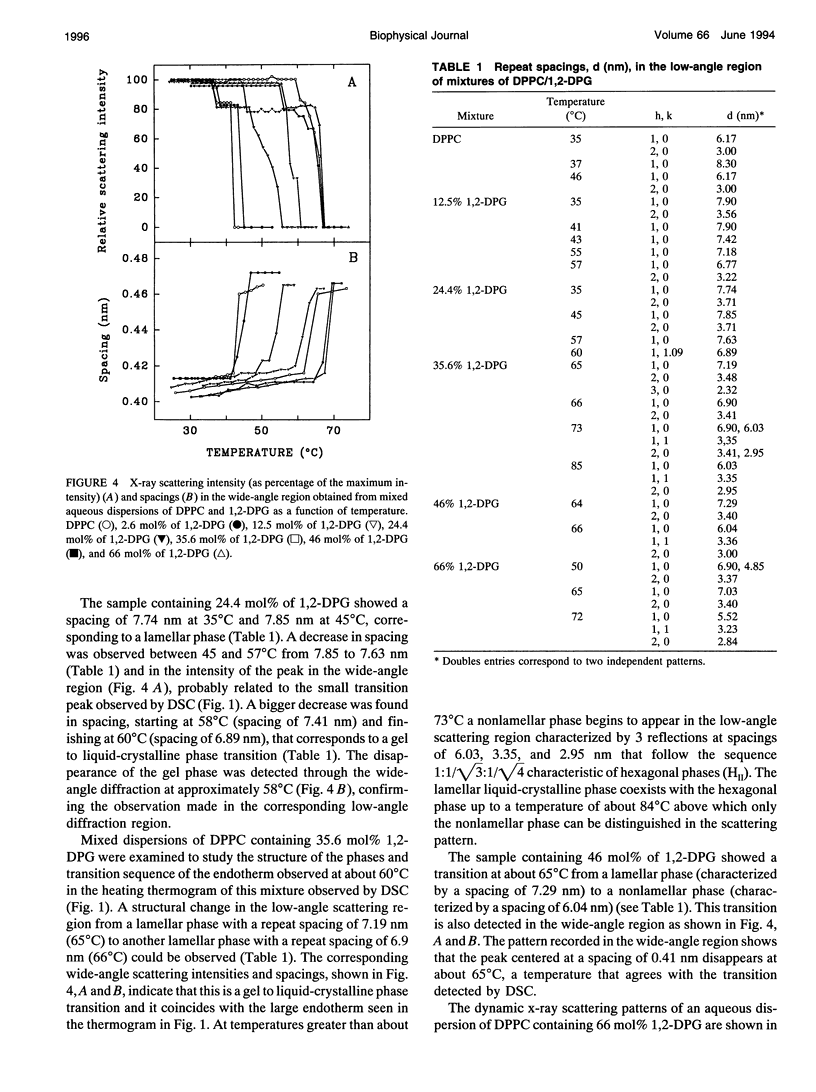
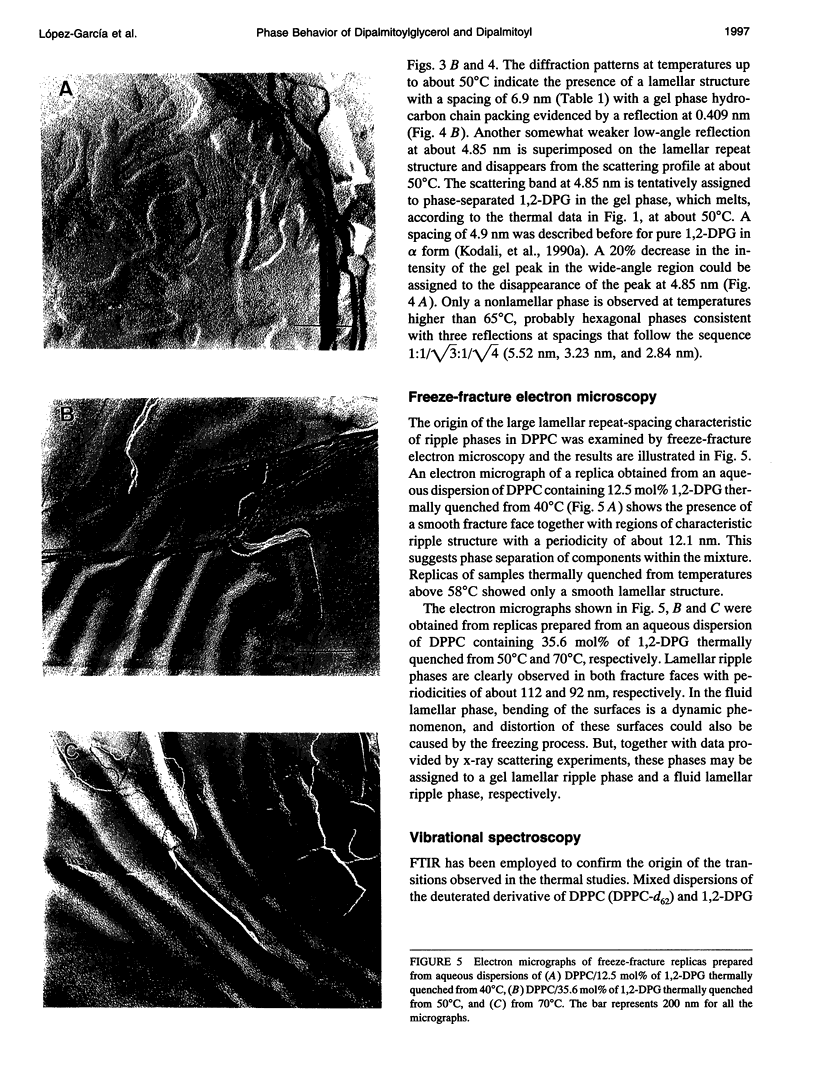
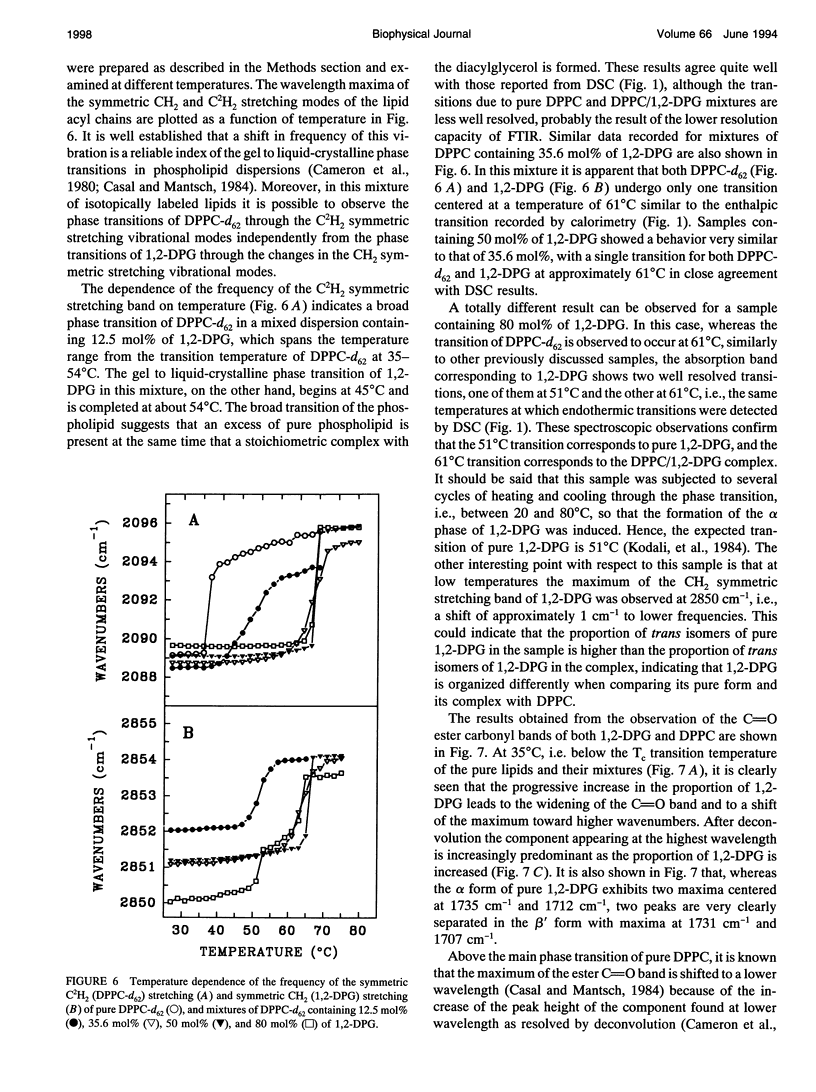
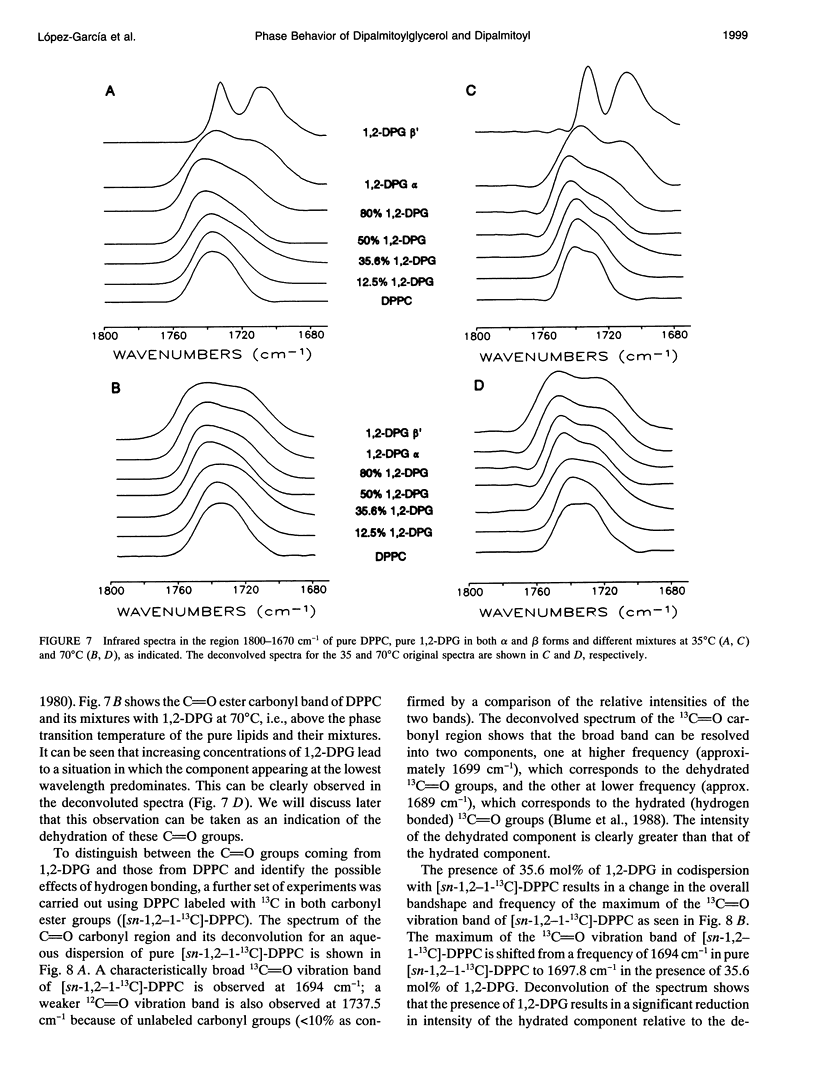
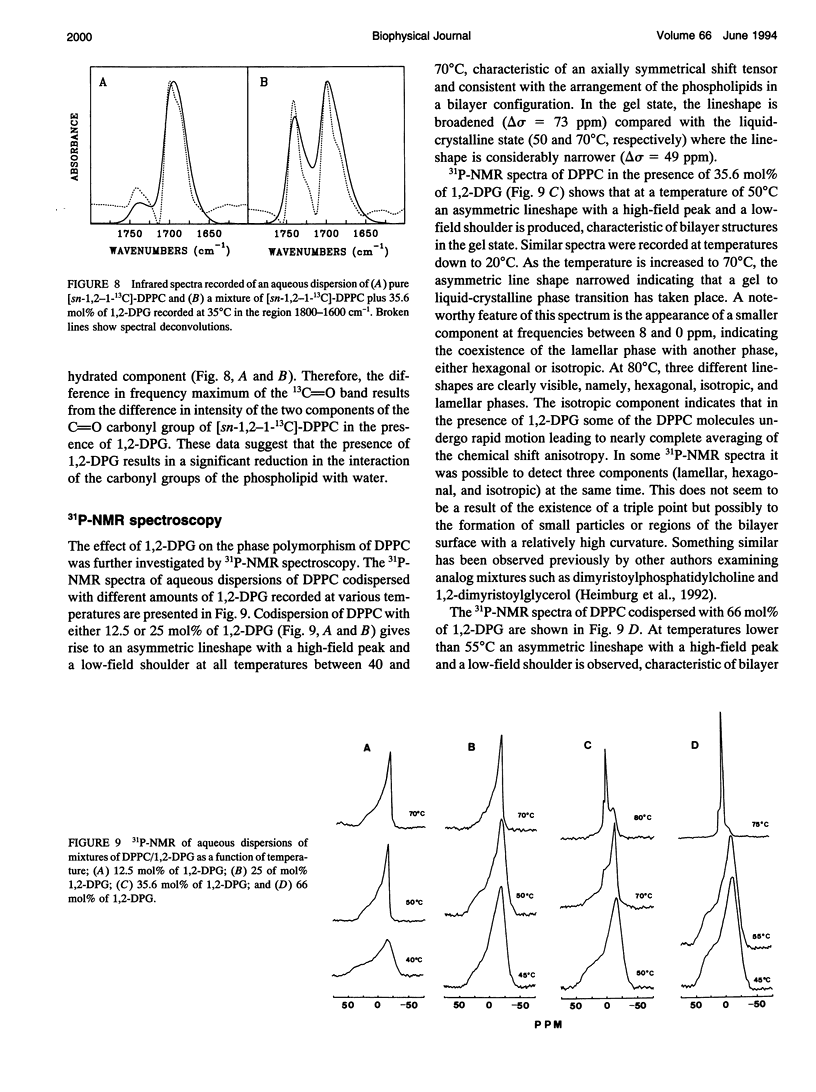
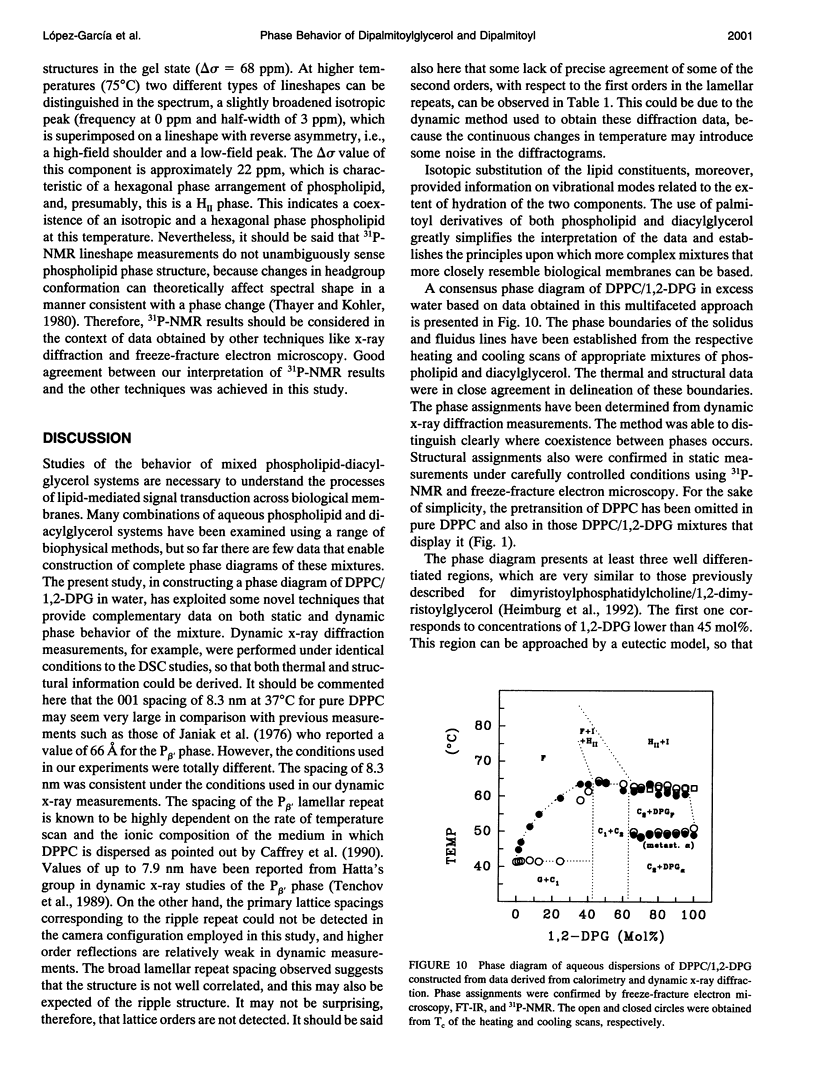
The phases and transition sequences for aqueous dispersions of mixtures of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-dipalmitoyl-sn-glycerol (1,2-DPG) have been studied by differential scanning calorimetry, dynamic x-ray diffraction, freeze-fracture electron microscopy, 31P-nuclear magnetic resonance spectroscopy, and Fourier-transform infrared spectroscopy. The results have been used to construct a dynamic phase diagram of the binary mixture as a function of temperature over the range 20 degrees-90 degrees C. It is concluded that DPPC and 1,2-DPG form two complexes in the gel phase, the first one with a DPPC/1,2-DPG molar ratio of 55:45 and the second one at a molar ratio of approximately 1:2, defining three different regions in the phase diagram. Two eutectic points are postulated to occur: one at a very low 1,2-DPG concentration and the other at a 1,2-DPG concentration slightly higher than 66 mol%. At temperatures higher than the transition temperature, lamellar phases were predominant at low 1,2-DPG concentrations, but nonlamellar phases were found to be predominant at high proportions of 1,2-DPG. A very important aspect of these DPPC/1,2-DPG mixtures was that, in the gel phase, they showed a ripple structure, as seen by freeze-fracture electron microscopy and consistent with the high lamellar repeat spacings seen by x-ray diffraction. Ripple phase characteristics were also found in the fluid lamellar phases occurring at concentrations up to 35.6 mol% of 1,2-DPG. Evidence was obtained by Fourier transform infrared spectroscopy of the dehydration of the lipid-water interface induced by the presence of 1,2-DPG. The biological significance of the presence of diacylglycerol in membrane lipid domains is discussed.
Full text
PDF





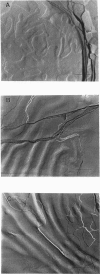
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Blume A., Hübner W., Messner G. Fourier transform infrared spectroscopy of 13C = O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988 Oct 18;27(21):8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- Burger K. N., Nieva J. L., Alonso A., Verkleij A. J. Phospholipase C activity-induced fusion of pure lipid model membranes. A freeze fracture study. Biochim Biophys Acta. 1991 Sep 30;1068(2):249–253. doi: 10.1016/0005-2736(91)90216-u. [DOI] [PubMed] [Google Scholar]
- Caffrey M., Fanger G., Magin R. L., Zhang J. Kinetics of the premelting (L beta'-P beta') and main transition (P beta'-L alpha) in hydrated dipalmitoylphosphatidylcholine. A time-resolved x-ray diffraction study using microwave-induced temperature-jumps. Biophys J. 1990 Sep;58(3):677–686. doi: 10.1016/S0006-3495(90)82410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D. G., Casal H. L., Mantsch H. H. Characterization of the pretransition in 1,2-dipalmitoyl-sn-glycero-3-phosphocholine by Fourier transform infrared spectroscopy. Biochemistry. 1980 Aug 5;19(16):3665–3672. doi: 10.1021/bi00557a005. [DOI] [PubMed] [Google Scholar]
- Casal H. L., Mantsch H. H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim Biophys Acta. 1984 Dec 4;779(4):381–401. doi: 10.1016/0304-4157(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Cevc G. Polymorphism of the bilayer membranes in the ordered phase and the molecular origin of the lipid pretransition and rippled lamellae. Biochim Biophys Acta. 1991 Feb 11;1062(1):59–69. doi: 10.1016/0005-2736(91)90335-6. [DOI] [PubMed] [Google Scholar]
- Cheng K. H., Hui S. W. Correlation between bilayer destabilization and activity enhancement by diacylglycerols in reconstituted Ca-ATPase vesicles. Arch Biochem Biophys. 1986 Jan;244(1):382–386. doi: 10.1016/0003-9861(86)90127-x. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Tsujita T., Brockman H. L. Enzymatic and physical characterization of diacylglycerol-phosphatidylcholine interactions in bilayers and monolayers. Biochemistry. 1989 Jan 10;28(1):32–40. doi: 10.1021/bi00427a006. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Modification by diacylglycerol of the structure and interaction of various phospholipid bilayer membranes. Biochemistry. 1986 May 20;25(10):2882–2889. doi: 10.1021/bi00358a022. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. L., Irvine R. F. Diacylglycerol potentiates phospholipase attack upon phospholipid bilayers: possible connection with cell stimulation. Biochem Biophys Res Commun. 1983 Nov 30;117(1):196–201. doi: 10.1016/0006-291x(83)91560-7. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Bray J., Quinn P. J. Long-chain unsaturated diacylglycerols cause a perturbation in the structure of phospholipid bilayers rendering them susceptible to phospholipase attack. Biochem Biophys Res Commun. 1984 Dec 14;125(2):836–842. doi: 10.1016/0006-291x(84)90615-6. [DOI] [PubMed] [Google Scholar]
- De Boeck H., Zidovetzki R. Effects of diacylglycerols on the structure of phosphatidylcholine bilayers: a 2H and 31P NMR study. Biochemistry. 1989 Sep 5;28(18):7439–7446. doi: 10.1021/bi00444a043. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Bhamidipati S. P., Kodali D. R., Small D. M. The interfacial conformation and transbilayer movement of diacylglycerols in phospholipid bilayers. J Biol Chem. 1991 Jan 15;266(2):1177–1186. [PubMed] [Google Scholar]
- Heimburg T., Würz U., Marsh D. Binary phase diagram of hydrated dimyristoylglycerol-dimyristoylphosphatidylcholine mixtures. Biophys J. 1992 Nov;63(5):1369–1378. doi: 10.1016/S0006-3495(92)81714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Nature of the Thermal pretransition of synthetic phospholipids: dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976 Oct 19;15(21):4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- Kodali D. R., Fahey D. A., Small D. M. Structure and polymorphism of saturated monoacid 1,2-diacyl-sn-glycerols. Biochemistry. 1990 Dec 4;29(48):10771–10779. doi: 10.1021/bi00500a008. [DOI] [PubMed] [Google Scholar]
- Kodali D. R., Tercyak A., Fahey D. A., Small D. M. Acyl migration in 1,2-dipalmitoyl-sn-glycerol. Chem Phys Lipids. 1990 Feb;52(3-4):163–170. doi: 10.1016/0009-3084(90)90111-4. [DOI] [PubMed] [Google Scholar]
- Luzzati V., Vargas R., Gulik A., Mariani P., Seddon J. M., Rivas E. Lipid polymorphism: a correction. The structure of the cubic phase of extinction symbol Fd-- consists of two types of disjointed reverse micelles embedded in a three-dimensional hydrocarbon matrix. Biochemistry. 1992 Jan 14;31(1):279–285. doi: 10.1021/bi00116a038. [DOI] [PubMed] [Google Scholar]
- Marsh D., Seddon J. M. Gel-to-inverted hexagonal (L beta-HII) phase transitions in phosphatidylethanolamines and fatty acid-phosphatidylcholine mixtures, demonstrated by 31P-NMR spectroscopy and x-ray diffraction. Biochim Biophys Acta. 1982 Aug 25;690(1):117–123. doi: 10.1016/0005-2736(82)90245-0. [DOI] [PubMed] [Google Scholar]
- Nieva J. L., Goñi F. M., Alonso A. Liposome fusion catalytically induced by phospholipase C. Biochemistry. 1989 Sep 5;28(18):7364–7367. doi: 10.1021/bi00444a032. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Aranda F. J., Villalaín J., San Martín C., Micol V., Gómez-Fernandez J. C. 1,2-Dioleoylglycerol promotes calcium-induced fusion in phospholipid vesicles. Chem Phys Lipids. 1992 Oct;62(3):215–224. doi: 10.1016/0009-3084(92)90058-w. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Villalaín J., Gómez-Fernández J. C. Interaction of diacylglycerols with phosphatidylcholine vesicles as studied by differential scanning calorimetry and fluorescence probe depolarization. Biochemistry. 1988 Dec 13;27(25):9030–9036. doi: 10.1021/bi00425a022. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Seddon J. M. An inverse face-centered cubic phase formed by diacylglycerol-phosphatidylcholine mixtures. Biochemistry. 1990 Aug 28;29(34):7997–8002. doi: 10.1021/bi00486a031. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Tenchov B. G., Yao H., Hatta I. Time-resolved x-ray diffraction and calorimetric studies at low scan rates: I. Fully hydrated dipalmitoylphosphatidylcholine (DPPC) and DPPC/water/ethanol phases. Biophys J. 1989 Oct;56(4):757–768. doi: 10.1016/S0006-3495(89)82723-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer A. M., Kohler S. J. Phosphorus-31 nuclear magnetic resonance spectra characteristic of hexagonal and isotropic phospholipid phases generated from phosphatidylethanolamine in the bilayer phase. Biochemistry. 1981 Nov 24;20(24):6831–6834. doi: 10.1021/bi00527a014. [DOI] [PubMed] [Google Scholar]
- van Dijck P. W., Kaper A. J., Oonk H. A., de Gier J. Miscibility properties of binary phosphatidylcholine mixtures. A calorimetric study. Biochim Biophys Acta. 1977 Oct 3;470(1):58–69. doi: 10.1016/0005-2736(77)90061-x. [DOI] [PubMed] [Google Scholar]
- van Gorkom L. C., Nie S. Q., Epand R. M. Hydrophobic lipid additives affect membrane stability and phase behavior of N-monomethyldioleoylphosphatidylethanolamine. Biochemistry. 1992 Jan 28;31(3):671–677. doi: 10.1021/bi00118a006. [DOI] [PubMed] [Google Scholar]